Abstract
Objectives
To evaluate efficacy and safety of combination therapy using certolizumab pegol (CZP) and methotrexate (MTX) as first-line treatment for MTX-naive, early rheumatoid arthritis (RA) with poor prognostic factors, compared with MTX alone.
Methods
MTX-naive, early RA patients with ≤12 months persistent disease, high anti-cyclic citrullinated peptide, and either rheumatoid factor positive and/or presence of bone erosions were enrolled in this multicentre, double-blind, randomised placebo (PBO)-controlled study. Patients were randomised 1:1 to CZP+MTX or PBO+MTX for 52 weeks. Primary endpoint was inhibition of radiographic progression (change from baseline in modified Total Sharp Score (mTSS CFB)) at week 52. Secondary endpoints were mTSS CFB at week 24, and clinical remission rates at weeks 24 and 52.
Results
316 patients randomised to CZP+MTX (n=159) or PBO+MTX (n=157) had comparable baseline characteristics reflecting features of early RA (mean disease duration: 4.0 vs 4.3 months; Disease Activity Score 28-joint assessment (DAS28)) (erythrocyte sedimentation rate (ESR)): 5.4 vs 5.5; mTSS: 5.2 vs 6.0). CZP+MTX group showed significantly greater inhibition of radiographic progression relative to PBO+MTX at week 52 (mTSS CFB=0.36 vs 1.58; p<0.001) and week 24 (mTSS CFB=0.26 vs 0.86; p=0.003). Clinical remission rates (Simple Disease Activity Index, Boolean and DAS28 (ESR)) of the CZP+MTX group were significantly higher compared with those of the PBO+MTX group, at weeks 24 and 52. Safety results in both groups were similar, with no new safety signals observed with addition of CZP to MTX.
Conclusions
In MTX-naive early RA patients with poor prognostic factors, CZP+MTX significantly inhibited structural damage and reduced RA signs and symptoms, demonstrating the efficacy of CZP in these patients.
Trial registration number
(NCT01451203).
Keywords: Early Rheumatoid Arthritis, Anti-TNF, Rheumatoid Arthritis, Inflammation, Disease Activity
Introduction
The emergence of biological agents targeting inflammatory cytokines such as tumour necrosis factor (TNF), which play key roles in the pathogenesis of rheumatoid arthritis (RA), has been of great importance. The effectiveness of these agents at inhibiting joint damage progression, in addition to providing symptom relief, has brought a paradigm shift to RA treatment.1 Since joint damage progression is rarely reversible,2 3 earlier treatment with effective drugs would be relevant in clinical practice.
Treatment guidelines and recommendations published by the European League Against Rheumatism (EULAR), the American College of Rheumatology (ACR) and the Japan College of Rheumatology recommend that all patients with RA should be treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) from the point of diagnosis.4–6 Methotrexate (MTX), either as monotherapy or in combination with other csDMARDs, should be given first-line unless contraindicated. For patients at risk of rapid disease progression, addition of a biologic can be considered if treatment targets are not achieved using csDMARDs alone. Earlier recognition of RA has become possible for many patients by application of the 2010 ACR/EULAR classification criteria.7 8 Ultimately, early diagnosis and intervention with effective therapeutics maximises the chance of preventing joint damage progression in order to maintain quality of life.9
Certolizumab pegol (CZP) is a humanised anti-TNF antibody fragment conjugated to polyethylene glycol, approved for treatment of inflammatory diseases, including RA. Efficacy and safety of CZP in established RA has been demonstrated in several studies10–13 but is previously unreported in MTX-naive early RA.
Herein, we conducted the Certolizumab–Optimal Prevention of joint damage for Early RA (C-OPERA) study, designed to include MTX-naive, early RA patients with poor prognostic factors. The study was double-blind (DB) for 1 year, with either CZP or placebo (PBO) administered concomitantly with MTX. Following this, the trial was open label for another year, whereby completers of the DB period were maintained on MTX monotherapy after discontinuing CZP. This report comprises results from the 1-year DB period.
Methods
Patients
Eligible patients were 20–64 years old with RA fulfilling the 2010 ACR/EULAR classification criteria. Patients had ≤12 months of persistent arthritic symptoms, at least moderate disease activity (Disease Activity Score 28-joint assessment (DAS28) with erythrocyte sedimentation rate (ESR) ≥3.2) and were MTX-naive. In addition, patients had poor prognostic factors: high anti-cyclic citrullinated peptide (anti-CCP) antibody (≥3× upper limit of normal (ULN)) and either positive rheumatoid factor (RF) and/or presence of bone erosions (based on radiographs of hands/feet, assessed by the investigator at each study site). Patients with high risk of infection (current use of antibiotics, history of serious/chronic infection treated by antibiotics within 6 months) or history of/active tuberculosis or malignancy, and patients previously exposed to MTX, leflunomide or biological DMARDs were excluded.
Study design
C-OPERA, a phase III multicentre study (NCT01451203), was DB and PBO-controlled to week 52, with a subsequent 52-week follow-up period when patients received MTX monotherapy. Patients were randomised 1:1 to either CZP+MTX or PBO+MTX (MTX monotherapy) via an interactive web-response system. Drug administration was performed by dedicated non-blinded persons due to distinguishability of CZP from PBO; however, these personnel were not permitted to engage in other study activities to maintain blinding. All investigators and healthcare professionals involved in safety/efficacy assessments were blind to study medications. Study drugs were subcutaneously administered as a loading dose of CZP 400 mg or PBO at weeks 0, 2 and 4, followed by CZP 200 mg or PBO every two weeks from week 6 to week 50. Oral MTX (8 mg/week) was initiated simultaneously. MTX dose was increased to 12 mg/week at week 4, 16 mg/week at week 8 and maintained at 16 mg/week thereafter. As per protocol, dose escalation of MTX could be postponed only for safety concerns or due to adverse events (AEs), in which case the dose was maintained at the highest tolerable dose. Patients who did not achieve an improvement of symptoms at or after week 24, that is, if moderate or higher disease activity (DAS28 (ESR) ≥3.2) persisted ≥4 weeks, in either treatment arm, were eligible to receive rescue treatment with open-label CZP after discontinuing DB period. Co-administration of any DMARD except MTX was prohibited during the study.
