Abstract
Background
Intrauterine contraception is a first-line option for young women, yet relatively few prospective studies have been performed in nulliparous women using currently available devices, and many providers are still reluctant to provide this option.
Methods
Between January 2012 and June 2014, 109 nulliparous women, aged 18–30 years, who had an intrauterine device (IUD) placed at a student health clinic [88 levonorgestrel-intrauterine system (LNG-IUS) users and 21 Cu T 380A (IUD) users] were surveyed at 1, 6, 12 and 18 months after insertion.
Results
Overall satisfaction was high; at follow-up survey 83% of 100 women (mean use 13.4 months) were ‘happy’ or ‘very happy’ with their IUD, and there were no differences in satisfaction between the two IUD types. Some 75% of participants stated that the insertion procedure went ‘very well’, despite 78% rating insertion pain as moderate to severe, and 46% experiencing vasovagal symptoms. The 12-month continuation rate was 89%, with discontinuations for expulsion (3%), side effects (6%), lack of anticipated benefit (1%) and pregnancy (1%). Users of the Cu T 380A were more likely to have heavy menses (74% vs 2%; p<0.0001) or moderate to severe cramping (68% vs 20%; p=0.0002) compared with LNG-IUS users. There were no uterine perforations or diagnoses of pelvic inflammatory disease. The rate of failed insertions during the study period was 6.2%.
Conclusions
Despite significant symptoms with insertion, intrauterine contraception is safe, effective and ultimately well tolerated in nulliparous women and should be provided to this population in both university and community health settings.
Keywords: intrauterine devices, intrauterine systems, long-acting reversible contraception
Key message points.
Satisfaction with both the levonorgestrel-releasing intrauterine system (LNG-IUS) and Copper T 380A intrauterine device is very high in young, nulliparous women, despite significant symptoms during the insertion procedure.
Copper T 380A users are more likely to have heavy menses and dysmenorrhoea than those who choose the LNG-IUS.
Introduction
Intrauterine contraception is convenient, safe and highly efficacious, and is recommended as a first-line option for all women, including adolescent and nulliparous women.1 Despite this recommendation, uptake is relatively low; only 5.6% of all contraceptive users and 3% of adolescents were using an intrauterine device (IUD) in a 2010 survey.2 Two reasons for this discrepancy have been proposed. First, many young women are unaware of the availability of IUDs; two studies found that fewer than 50% of adolescents and young women had knowledge of them.3 4 Second, despite evidence-based guidelines promoting their use and studies demonstrating their safety in nulliparous and adolescent women, many providers are still reluctant to recommend IUDs. In a recent survey of obstetrician-gynaecologists, only 67% considered nulliparous women to be appropriate candidates, and just 43% felt that IUDs should be considered as first-line options for adolescents.5
We were able to identify only five prospective studies of nulliparous women, representing a total of 818 subjects, that were published after 1980 and used devices currently available in the USA.6–10 The CHOICE study of Long Acting Reversible Contraception in St Louis has a very sizeable population of 853 nulliparous subjects using IUDs but has not to date reported their outcomes separately from parous women; differences in satisfaction and continuation based on parity are stated to be less than 10%.11
The aim of our study was to follow nulliparous college students who chose intrauterine contraception and measure continuation rates, satisfaction and subjective experiences of both insertion and ongoing use, with the goal of further educating both providers and patients on the expected outcomes in this population.
Methods
Gannett Health Services provides primary care to the roughly 22 000 students of Cornell University's Ithaca, New York campus. We offered enrollment in the study to all women aged 18 years and over with no history of pregnancy beyond 20 weeks who had an IUD, either the Paragard® Copper T 380A (Cu T 380A) or the Mirena® levonorgestrel intrauterine system (LNG-IUS) electively placed at Gannett Health Services between 20 January 2012 and 20 June 2013. Informed consent was obtained from all subjects, and the study was conducted in compliance with and with approval from the Institutional Review Boards of both authors’ institutions.