The study was conducted from October 2011 to August 2013 at 73 sites in Japan after approval by the Institutional Review Board designated by each site, in compliance with ethical principles of the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent.
Efficacy assessments
The primary efficacy endpoint was inhibition of joint damage progression, assessed as change from baseline (CFB) in van der Heijde modified Total Sharp Score (mTSS) at week 52. The same measure at week 24 was a secondary efficacy endpoint. mTSS was evaluated by two independent readers in accordance with previously reported methods.14 15 In addition to mTSS CFB, non-progression (defined: mTSS CFB ≤0.5) and the rapid radiographic progression rate (RRP; defined: yearly progression (YP) >5)16 17 were analysed. Other secondary efficacy endpoints included clinical remission rates, assessed by ACR/EULAR criteria (Simple Disease Activity Index (SDAI)-based and Boolean-based) and DAS28 (ESR) at weeks 24 and 52.
Signs and symptoms were assessed by clinical remission rates (SDAI, Boolean and DAS28 (ESR)), functional remission rates (Health Assessment Questionnaire Disability Index (HAQ-DI)) and ACR20/50/70 responses, evaluated at each time point.
Safety assessments
All undesirable events during the DB period were recorded as AEs or serious AEs. Safety was evaluated by laboratory tests (haematological, blood chemistry, urinalysis), chest radiographs and ECGs.
Statistical analyses
Sample size was based on expected difference in mTSS CFB at week 52 between CZP and PBO groups of 2.57±6.75. Verification of superiority of CZP+MTX over MTX monotherapy for primary endpoint would then have 90% power at a two-sided significance level of 5% with 146 patients per group (thus the planned number was 150 patients).
Primary analyses used the full analysis set, defined as patients who received ≥1 dose of study drug and provided any efficacy data thereafter. For the imputation of missing data, linear extrapolation was used for mTSS and last observation carried forward used for other efficacy variables. Non-responder imputation was added as a sensitivity analysis for clinical remission analyses. For the primary endpoint, an analysis of covariance (ANCOVA) model was used for mTSS CFB by converting measured values to rank scores and using treatment group as a factor and baseline rank score as a covariate. Fisher's exact test was used for analyses of non-progression, RRP in mTSS, clinical remission and ACR20/50/70 response.
Results
Patient baseline demographics/characteristics
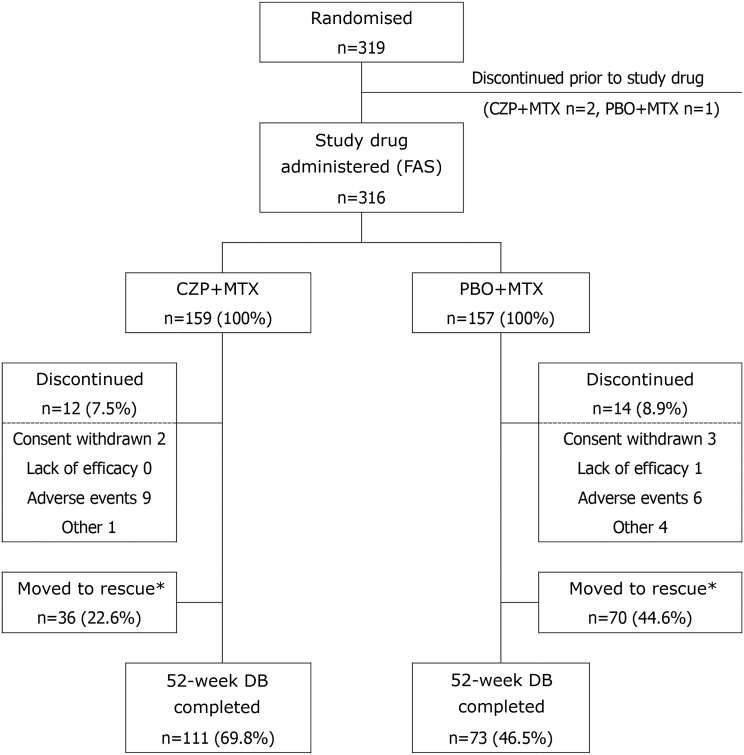
Of 319 patients randomised, 316 (159, CZP+MTX; 157, PBO+MTX) received study drug. Of these, 111 patients (69.8%) in the CZP+MTX group and 73 patients (46.5%) in the PBO+MTX group completed the 52-week DB period (figure 1). Fewer PBO+MTX patients completed DB period than CZP+MTX patients, mainly due to the increased number of discontinuations (figure 1).
Figure 1.
Patient disposition. *Patients who did not achieve an improvement of RA symptoms (defined as the persistence of DAS28[ESR] ≥3.2 for 4 weeks or longer) after Week 24 were eligible to withdraw from DB and move to rescue treatment with open label CZP. CZP, certolizumab pegol; DAS28, Disease Activity Score 28-joint assessment; DB, double blind; ESR, erythrocyte sedimentation rate; FAS, full analysis set; MTX, methotrexate; PBO, placebo; RA, rheumatoid arthritis.
Treatment groups were generally balanced with respect to demographic and baseline characteristics (table 1). Overall, patients' mean age was 49 years (range 21–64 years). Mean RA duration (time from onset of persistent arthritic symptoms) was approximately 4 months in both groups. All patients had high titre (≥3 times ULN) anti-CCP antibody; approximately 95% were RF positive. Bone erosion was confirmed in 50% of patients. Mean±SD DAS28 (ESR) was 5.4±1.1 for CZP+MTX and 5.5±1.2 for PBO+MTX. Mean (median) mTSS in CZP+MTX and PBO+MTX groups was 5.2 (1.5) and 6.0 (1.5), and no radiographic damage (mTSS ≤0.5) was observed in 35.2% and 35.7% of patients, respectively. There was no difference between groups in mean baseline body weight (57.4±11.3 in CZP+MTX, 57.4±10.6 in PBO+MTX; kg, mean±SD) or average weekly MTX dose throughout the study period (11.6±3.0 in CZP+MTX, 11.6±2.7 in PBO+MTX; mg/week).
Table 1.