Online surveys were sent to the participants by email using Qualtrics® software (licensed by Cornell University) at 1, 6, 12 and 18 months after IUD placement. The initial survey asked questions regarding gynaecological history, as well as the woman's experience of the IUD insertion. Subsequent surveys were identical to each other and assessed complications, bleeding patterns, continuation and overall satisfaction. Women who discontinued the use of the IUD were excluded from subsequent surveys. Women who did not respond were still invited to take future surveys. Months of observed use were calculated by using the latest survey received (6-, 12- or 18-month) for continuing users, or for actual duration of use for those who discontinued. Satisfaction was assessed using five-point Likert-type scales and was taken from the most recent survey received from each subject for both continuers and discontinuers. All participants received a US$20 gift card for each completed survey. The electronic medical record was used to clarify subjective data when necessary. Event rates are reported for the first 12 months of observation and using the Pearl index for the entire study period.12 Data were analysed using Microsoft Excel® and GraphPad® software. For categorical data, Fisher's exact test was performed using a p<0.05 to determine significance.
This was an observational study; participants received standard contraceptive care as practised at Gannett Health Services at the time. Women interested in an IUD were first scheduled for a consultation visit for education about the two types available, discussion of the procedure, and review of medical eligibility according to the USA Centers for Disease Control (CDC) guidelines.13 Extensive written material was also provided about the method as well as the insertion procedure itself. Insertions were scheduled within 5–7 days of onset of menses and performed by one of three providers: two physicians and a nurse practitioner, all family medicine trained. Chlamydia and gonorrhoea testing was recommended if appropriate as per CDC screening guidelines but was not mandatory.
In accordance with clinic protocol at the time, all women were instructed to take 800 mg ibuprofen and/or 1000 mg paracetamol 1 hour prior to the procedure and were prescribed misoprostol 200 μg to be taken orally the night before and the morning of the insertion. Topical anaesthetic spray was applied to the cervix before tenaculum placement, and an endometrial pipelle was used as a uterine sound, pre-filled with 1 cc 2% lidocaine, which was then instilled in the uterine cavity. Dilators and cervical blocks were not used, as they were beyond the scope of practice of our practitioners. In other aspects, insertion was carried out in accordance with the manufacturer's instructions. Women were observed for 15 minutes or until they felt ready to leave and then scheduled for a recheck visit in 6 weeks.
Women in whom an IUD insertion was attempted but was unsuccessful were not included in the study population. During the study period, a total of 198 insertions were scheduled at Gannett Health Services. For 15 of these, no placement occurred; three were not attempted (existing pregnancy, cervicitis at time of visit, and syncope before the procedure began), and 12 were attempted but were not successful due to either cervical stenosis or insufficient uterine depth. Therefore, at Gannett Health Services during this period, a total of 6.2% of attempted insertions were unsuccessful.
Results
A total of 116 women enrolled in the study, of whom 109 (88 LNG-IUS and 21 Cu T 380A users) completed at least one study survey, for an overall response rate of 94%. Continuation data (at least 1-, 6-, 12- or 18-month survey) was provided by 86% of all enrolled. (For detailed response rate data see online Supplementary Material: Response rates) Mean age was 24.7 (range 18–30) years. All women were enrolled at Cornell University and thus had high levels of education. Data on race/ethnicity was provided by 104 participants as follows: Asian 7%, Black 3%, Hispanic 1%, Multiracial 13%, Unknown 13% and White 64%. Current level of condom use was reported as ‘never’ by 46% and ‘always’ by 18% of subjects. Number of sexual partners in the preceding 12 months ranged from 1 to 10, with a mean of 1.9.
Insertion visit
The vast majority of women (85%) felt ‘very well informed’ for their insertion visit. Symptoms from the pre-medication (misoprostol, ibuprofen and/or paracetamol) were relatively infrequent, with 87% of women reporting no nausea and 74% reporting no cramping.
For symptoms related to insertion see Table 1. There were no significant differences in symptoms between the two device types. One participant reported brief loss of consciousness, which occurred 15 minutes after insertion. Three women reported that they felt fine during the procedure but developed significant vasovagal symptoms after leaving the office.
Table 1.
Symptoms related to intrauterine device insertion
| Symptom | n (%) |
|---|---|
| Pain with IUD insertion | |
| None | 0 (0) |
| Mild | 19 (23) |
| Moderate | 29 (35) |
| Severe | 36 (42) |
| Light-headedness, nausea, or sweating during procedure* | |
| None | 45 (54) |
| Mild | 16 (19) |
| Moderate | 20 (24) |
| Severe | 3 (4) |
| Pain in first 24 hours | |
| None | 4 (5) |
| Mild | 30 (36) |
| Moderate | 34 (40) |
| Severe | 16 (19) |
| Pain at 24–72 hours | |
| None | 19 (23) |
| Mild | 39 (46) |
| Moderate | 16 (19) |
| Severe | 10 (12) |
| Pain after 1 week | |
| None | 52 (62) |
| Mild | 15 (18) |
| Moderate | 13 (15) |
| Severe | 4 (5) |
*Percentages do not add up to 100% due to rounding.