Demographics and baseline characteristics (FAS population)
| Parameter | CZP+MTX n=159 |
PBO+MTX n=157 |
|---|---|---|
| Age (years) | 49.4±10.6 | 49.0±10.3 |
| Female, n (%) | 129 (81.1) | 127 (80.9) |
| Weight (kg) | 57.4±11.3 | 57.4±10.6 |
| BMI (kg/m2) | 22.4±3.9 | 22.5±3.7 |
| RA duration (months)* | 4.0±2.9 | 4.3±2.8 |
| <3 months, n (%) | 60 (37.7) | 57 (36.3) |
| 3–<6 months, n (%) | 60 (37.7) | 56 (35.7) |
| 6–12 months, n (%) | 39 (24.5) | 44 (28.0) |
| Previous DMARDs use, n (%) | 31 (19.5) | 29 (18.5) |
| Steroid use at baseline, n (%) | 26 (16.4) | 31 (19.7) |
| Anti-CCP antibody positive, n (%) | 159 (100.0) | 157 (100.0) |
| High titre (≥3 times of ULN), n (%) | 159 (100.0) | 157 (100.0) |
| Titre (U/mL)† | 176.7±107.5 | 185.2±107.7 |
| RF positive, n (%) | 153 (96.2) | 146 (93.0) |
| High titre (≥3 times of ULN), n (%) | 119 (74.8) | 117 (74.5) |
| Titre (U/mL)† | 182.5±177.4 | 167.3±166.5 |
| Bone erosion (judged by physician), n (%) | 79 (49.7) | 80 (51.0) |
| TJC (/28 joints) | 8.4±6.1 | 8.9±6.5 |
| SJC (/28 joints) | 8.3±5.3 | 8.4±5.3 |
| PtGADA (mm) | 50.4±22.4 | 52.9±22.7 |
| PhGADA (mm) | 56.7±20.5 | 58.4±21.4 |
| ESR (mm/h) | 38.4±25.3 | 43.7±28.2 |
| CRP (mg/dL) | 1.3±1.8 | 1.5±1.9 |
| MMP-3 (ng/mL)‡ | 130.4±135.4 | 185.4±214.9 |
| DAS28 (ESR) | 5.4±1.1 | 5.5±1.2 |
| SDAI | 28.7±12.5 | 30.0±13.6 |
| HAQ-DI score | 1.0±0.6 | 1.1±0.7 |
| mTSS | 5.2±8.8 | 6.0±15.3 |
| Negative (≤0.5), n (%) | 56 (35.2) | 56 (35.7) |
| Erosion score | 2.2±4.4 | 2.8±7.9 |
| Negative (≤0.5), n (%) | 82 (51.6) | 80 (51.0) |
| Joint space narrowing score | 2.9±5.8 | 3.2±8.6 |
| Negative (≤0.5), n (%) | 87 (54.7) | 82 (52.2) |
| Average weekly MTX dose (mg/week) | 11.6 (3.0) | 11.6 (2.7) |
Values are mean±SD unless otherwise indicated.
*Time from onset of persistent arthritic symptoms.
†Data exceeding measurement upper limit (≥300 U/mL) are regarded as 300 U/mL.
‡Normal range: 36.9–121 (male), 17.3–59.7 (female) ng/mL.
BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C reactive protein; CZP, certolizumab pegol; DAS28 (ESR), Disease Activity Score 28-joint assessment; DMARDs, disease-modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; FAS, full analysis set; HAQ-DI, Health Assessment Questionnaire Disability Index; MMP-3, matrix metalloproteinase-3; mTSS, modified Total Sharp Score; MTX, methotrexate; PBO, placebo; PhGADA, physician global assessment of disease activity; PtGADA, patient's global assessment of disease activity; RA, rheumatoid arthritis; RF, rheumatoid factor; SDAI, Simple Disease Activity Index; SJC, swollen joint count; TJC, tender joint count; ULN, upper limit of normal.
Inhibition of joint damage progression
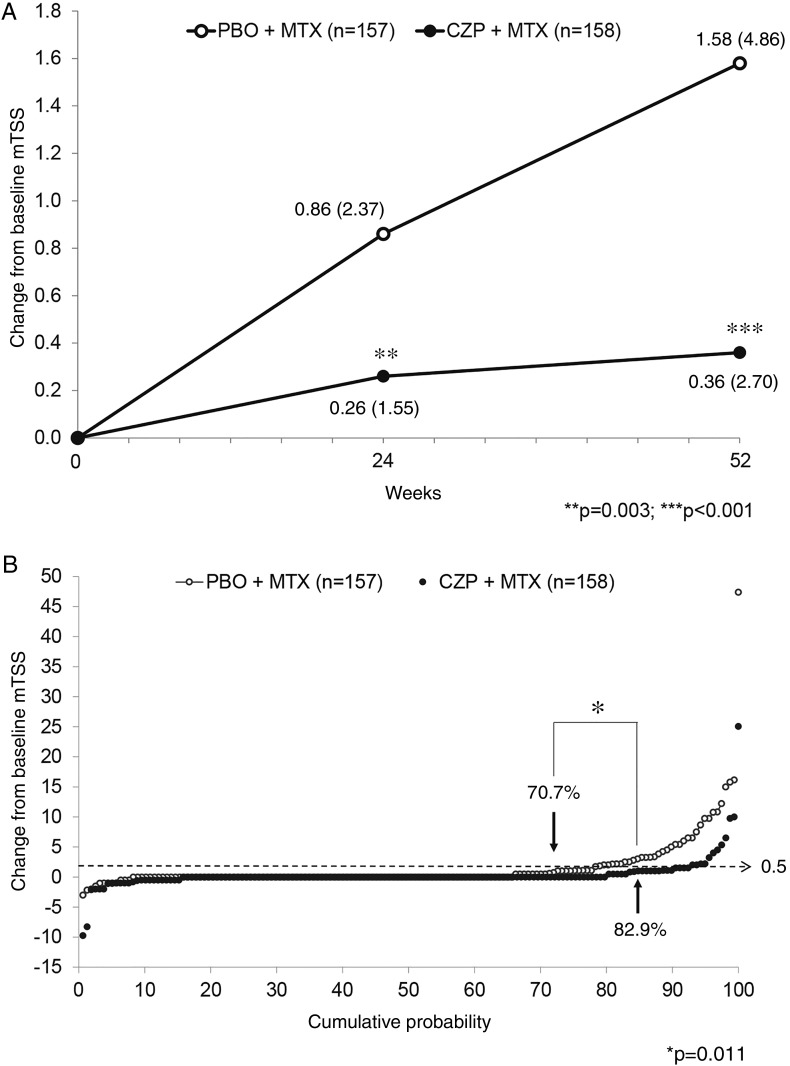
For the primary endpoint, mTSS CFB (mean±SD) at week 52 was 0.36±2.70 with CZP+MTX and 1.58±4.86 with PBO+MTX, statistically significant by ANCOVA on the ranks (p<0.001). At week 24, smaller mTSS CFB was observed with CZP+MTX compared with PBO+MTX (0.26±1.55 vs 0.86±2.37; p=0.003) (figure 2A).