IUD, intrauterine device.
Seventy-five percent reported that they felt the insertion procedure went ‘very well’. Only one woman stated that the procedure went poorly, as her insertion required two attempts to be successful.
Seventeen subjects reported moderate or severe cramping after 1 week. Of these women, one was found to have a partial expulsion at the 6-week follow-up visit, one had endometritis due to bacterial vaginosis, one was lost to follow-up, and the remaining 14 denied any continued pain at the 6-week visit.
Continuation
A total of 11 expulsions or discontinuations were recorded during the study period (Table 2). Pearl index and 12-month rates were calculated for all expulsion and discontinuation events in the 100 women who provided follow-up surveys (Table 3).
Table 2.
Details of expulsion and discontinuation events
| Time (months) | Reason | IUD type | Comments |
|---|---|---|---|
| 1 | Expulsion | LNG-IUS | |
| 2 | Expulsion | Cu T 380A | |
| 4 | Expulsion | Cu T 380A | |
| 4 | Side effects | Cu T 380A | Heavy bleeding and spotting |
| 4 | Lack of benefit | LNG-IUS | Breast size did not decrease |
| 5 | Pregnancy | Cu T 380A | Intrauterine pregnancy |
| 6–12* | Side effects | LNG-IUS | Pain, daily cramping, acne, decreased libido, continual spotting, headaches |
| 6–12* | Side effects | Cu T 380A | Heavy bleeding, severe cramps |
| 8 | Side effects | LNG-IUS | Acne, decreased libido, headaches |
| 12 | Side effects | LNG-IUS | Acne, pain, mood changes |
| 12–18† | Side effects | LNG-IUS | Cramps, heavy bleeding, pain with intercourse |
*Exact timing of discontinuation unknown. Six months used for calculation of observed use.
†Exact timing of discontinuation unknown. Twelve months used for calculation of observed use.
Cu T 380A, copper intrauterine device; IUD, intrauterine device; LNG-IUS, levonorgestrel intrauterine system.
Table 3.
Continuation, expulsion and discontinuation: 12-month rates and study period Pearl indices
| Total | LNG-IUS | Cu T 380A | p | ||||
|---|---|---|---|---|---|---|---|
| 12-month n (%) |
Study period n (PI) |
12-month n (%) |
Study period n (PI) |
12-month n (%) |
Study period n (PI) |
||
| Continuation | 81 (89) | 67 (93) | 14 (74) | 0.03 | |||
| Expulsion | 3 (3.3) | 3 (2.7) | 1 (1.4) | 1 (1.1) | 2 (10.5) | 2 (11.2) | |
| Discontinuation | 7 (7.7) | 8 (7.1) | 4 (5.6) | 5 (5.3) | 3 (15.8) | 3 (16.9) | |
| Side effects | 5 (5.5) | 6 (5.3) | 3 (4.2) | 4 (4.2) | 2 (10.5) | 2 (16.9) | |
| Pregnancy | 1 (1.1) | 1 (0.9) | 0 | 0 | 1 (5.3) | 1 (5.6) | |
| Lack of benefit | 1 (1.1) | 1 (0.9) | 1 (1.4) | 1 (1.1) | 0 | 0 | |
| Total n for 12-month rates | 91 | 72 | 19 | ||||
| Woman-years observation for PI | 112.8 | 95.0 | 17.8 | ||||
Two-tailed p value calculated with Fisher's exact test for difference between intrauterine device types. p values not shown if >0.05.
Cu T 380A, copper intrauterine device; LNG-IUS, levonorgestrel intrauterine system; PI, Pearl index (=number of events per 100 woman-years).