Figure 2.
(A) Change from baseline in modified Total Sharp Score (mTSS CFB) at weeks 24 and 52. For calculation of p values, an ANCOVA model was used for mTSS CFB by converting measured values to rank scores and using treatment group as a factor and baseline rank score as a covariate. Values in the figure indicate mean (SD) at each time point and treatment group. (B) Cumulative probability plot of mTSS CFB at week 52. Percentages in the figure indicate non-progression (mTSS CFB ≤0.5) rates of each treatment group. P value is calculated by Fisher's exact test. The mTSS data used in (A) and (B) are all imputed using linear extrapolation (LINEAR) for FAS. The number of patients in the CZP+MTX group is 158 despite the FAS reported as 159 because one patient in the group had no mTSS data after treatment. CZP, certolizumab pegol; MTX, methotrexate; mTSS, modified total Sharp score; PBO, placebo.
The percentage of patients with non-progression (mTSS CFB ≤0.5) at week 52 was higher with CZP+MTX than with PBO+MTX (82.9% vs 70.7%; p=0.011 by Fisher's exact test). Individual patient data are presented in the cumulative probability plot of mTSS CFB at week 52 (figure 2B). In addition, 3.2% of patients with CZP+MTX exhibited RRP (defined as YP >5), compared with 10.8% with PBO+MTX (p=0.008).
Clinical responses
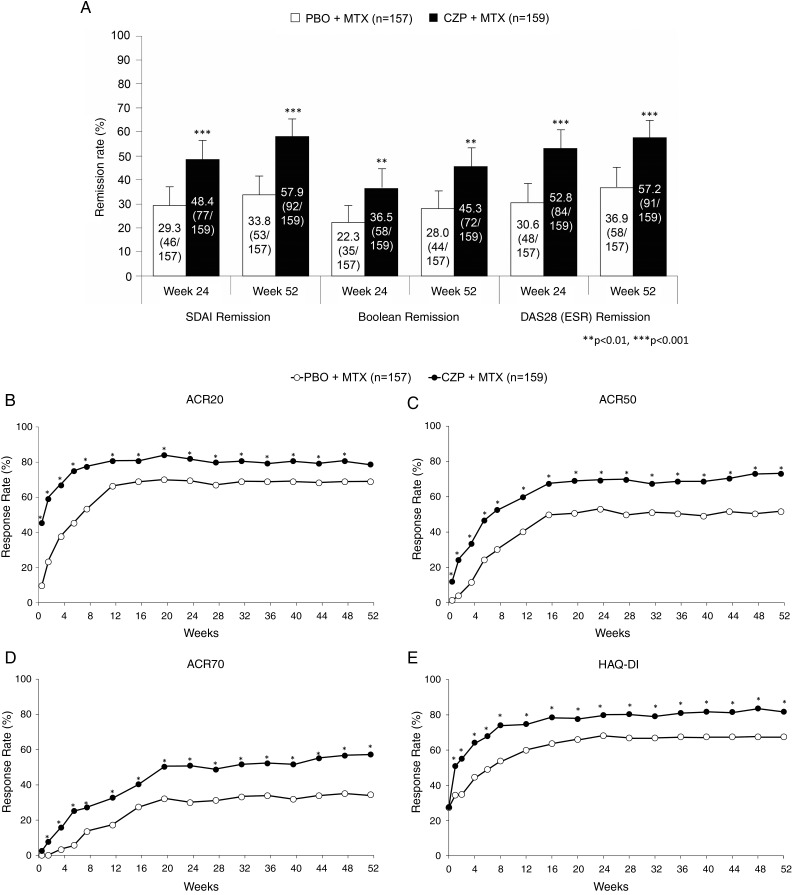
Higher ACR/EULAR remission rates were observed with CZP+MTX compared with PBO+MTX (SDAI remission at week 24: 48.4% vs 29.3%, p<0.001; at week 52: 57.9% vs 33.8%, p<0.001, respectively, and Boolean remission at week 24: 36.5% vs 22.3%, p=0.007; at week 52: 45.3% vs 28.0%, p=0.002, respectively). Similarly, DAS28 (ESR) remission rates at week 24 were approximately 20% higher with CZP+MTX than PBO+MTX (52.8% vs 30.6%; p<0.001); this difference was maintained until week 52 (57.2% vs 36.9%; p<0.001) (figure 3A).
Figure 3.
(A) Clinical remission rates at weeks 24 and 52 by Simple Disease Activity Index (SDAI), Boolean and Disease Activity Score 28-joint assessment (DAS28) (erythrocyte sedimentation rate (ESR)) criteria analysed using full analysis set (FAS), last observation carried forward (LOCF) data set. Error bars indicate 95% confidence interval of each remission rate. P values are calculated by Fisher's exact test. (B-E) Time course of American College of Rheumatology (ACR) response rates of (B) ACR20, (C) ACR50, (D) ACR70 and (E) Health Assessment Questionnaire Disability Index (HAQ-DI) remission rates. *p<0.05 between the groups at each particular time point, calculated by Fisher's exact test. CZP, certolizumab pegol; MTX, methotrexate; PBO, placebo.
ACR responses were higher at all time points with CZP+MTX compared with PBO+MTX, and a significant difference between the two arms was observed from week 1 in ACR20 and ACR50, and week 2 in ACR70 (figure 3B–D). ACR responses at week 52 in CZP+MTX vs PBO+MTX groups were 78.6% vs 68.8% (p=0.055 by Fisher's exact test) in ACR20, 73.0% vs 51.6% (p<0.001) in ACR50 and 57.2% vs 34.4% (p<0.001) in ACR70, respectively. A similar time course for HAQ-DI remission rates is shown in figure 3E.