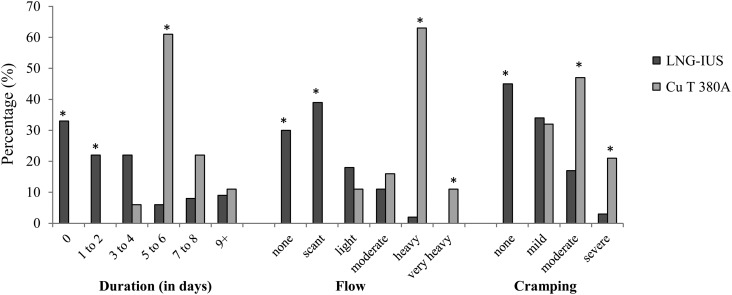
Menstrual symptoms
As expected, menstrual duration, flow and cramping were different for the two groups (Figure 1). At 6 months, 74% of Cu T 380A users reported regular menses versus 28% of LNG-IUS users (p=0.0003). Approximately one-third of LNG-IUS users reported amenorrhoea and a further third reported only scant menstrual bleeding (defined as requiring at most a small panty liner) at all surveys. Inter-menstrual spotting was not significantly different between the two groups, and was described as none (41%), rare (35%), occasional (17%), frequent (6%) and continual (1%) at 6 months of use.
Figure 1.
Menstrual symptoms by intrauterine device type. Percentage of women reporting symptoms at 6 months of 64 levonorgestrel intrauterine system (LNG-IUS) users and 19 Cu T 380A intrauterine device users. *p<0.05 for difference between LNG-IUS and Cu T 380A.
Complications
There was one pregnancy as mentioned previously. There were no cases of uterine perforation, pelvic inflammatory disease, or chlamydia or gonorrhoea infection during the study period. There was one case of endometritis due to bacterial vaginosis, which may have been present at the time of insertion.
Satisfaction
Overall, including those women who had discontinued the IUD, 83% stated that they were ‘very happy’ or ‘happy’ with the IUD, and 87% reported that they were ‘very likely’ or ‘likely’ to recommend it to a friend. There were no statistically significant differences in satisfaction between LNG-IUS and Cu T 380A users. A total of eight ‘neutral’ and one ‘unhappy’ participants continued use and cited the following reasons for relative dissatisfaction: mood symptoms (2 women), acne (2), pain with intercourse (2), irregular menses (3), menstrual cramping (5), spotting (3), heavy bleeding (1), decreased libido (1) and weight gain (1).
Discussion
The data from our study contribute to a growing body of research that supports the premise that intrauterine contraception in young nulliparous women is safe, effective and ultimately very well tolerated. We also obtained detailed information about typical experiences of our study population, related to both the insertion procedure itself and the first 6–18 months of IUD use, which can be used to educate both patients and providers. Due to our relatively small sample size, however, the precision of our estimates is low, and we may not have been sufficiently powered to detect smaller differences between the two IUD types. Additionally, response rates in our study dropped to only 35–46% after 12 months, likely due to students moving off campus, and so the 18-month data may be skewed due to loss to follow-up. The university population also tends to differ from the general population in terms of both socioeconomic status and access to contraceptive resources (in the USA, most universities have on-campus, confidential, and low- or no-cost contraceptive services for students, a resource which is not available to many community-dwelling residents), and so our findings may not be broadly generalisable.
Despite multiple interventions to try to reduce insertion-related pain, there was still a high level of discomfort with the procedure, which is not unexpected given that all of our subjects were nulliparous. Other studies have reported widely ranging mean and median pain scores, ranging from 2.7 to 6.8 out of 10 in nulliparous women.14–19 These highly varied scores, and the fact that many authors report a wide variation of pain scores within their cohorts, raise the question of whether categorical values, such as those used in this study, might yield more consistent results than numerical scores. A significant limitation of our study is that insertion-related symptoms were assessed retrospectively after several weeks had passed. Although this allowed for assessment of delayed or persistent pain, it likely diminished the accuracy of data related to acute procedure-related symptoms.
At the time of our study, our local gynaecologists recommended the routine use of misoprostol and topical anaesthetics, but the literature to date now shows that they likely were not helpful. Despite widespread use of misoprostol by providers,20 multiple studies show no efficacy for reduction of insertion-related pain,16 and although none of the trials thus far have evaluated the dosing regimen used in this cohort, use of misoprostol in our clinic has since been discontinued due to lack of evidence. Topical lidocaine gel has been studied at multiple doses and also found to be ineffective,21 and so our providers likewise no longer use topical anaesthetic spray. A single trial of intrauterine lidocaine has been published, showing no benefit, but the numbers were relatively small and did appear to show a trend toward reduced pain (2.95 vs 3.75 out of 10 mean pain scores).22 Pre-procedure ibuprofen 400 mg has also been shown to be of no benefit.19 Given that the literature to date shows no efficacy of the pain medications used in our clinic during the study period, the insertional pain data from our study are likely generalisable to a setting where such medications are not used.
There was only one syncopal event reported in our study, but vasovagal symptoms (reported as light-headedness, nausea or sweating) were reported by 46% of subjects. This is higher than much of the published literature for nulliparous women; 1–8% in those that relied on provider report15 23 24 and 12–39% in those that directly surveyed patients.14 17 18 It is possible that the 1-month retrospective timing of our survey or the phrasing of our question, which included even mild symptoms, resulted in a higher response rate. It is unlikely that the symptoms were due to the routine use of misoprostol, as placebo-controlled studies of misoprostol show no difference in rates of vasovagal symptoms.17 18 Alternatively, the vasovagal reactions could be caused by the topical anaesthetics themselves; we were unable to identify any published data in regard to this outcome.
Previous studies also described vasovagal reactions as occurring within 10 minutes of insertion, but in our cohort three women reported the onset of symptoms 15–45 minutes after the procedure and continuing for several hours. One woman described a tortuous solo journey home, as her symptoms began at the bus stop. Again, this new finding could be due to the delayed timing of our survey and therefore increased case-finding. It could, however, also represent the cessation of intrauterine anaesthetic effect. Further studies assessing delayed symptoms would be welcome, as this would be an important element of patient counselling and management.
Many women continued to have moderate to severe discomfort for the first 24 hours after the procedure, and 20% continued to have high levels of discomfort for more than 1 week. Women need to be prepared for this eventuality, so that they can make accommodations in terms of work, school and family schedules and duties. Therefore, pre-procedure counselling in regard to not only risks and benefits of intrauterine contraception but also the potential discomfort and side effects should continue to be an important part of contraceptive care.
Despite the high rates of symptoms associated with IUD insertion, the majority of women in our study stated both that they felt well prepared and that the procedure went well. This is consistent with other studies, which have reported pain as ‘mostly’ to ‘completely’ acceptable in 73% of women with no history of vaginal delivery14 and overall satisfaction rates of 77% at 1 week.25
The expulsion and contraceptive failure rates seen in this study are very similar to those seen in other comparable studies (see online Supplementary Material: Pregnancy, expulsion, and continuation rates in other studies).6–11 15 23 26–33 Our overall continuation rate, however, is slightly higher than the 71–79% seen in most other prospective studies7 8 9 26 and most similar to the 84–88% seen in the CHOICE study and the one published study of college students.6 11 In both these studies, as well as our own, thorough contraceptive counselling was provided to all subjects, which may increase continuation rates by allowing women for whom potential side effects might be highly undesirable to self-select out before placement. In this age of burgeoning enthusiasm for same-day placement of IUDs,34 the role of thorough anticipatory guidance and the potential impact of same-day insertions on continuation rates would be important areas for further study.
We did find a small difference in 12-month continuation rates between the two IUD types, but when analysed by individual reason for discontinuation or expulsion, the difference was no longer statistically significant. The CHOICE study, which enrolled 853 nulliparous women, found no statistically significant difference in continuation rates between the LNG-IUS and the Cu T 380A.11
As expected, there were significant differences in menstrual bleeding between users of the LNG-IUS versus the Cu T 380A. While these general differences have been previously known, we find it useful to present them in detailed and comparative form to assist women in choosing their method.
Cramping was much more significant in the Cu T 380A than the LNG-IUS users despite no difference in pre-IUD dysmenorrhoea or insertion-related pain, making baseline differences in pain perception between the two groups less likely. The Cu T 380A is approximately the same size and shape as the LNG-IUS 20 (only 2 mm longer in the stem), and data from a study comparing 28×28 mm with 32×32 mm LNG-IUS systems found no difference in dysmenorrhoea based on device size,26 so size would not be expected to be causative. Thus, the role of the presence of copper versus the absence of levonorgestrel in causing dysmenorrhoea may be worthy of further investigation.