Subgroup analyses for joint damage
Subgroup analyses of mTSS CFB at week 52, stratified by baseline parameters including anti-CCP antibody, RF, C-reactive protein (CRP), matrix metalloproteinase (MMP)-3, HAQ-DI, DAS28 (ESR), mTSS and average concomitant MTX dose are shown in table 2. A comparison of mTSS CFB between treatment groups consistently showed less progression with CZP+MTX compared with PBO+MTX in all categories of these parameters, except for patients with baseline DAS28 (ESR) <3.2 (a small number of patients, n=8). Meanwhile, intra-parameter comparison of mTSS CFB revealed a trend of greater mTSS CFB with higher titres of anti-CCP and RF, higher serum CRP and MMP-3, and higher HAQ-DI, DAS28 (ESR) and mTSS at baseline, which was greater in the PBO+MTX group relative to CZP+MTX. In contrast, with regard to concomitant MTX dose, the expected dose-dependent inhibitory effect was not found in either group.
Table 2.
Subgroup analysis of mTSS CFB at week 52 by baseline parameters and concomitant MTX dose (FAS, LINEAR)
| Parameter at baseline | Subgroup | CZP+MTX | PBO+MTX | ||
|---|---|---|---|---|---|
| n | mTSS CFB mean±SD |
n | mTSS CFB mean±SD |
||
| Anti-CCP antibody (U/mL) | <100 | 51 | −0.03±0.69 | 51 | 1.34±3.11 |
| 100–<300 | 57 | 0.11±1.99 | 56 | 1.52±3.79 | |
| ≥300 | 50 | 1.05±4.21 | 50 | 1.91±7.01 | |
| RF (IU/mL) | <20 | 6 | −0.26±0.45 | 11 | 2.20±5.14 |
| 20–<60 | 33 | 0.06±1.09 | 29 | 0.82±3.07 | |
| ≥60 | 119 | 0.48±3.06 | 117 | 1.72±5.20 | |
| CRP (mg/dL) | ≤0.5 | 75 | 0.13±0.74 | 69 | 0.39±2.12 |
| >0.5–≤1.0 | 22 | 0.00±0.52 | 27 | 1.85±3.23 | |
| >1.0 | 61 | 0.78±4.25 | 61 | 2.82±6.97 | |
| MMP-3 (ng/mL) | <50 | 36 | 0.09±0.50 | 33 | 0.01±0.42 |
| 50–<100 | 59 | 0.31±0.97 | 50 | 1.44±3.17 | |
| ≥100 | 63 | 0.57±4.17 | 74 | 2.38±6.47 | |
| HAQ-DI | ≤0.5 | 43 | 0.27±1.61 | 43 | 0.52±2.71 |
| >0.5–≤1.0 | 44 | 0.10±0.98 | 41 | 1.60±4.09 | |
| >1.0 | 71 | 0.58±3.76 | 73 | 2.21±6.04 | |
| DAS28 (ESR) | <3.2 | 5 | 0.10±0.22 | 3 | 0.00±0.00 |
| 3.2–5.1 | 60 | 0.20±0.83 | 54 | 0.71±3.14 | |
| >5.1 | 93 | 0.49±3.46 | 100 | 2.10±5.59 | |
| mTSS | ≤0.5 | 55 | 0.20±0.64 | 56 | 0.42±0.99 |
| >0.5 | 103 | 0.45±3.32 | 101 | 2.23±5.93 | |
| Concomitant MTX—average dose (mg/week) | 0–8 | 18 | 0.07±0.88 | 21 | 0.61±2.37 |
| 8–≤12 | 64 | 0.38±4.01 | 59 | 1.40±2.98 | |
| >12–16 | 76 | 0.42±1.27 | 77 | 1.99±6.31 | |
CCP, cyclic citrullinated peptide; CFB, change from baseline; CRP, C reactive protein; CZP, certolizumab pegol; DAS28, Disease Activity Score 28-joint assessment; ESR, erythrocyte sedimentation rate; FAS, full analysis set; HAQ-DI, Health Assessment Questionnaire Disability Index; LINEAR, linear extrapolation; MMP-3, matrix metalloproteinase-3; mTSS, modified Total Sharp Score; MTX, methotrexate; PBO, placebo; RF, rheumatoid factor.
Safety
Study drug exposure during treatment period was greater in the CZP+MTX group (136.2 patient-years) compared with the PBO+MTX group (116.0 patient-years), as more PBO+MTX -treated patients withdrew (mainly due to lack of efficacy). Overall, 153 patients (96.2%) in the CZP+MTX group and 148 patients (94.3%) in the PBO+MTX group reported any AEs. Serious AEs were reported by 13 patients (8.2%) in the CZP+MTX group and 14 patients (8.9%) in the PBO+MTX group. No clinically relevant difference between groups was observed in overall incidence of AEs and serious AEs (table 3).
Table 3.
Summary of treatment-emergent adverse events
| Parameter | CZP+MTX n=159 PY=136.2 n (%) |
PBO+MTX n=157 PY=116.0 n (%) |
|---|---|---|
| AE summary | ||
| Any AEs | 153 (96.2) 542.0* |
148 (94.3) 548.2* |
| Serious AEs | 13 (8.2) 11.0* |
14 (8.9) 12.9* |
| Deaths | 0 | 0 |
| AEs of interest | ||
| Infections and infestations | 97 (61.0) | 87 (55.4) |
| Serious infection | 5 (3.1) | 7 (4.5) |
| Pneumocystis jiroveci pneumonia | 3 (1.9) | 2 (1.3) |
| Bronchitis | 1 (0.6) | 0 |
| Meningitis fungal | 1 (0.6) | 0 |
| Pneumonia bacterial | 1 (0.6) | 2 (1.3) |
| Pneumonia | 0 | 1 (0.6) |
| Pneumonia mycoplasmal | 0 | 1 (0.6) |
| Pyelonephritis acute | 0 | 1 (0.6) |
| Pneumonia | 7 (4.4) | 8 (5.1) |
| Pneumonia bacterial | 4 (2.5) | 2 (1.3) |
| Pneumocystis jiroveci pneumonia | 3 (1.9) | 3 (1.9) |
| Bronchopneumonia | 1 (0.6) | 0 |
| Pneumonia | 0 | 2 (1.3) |
| Pneumonia mycoplasmal | 0 | 1 (0.6) |
| Tuberculosis | 0 | 0 |
| Interstitial lung disease | 5 (3.1) | 1 (0.6) |
| Malignancies† | 1 (0.6) | 0 |
| Hepatic disorders‡ | 68 (42.8) | 70 (44.6) |
| Hematopoietic cytopenias§ | 12 (7.5) | 13 (8.3) |
| Nausea/vomiting/decreased appetite | 39 (24.5) | 32 (20.4) |
| Stomatitis | 19 (11.9) | 26 (16.6) |
| Injection site reaction | 5 (3.1) | 2 (1.3) |
*Event rate: per 100 patients-years.