For method-related discontinuations, the most common reasons were pain and unacceptable bleeding patterns, often present together. Three LNG-IUS users also reported hormonal side effects, such as acne, mood changes and decreased libido as reasons for discontinuation, and these side effects were also cited by women who continued use but were less than happy with the method. One LNG-IUS user in our study developed a complex 7 cm ovarian cyst which resolved without treatment, an occurrence that has previously been reported in the literature as a dose-dependent phenomenon with the LNG-IUS.26
Overall, this study demonstrates that intrauterine contraception in nulliparous women is safe, effective and very well tolerated. Detailed symptom data are provided to help counsel women as to what they are likely to experience, in terms of insertion-related pain and discomfort as well as ongoing menstrual symptoms. Areas for further study include the role of intrauterine anaesthetic for insertion, the prevalence of immediate and delayed vasovagal symptoms related to insertion, and the impact of separate consultation visits versus same-day insertion protocols on overall continuation rates and satisfaction.
Supplementary Material
Acknowledgments
The authors would like to thank Haley Frater and Jennifer Johnson for their assistance, and all the staff at Gannett Health Services for their encouragement.
Footnotes
Funding: This study was supported financially by Gannett Health Services at Cornell University.
Competing interests: None.
Ethics approval: Cornell University IRB and UW-Stout IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120:983–988. 10.1097/AOG.0b013e3182723b7d [DOI] [PubMed] [Google Scholar]
- 2.Guttmacher Institute. Fact Sheet: Contraceptive use in the United States. June 2014. http://www.guttmacher.org/pubs/fb_contr_use.html [accessed 9 July 2014].
- 3.Fleming K, Sokoloff A, Raine T. Attitudes and beliefs about the intrauterine device among teenagers and young women. Contraception 2010;82:178–182. 10.1016/j.contraception.2010.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitaker A, Johnson L, Harwood B, et al. Adolescent and young adult women's knowledge of and attitudes toward the intrauterine device. Contraception 2008;78:211–217. 10.1016/j.contraception.2008.04.119 [DOI] [PubMed] [Google Scholar]
- 5.Luchowski A, Anderson B, Poer M, et al. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception 2014;89:572–577. 10.1016/j.contraception.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 6.Armitage C, Mitchell C, Wigan C, et al. Uptake and continuation rates of the intrauterine system in a university student general practice population in the UK. J Fam Plann Reprod Health Care 2013;39:186–189. 10.1136/jfprhc-2012-100392 [DOI] [PubMed] [Google Scholar]
- 7.Marions L, Lövkvist L, Taube A, et al. Use of the levonorgestrel releasing-intrauterine system in nulliparous women – a non-interventional study in Sweden. Eur J Contracept Reprod Health Care 2011;16:126–134. 10.3109/13625187.2011.558222 [DOI] [PubMed] [Google Scholar]
- 8.Brockmeyer A, Kishen M, Webb A. Experience of IUD/IUS insertions and clinical performance in nulliparous women – a pilot study. Eur J Contracept Reprod Health Care 2008;13:248–254. 10.1080/02699200802253706 [DOI] [PubMed] [Google Scholar]
- 9.Suhonen S, Haukkama M, Jakobsson T, et al. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception 2004;69:407–412. 10.1016/j.contraception.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 10.Otero-Flores J, Guerrero-Carreño L, Vázquez-Estrada L. A comparative randomized study of three different IUDs in nulliparous Mexican women. Contraception 2003;67:273–276. 10.1016/S0010-7824(02)00519-X [DOI] [PubMed] [Google Scholar]
- 11.Peipert J, Zhao Q, Allsworth J, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol 2011;117:1105–1113. 10.1097/AOG.0b013e31821188ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shelton J, Taylor R. The Pearl Pregnancy Index reexamined: still useful for clinical trials of contraceptives. Am J Obstet Gynecol 1981;139:592–596. [DOI] [PubMed] [Google Scholar]
- 13.Centres for Disease Control. US Medical Eligibility Criteria for Contraceptive Use 2010. MMWR Recomm Rep 2010;59:1–86. [PubMed] [Google Scholar]
- 14.Allen R, Carey M, Raker C, et al. A prospective cohort study of pain with intrauterine device insertion among women with and without vaginal deliveries. J Obstet Gynaecol 2014;34:263–267. 10.3109/01443615.2013.868424 [DOI] [PubMed] [Google Scholar]