†Cervix carcinoma.
‡Including following preferred terms: alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyltransferase increased, hepatic function abnormal, hepatic enzyme increased, hepatic steatosis, hyperbilirubinaemia, liver disorder, liver function test abnormal.
§Including following preferred terms: granulocytopenia, leucopenia, lymphopenia, lymphocyte count decreased, white blood cell count decreased.
AE, adverse event; CZP, certolizumab pegol; MTX, methotrexate; PBO, placebo; PY, total summation of individual patient-years.
Overall incidence of infections and infestations was higher with CZP+MTX (61.0%) compared with PBO+MTX (55.4%), with no difference in the rate of serious infections (3.1% in CZP+MTX vs 4.5% in PBO+MTX). Similar incidences were observed for pneumonia (10 events reported in seven patients [4.4%] for CZP+MTX vs 10 events in eight patients [5.1%] for PBO+MTX), including three cases of Pneumocystis jiroveci pneumonia in each group.
There was no difference in the severity pattern of pneumonia events between CZP+MTX (four serious events) and PBO+MTX (six serious events). There was an apparent correlation between MTX dose and the occurrence of pneumonia since only one patient in each group experienced an event of bacterial pneumonia while receiving low MTX dose (0–8 mg/week) versus five and four patients in the CZP+MTX and PBO+MTX groups, respectively, who experienced ≥1 pneumonia event with high MTX dose (>12–16 mg/week).
The incidence of hepatic events was high (mostly abnormal hepatic function) although it was similar between groups (hepatic disorders: 42.8% with CZP+MTX, 44.6% with PBO+MTX; ‘investigations’ system organ class in hepatic disorders: 6.9% with CZP+MTX; 8.9% with PBO+MTX), indicating that there was no increased risk with the addition of CZP. No patients were withdrawn from the study due to hepatic events, and almost all events were resolved by temporarily discontinuing or reducing MTX dose. No cases of tuberculosis, demyelinating disorders, lupus-like syndrome, serious allergic reactions or serious haematological disorders were reported.
Discussion
Compared with similar studies of anti-TNF agents in MTX-naive early RA patients, C-OPERA is characterised by two unique features. First, as far as we know, this is the first randomised controlled trial (RCT) to employ the 2010 ACR/EULAR classification criteria as the main inclusion criteria. Thus, patients enrolled in C-OPERA had very early stages of disease, strictly defined as the time from initiation of persistent arthritic symptoms identified by medical interview (RA duration ≤12 months). Approximately 35% of patients had no joint damage (mTSS ≤0.5) in baseline radiographs, and mean baseline mTSS of 5.6 units (5.2–6.0) was the lowest among similar RCTs of biologics (approximately 10–20 units).18–22 Second, we intentionally enrolled only patients with high anti-CCP antibody titres, which is highly specific for RA,23 24 compensating for a relatively low specificity of classification criteria. Since positive anti-CCP antibody predicts poor prognosis and rapid progression,25–29 these patients are more likely to require and benefit from aggressive treatment during early disease.
Regarding radiographic joint damage, a statistically significant inhibitory effect was consistently confirmed in patients receiving CZP by analyses of mTSS CFB, non-progression rate, YP and RRP rate. In addition, an absolutely small mean YP (0.37) and high non-progression rate (82.9%) at week 52 in patients with CZP indicate that concomitant use of CZP with MTX brings proven benefits for inhibition of joint damage progression.
Overall, clinical remission rates were relatively high in patients receiving MTX monotherapy (SDAI: 33.8%; Boolean: 28.0%; DAS28 (ESR): 36.9% at week 52; figure 3A) compared with similar RCTs of biologics,18–22 but were higher in the group receiving CZP (SDAI: 57.9%; Boolean: 45.3%; DAS28 (ESR): 57.2%). Moreover, patients receiving CZP had better ACR responses and HAQ-DI remission rates as early as week 1.
By protocol, MTX dose was increased to 16 mg/week at week 8, unless there were safety concerns. Consequently, average MTX dose throughout the 52 weeks was approximately 12 mg/week, relatively low compared with reports from similar early RA studies, mainly conducted in the USA or the EU (15–17 mg/week).18–22 However, considering the difference in average patient body weight between C-OPERA (57 kg) and the above studies (74–79 kg), actual MTX dose per body weight was similar. Moreover, it has been reported that concentrations of MTX polyglutamates, a potential marker for MTX use, in red blood cells are relatively higher in the Japanese study compared with the US study, suggesting a lower dose of MTX may be sufficient in Japanese patients.30 This is the first Japanese study to mandate use of maximum MTX dose (16 mg/week) by protocol, which may explain better MTX monotherapy results relative to those in previous Japanese studies.
Results of subgroup analyses stratified by MTX dose for mTSS CFB at week 52 (table 2) failed to prove the dose-dependent effect of MTX on joint damage inhibition, regardless of concomitant CZP. This was despite higher DAS28 (ESR) remission rates at week 52 with high-dose MTX (>12–16 mg/week) (42.9%) compared with lower doses (8–≤12 mg/week) (30.5%) in patients on MTX monotherapy. Alternatively, the DAS28 (ESR) remission rates in patients with concomitant CZP were not different between high-dose and low-dose MTX groups (59.2% and 56.9%, respectively). It should be noted that MTX dose was not randomly selected, but only adjusted if there were issues of tolerability. There were some variations in baseline characteristics among the subgroups that could have affected the outcomes.