- 15.Brown W, Trouton K. Intrauterine device insertions: which variables matter? J Fam Plann Reprod Health Care 2014;40:117–121. 10.1136/jfprhc-2012-100383 [DOI] [PubMed] [Google Scholar]
- 16.Espey E, Singh R, Leeman L, et al. Misoprostol for intrauterine device insertion in nulliparous women: a randomized controlled trial. Am J Obstet Gynecol 2014;210:208.e1–208.e5. 10.1016/j.ajog.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 17.Dijkhuizen K, Dekkers O, Holleboom C, et al. Vaginal misoprostol prior to insertion of an intrauterine device: an RCT. Hum Reprod 2011;26:323–329. 10.1093/humrep/deq348 [DOI] [PubMed] [Google Scholar]
- 18.Sääv I, Aronsson A, Marions L, et al. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Hum Reprod 2007;22:2647–2652. 10.1093/humrep/dem244 [DOI] [PubMed] [Google Scholar]
- 19.Hubacher D, Reyes V, Lillo S, et al. Pain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofen. Am J Obstet Gynecol 2006;195: 1272–1277. 10.1016/j.ajog.2006.08.022 [DOI] [PubMed] [Google Scholar]
- 20.Ward K, Jacobson J, Turok D, et al. A survey of provider experience with misoprostol to facilitate intrauterine device insertion in nulliparous women. Contraception 2011;84:594–599. 10.1016/j.contraception.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 21.Allen R, Raker C, Goyal V. Higher dose cervical 2% lidocaine gel for IUD insertion: a randomized controlled trial. Contraception 2013;88:730–736. 10.1016/j.contraception.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 22.Nelson A, Fong J. Intrauterine infusion of lidocaine does not reduce pain scores during IUD insertion. Contraception 2013;88:37–40. 10.1016/j.contraception.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 23.Bayer L, Jensen J, Li H, et al. Adolescent experience with intrauterine device insertion and use: a retrospective cohort study. Contraception 2012;86:443–451. 10.1016/j.contraception.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 24.Berger G, Edelman D, Regenie S. Patients’ responses to IUD insertion. Int J Gynaecol Obstet 1976;14:147–152. [DOI] [PubMed] [Google Scholar]
- 25.Lathrop E, Haddad L, McWhorter C, et al. Self-administration of misoprostol prior to intrauterine device insertion among nulliparous women: a randomized controlled trial. Contraception 2013;88:725–729. 10.1016/j.contraception.2013.07.011 [DOI] [PubMed] [Google Scholar]
- 26.Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril 2012;97:616–622. 10.1016/j.fertnstert.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 27.Winner B, Peipert J, Zhao Q, et al. effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. 10.1056/NEJMoa1110855 [DOI] [PubMed] [Google Scholar]
- 28.Aoun J, Dines V, Stovall D, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol 2014;123:585–592. 10.1097/AOG.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 29.Berenson A, Tan A, Hirth J, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol 2013;121:951–958. 10.1097/AOG.0b013e31828b63a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldhuis H, Vos A, Lagro-Janssen A. Complications of the intrauterine device in nulliparous and parous women. Eur J Gen Pract 2004;10:82–87. 10.3109/13814780409044540 [DOI] [PubMed] [Google Scholar]
- 31.Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care 2003;29:227–231. 10.1783/147118903101197854 [DOI] [PubMed] [Google Scholar]
- 32.Godfrey E, Memmel L, Neustadt A, et al. Intrauterine contraception for adolescents aged 14–18 years: a multicenter randomized pilot study of levonorgestrel-releasing intrauterine system compared to the Copper T 380A. Contraception 2010;81:123–127. 10.1016/j.contraception.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 33.Lara-Torre E, Spotswood L, Correia N, et al. Intrauterine contraception in adolescents and young women: a descriptive study of use, side effects, and compliance. J Pediatr Adolesc Gynecol 2011;24:39–41. 10.1016/j.jpag.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Worcester S. Data support same-day IUD placement in women seeking contraceptive services. Ob.Gyn.News. 3 June 2014. http://www.obgynnews.com/home/article/data-support-same-day-iud-placement-in-women-seeking-contraceptive-services/daac2d 183ed60e64d773d0ddd68d52b8.html [accessed 9 July 2014]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.