The Combination Therapy with Adalimumab in Subjects with Early Rheumatoid Arthritis (CONCERTO) study31 of adalimumab in early RA demonstrated a statistically significant trend of improved efficacy with increasing concomitant MTX dose, from 2.5 to 20 mg/week. However, clinical, functional and radiographic assessments at week 26 were similar between groups receiving 10 and 20 mg/week of concomitant MTX. This is consistent with our current findings from C-OPERA in terms of the lack of clear association between MTX dose and efficacy on joint damage inhibition, suggesting that higher doses of MTX may not always be necessary when administered with concomitant anti-TNF agents. However, this is far from conclusive, requires further investigation and may be limited to effects on joint damage progression.
The number of AEs per 100 patient-years was approximately 1.3–1.5 times higher in C-OPERA than the Japan RA Prevention of Structural Damage (J-RAPID) study.12 J-RAPID was similar to C-OPERA; it was conducted in Japanese patients with RA (although these patients had established RA and previous inadequate response to MTX), but average weekly MTX dose was lower (J-RAPID: 6–8 mg/week; C-OPERA: 12 mg/week). In the system, organ classes ‘infections and infestations’, ‘gastrointestinal disorders’ and ‘hepatobiliary disorders’ AEs were more frequently observed in C-OPERA than J-RAPID; these AEs were increased in both PBO and CZP arms, and there was no meaningful difference between the groups. This suggests that the increased frequency of these AEs in C-OPERA may have been associated with the higher MTX dose. Moreover, as all patients were MTX-naive at study entry, their tolerance to MTX treatment could not be anticipated. Of note, hepatic events, including abnormal investigations, were resolved by temporarily discontinuing or reducing MTX dose and no additional safety risk was identified with CZP, based on the lack of a clinically significant difference between the two treatment groups in terms of the incidence or pattern of AEs.
Study limitations included not assessing the effect of CZP monotherapy, which is of interest in early RA treatment. As the current RA treatment recommendations suggest that MTX and/or conventional synthetic DMARDs should be used for initial treatment,5 32 a CZP monotherapy arm was not included in this study.
These efficacy and safety findings from C-OPERA in MTX-naive early RA suggest that CZP could be used as possible first-line treatment concomitantly with MTX in patients with poor prognostic factors, as typified by high-titre anti-CCP antibody. Patients with higher disease activity, functional disability or bone erosion in the early stages of RA will have a higher chance of preventing joint damage and disease progression.
Acknowledgments
The authors would like to acknowledge all the patients, investigators and support staff who participated in the study. The authors also acknowledge Costello Medical Consulting, Cambridge, UK, for writing assistance, which was funded by UCB Pharma, and ‘Matladi N. Ndlovu, PhD, UCB Pharma, Brussels, Belgium and Tomoko Suzuki, UCB Pharma, Tokyo, Japan, for publication coordination.
Correction notice: This article has been corrected since it was published Online First. Figure 3E has been corrected.
Contributors: All the authors made substantial contributions to evaluation of the study results and to develop and review the manuscript.
Funding: This study was sponsored by Otsuka Pharmaceutical Co. (Tokyo, Japan), Astellas Pharma (Tokyo, Japan) and UCB Pharma (Tokyo, Japan). UCB Pharma sponsored the study and the development of the manuscript. In addition to content approval by the authors, UCB signed off on the manuscript following a full review to ensure that the publication did not contain any information that has the potential to damage the intellectual property of UCB.
Competing interests: TA has taken part in speakers’ bureaus for Astellas, Bristol-Myers, Chugai and Mitsubishi-Tanabe; KY has received consultancy fees from Abbott, BMS, Chugai, Eisai, Mitsubishi-Tanabe, Pfizer, Roche and UCB Pharma, and has received research grants from Abbott, Eisai, Mitsubishi-Tanabe, Pfizer, Santen and UCB Pharma; TT has received consultancy fees from AstraZeneca, Asahi Kasei, Eli Lilly, Mitsubishi-Tanabe and Novartis, research grants from Abbott, Astellas, BMS, Chugai, Daiichi-Sankyo, Eisai, Janssen, Mitsubishi-Tanabe, Nippon Shinyaku, Otsuka, Pfizer, Sanofi-Aventis, Santen, Takeda and Teijin, and has taken part in speakers’ bureaus for Abbott, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer and Takeda and UCB Pharma; HY has received consultancy fees from Abbott, Astellas, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer, Takeda and UCB Pharma, and has received research grants from Abbott, Astellas, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer, Takeda and UCB Pharma; NI has received research grants from Abbott, Astellas, BMS, Takeda, Chugai, Eisai, Janssen, Kaken Mitsubishi-Tanabe and Pfizer, and has taken part in speakers’ bureaus for Abbott, Astellas, BMS, Chugai, Eisai, Janssen, Kaken, Mitsubishi-Tanabe, Otsuka, Pfizer, Taisho-Toyama and Takeda; YT has received research grants from Astellas, AbbVie, BMS, Chugai, Daiichi-Sankyo, Mitsubishi-Tanabe, MSD, has received consultancy fees from Abbott, AbbVie, Asahi Kasei, Astellas, AstraZeneca, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, GSK, Janssen, Mitsubishi-Tanabe, MSD, Pfizer, Quintiles, Takeda and UCB Pharma, and has taken part in speakers’ bureaus for Abbott, AbbVie, Asahi Kasei, Astellas, AstraZeneca, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, GSK, Janssen, Mitsubishi-Tanabe, MSD, Pfizer, Quintiles, Takeda and UCB Pharma; KE has received consultancy fees from UCB Pharma; AW has received research grants from Daiichi-Sankyo, Dainippon-Sumitomo, Kyorin, Meiji Seika; Shionogi, Taiho, Taisho and Toyama Chemical, and has taken part in speakers’ bureaus for Daiichi-Sankyo, Dainippon-Sumitomo, GSK, Mitsubishi-Tanabe, MSD, Pfizer, Shionogi and Taisho-Toyama; HO has received consultancy fees from Astellas and UCB Pharma; SY has received research grant from BMS and taken part in speakers’ bureaus for AbbVie, Astellas, Chugai, Eizai, Pfizer, Mitsubishi-Tanabe and Takeda; YY has no competing interests to disclose; YK has received speakers’ bureau from Astellas, Chugai, and Ono; TM has received speaker honoraria from Pfizer Japan, Janssen Pharmaceutical Co. and Astellas Pharma; and research grants form Quintiles Transnational Japan K.K, Janssen Pharmaceutical Co., Takeda Chemical Industries, Daiichi Sankyo Co., Astellas Pharma, Eli Lilly Japan K.K., MSD Co., Nippon Kayaku Co., Parexel International Corp., Pfizer Japan and Bristol-Myers Squibb; MI has received payment for lectures from Astellas, Chugai, Ono and Tanabe-Mitsubishi, has received research grants from Pfizer and a royalty fee from Chugai; TS is an employee of UCB Pharma; TO is an employee of Astellas; DvdH has received consultancy fees from AbbVie, Amgen, AstraZeneca, Augurex, BMS, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, Galapagos, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB Pharma and Vertex; and is the Director of Imaging Rheumatology bv; NM has received research grants from Abbott, Astellas, Chugai, Eisai, Mitsubishi-Tanabe, Pfizer and Takeda; TK has received consultancy fees from AbbVie, Astellas, BMS, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe, Pfizer, Santen, Taisho-Toyama, Takeda, Teijin and UCB Pharma, and has taken part in speakers’ bureaus for Abbott, Astellas, BMS, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe, Pfizer, Santen, Taisho-Toyama, Takeda, Teijin and UCB Pharma.
Patient consent: Obtained.
Ethics approval: This study was conducted after approval by the Institutional Review Board designated by each study site, in compliance with the ethical principles described in the Declaration of Helsinki and Good Clinical Practice.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Nam JL, Winthrop KL, van Vollenhoven RF, et al. . Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis 2010;69:976–86. 10.1136/ard.2009.126573 [DOI] [PubMed] [Google Scholar]
- 2.van der Heijde DM. Radiographic imaging: the ‘gold standard’ for assessment of disease progression in rheumatoid arthritis. Rheumatology (Oxford) 2000;39(Suppl 1):9–16. 10.1093/oxfordjournals.rheumatology.a031496 [DOI] [PubMed] [Google Scholar]
- 3.Quinn MA, Emery P. Window of opportunity in early rheumatoid arthritis: possibility of altering the disease process with early intervention. Clin Exp Rheumatol 2003;21(5 Suppl 31):S154–7. [PubMed] [Google Scholar]
- 4.Smolen JS, Landewe R, Breedveld FC, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JA, Furst DE, Bharat A, et al. . 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res 2012;64:625–39. 10.1002/acr.21641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koike R, Takeuchi T, Eguchi K, et al. . Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol 2007;17:451–8. 10.3109/s10165-007-0626-3 [DOI] [PubMed] [Google Scholar]
- 7.Aletaha D, Neogi T, Silman AJ, et al. . 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 8.Aletaha D, Neogi T, Silman AJ, et al. . 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 9.Nell VP, Machold KP, Eberl G, et al. . Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2004;43:906–14. 10.1093/rheumatology/keh199 [DOI] [PubMed] [Google Scholar]
- 10.Smolen J, Landewe RB, Mease P, et al. . Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797–804. 10.1136/ard.2008.101659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keystone E, Heijde D, Mason D Jr, et al. . Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319–29. 10.1002/art.23964 [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Takeuchi T, Yamanaka H, et al. . Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: the J-RAPID randomized, placebo-controlled trial. Mod Rheumatol 2014;24:715–24. 10.3109/14397595.2013.864224 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K, Takeuchi T, Yamanaka H, et al. . Efficacy and safety of certolizumab pegol without methotrexate co-administration in Japanese patients with active rheumatoid arthritis: the HIKARI randomized, placebo-controlled trial. Mod Rheumatol 2014;24:552–60. 10.3109/14397595.2013.843764 [DOI] [PubMed] [Google Scholar]
- 14.van der Heijde DM, van Riel PL, Nuver-Zwart IH, et al. . Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet 1989;1:1036–8. 10.1016/S0140-6736(89)92442-2 [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- 16.Tanaka Y, Takeuchi T, Mimori T, et al. . Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis 2010;69:1286–91. 10.1136/ard.2009.121491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi T, Tanaka Y, Amano K, et al. . Clinical, radiographic and functional effectiveness of tocilizumab for rheumatoid arthritis patients—REACTION 52-week study. Rheumatology (Oxford) 2011;50:1908–15. 10.1093/rheumatology/ker221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Clair EW, van der Heijde DM, Smolen JS, et al. . Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432–43. 10.1002/art.20568 [DOI] [PubMed] [Google Scholar]
- 19.Bathon JM, Martin RW, Fleischmann RM, et al. . A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586–93. 10.1056/NEJM200011303432201 [DOI] [PubMed] [Google Scholar]
- 20.Breedveld FC, Weisman MH, Kavanaugh AF, et al. . The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 21.Kavanaugh A, Fleischmann RM, Emery P, et al. . Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64–71. 10.1136/annrheumdis-2011-201247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi T, Yamanaka H, Ishiguro N, et al. . Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis 2014;73:536–43. 10.1136/annrheumdis-2012-202433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura K, Sugiyama D, Kogata Y, et al. . Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007;146:797–808. 10.7326/0003-4819-146-11-200706050-00008 [DOI] [PubMed] [Google Scholar]
- 24.van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, et al. . Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 2004;50:709–15. 10.1002/art.20044 [DOI] [PubMed] [Google Scholar]
- 25.Syversen SW, Gaarder PI, Goll GL, et al. . High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 2008;67:212–17. 10.1136/ard.2006.068247 [DOI] [PubMed] [Google Scholar]
- 26.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, et al. . Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005;7:R949–58. 10.1186/ar1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berglin E, Johansson T, Sundin U, et al. . Radiological outcome in rheumatoid arthritis is predicted by presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA-RF at disease onset. Ann Rheum Dis 2006;65:453–8. 10.1136/ard.2005.041376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer O, Labarre C, Dougados M, et al. . Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 2003;62:120–6. 10.1136/ard.62.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vencovsky J, Machacek S, Sedova L, et al. . Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 2003;62:427–30. 10.1136/ard.62.5.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi C, Kaneko Y, Okano H. Methotrexate polyglutamates in erythrocytes correlates with clinical response in Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2014;73(Suppl 2):213–19. [Google Scholar]
- 31.Burmester GR, Kivitz AJ, Kupper H, et al. . Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 2015;74:1037–44. 10.1136/annrheumdis-2013-204769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smolen JS, Landewe R, Breedveld FC, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. 10.1136/ard.2009.126532 [DOI] [PMC free article] [PubMed] [Google Scholar]