Dabigatran is the first of a series of new direct acting oral anticoagulants that was clinically introduced for the prevention of ischaemic stroke in patients with atrial fibrillation (AF). In the randomized evaluation of long-term anticoagulation therapy (RE-LY) study, dabigatran was shown to be superior or non-inferior to warfarin in preventing ischaemic stroke, depending on the dose administered (150 or 110 mg twice daily, respectively).1 This phase III trial opened the door to the clinical introduction of this direct thrombin inhibitor, resulting in a swift clinical uptake around the globe. This was rapidly followed by the introduction of a number of direct factor Xa antagonists, after similar large warfarin-controlled trials showed non-inferiority (or superiority) of rivaroxaban, apixaban and finally edoxaban for preventing ischaemic stroke in patients with AF. The RE-LY study was accompanied by a number of substudies on safety aspects, including genetic and other determinants of dabigatran blood concentrations in relation to clinical outcomes.
In the Heart publication, another analysis of RE-LY is presented, showing that the use of dabigatran is associated with a reduction in plasma apoB levels, suggesting an unexpected pleiotropic side effect with potential clinical consequences.2 The authors observed a ±7% reduction in apoB, an effect that was not dose-dependent, but is clinically relevant when compared with the effects of statins (estimated by the authors as about 25% of the effect obtained with high-dose statin treatment). Importantly, the effect was still evident in subjects that were on actual statin treatment. ApoB is an important cofactor in atherogenesis and an elevated apoB-to-apoA1 ratio has been associated with cardiovascular disease. In contrast to the observed change in apoB, apoA1 levels were not affected by dabigatran. Although, from this substudy, no underlying mechanisms explaining the reduction in apoB were derived, the authors propose that most likely the conversion of dabigatran etexilate to the active dabigatran by carboxylesterases is a causal factor. The reasoning is that the conversion of dabigatran etexilate may influence apolipoprotein metabolism that is also regulated by microsomal carboxylesterases; changes in apoB may result from this competing activity.2 The present paper does not provide any direct evidence to support this theory, but there also are no strong data to refute this hypothesis. In particular, there are no dates to suggest that the inhibitory effect of dabigatran on thrombin may (in)directly affect apoB levels. Additional analyses by the authors did not show any evidence for associations between markers for thrombin formation (prothrombin fragment F1+2) and changes in apoB levels. On the other hand, the lack of a dose–response effect of dabigatran is a certain weakness in this story, but it can be argued that the discrepancy between peak and trough measurements at 1 month and the lipoprotein determinations at 3 months does not provide the ideal setting for assessing such dose-response relationships.
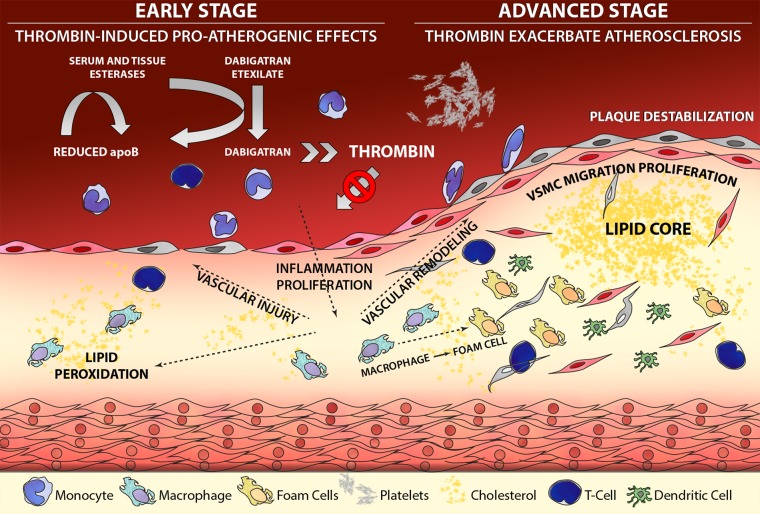
Curious as this unexpected side effect may be, an effect of dabigatran on the vascular outcome of dyslipidaemia (atherosclerosis) could be anticipated, based on experimental studies. First, hypercoagulability is associated with increased atherogenesis in experimental models; in human beings, markers of clotting activity are also linked to cardiovascular disease outcomes.3 Second, anticoagulation may delay atherogenesis; indeed, several studies from different teams have demonstrated that dabigatran attenuates atherosclerosis in mice susceptible to develop atherosclerosis based on an apoE null genetic background. Although in atherosclerosis research the use of such mouse models is a matter of debate, the effects of thrombin inhibition with dabigatran on the development and phenotype of experimental atherosclerosis is quite striking. Improving endothelial function, reducing oxidative stress and delaying or almost completely preventing atherosclerosis and modifying plaque phenotypes have all been reported (and by independent groups, which makes publication bias less likely).4–7 Interestingly, in none of these studies a clear effect on cholesterol profile was found (or looked for) as explanation for the protective effects of dabigatran. Most, if not all, of the effects of the thrombin inhibitor have been ascribed to inhibition of thrombin, known to be a potent and pleiotropically active enzyme from the coagulation cascade.3 Under conditions of inflammatory pressure that diminish the vascular anticoagulant reserve, thrombin may be enabled to act in more pro-inflammatory and prothrombotic directions, through interacting with protease-activated receptors (PAR; in particular PAR-1). These effects are markedly attenuated by the treatment with dabigatran in the mouse models of atherosclerosis. Is the protective effect limited to direct thrombin inhibition? From a coagulation protease perspective this seems unlikely; indeed few studies with rivaroxaban (a direct factor Xa inhibitor) suggested similar protective effects on experimental atherosclerosis, but these effects need to be confirmed. Theoretically, it is likely that any agent that inhibits thrombin generation will also have the potential to inhibit experimental atherosclerosis. Could the esterase effect of dabigatran also have played a role in the mouse studies? We do not know the answer but it may well be the case (figure 1).
Figure 1.
Thrombin is involved in many pathways in atherogenesis (including inflammation and proliferation); blocking thrombin's catalytic activity with dabigatran will also diminish the potential of thrombin to bind and activate protease activated receptor-1 (PAR-1), resulting in attenuated effects of thrombin on atherogenesis (indicated by the blocked arrow). The potentially pleiotropic actions of thrombin in vivo may be supported by the observed effect of dabigatran lowering apoB levels in blood, presumably by competitive use of carboxylesterases for activation of the prodrug dabigatran etexilate to the active dabigatran (indicated in the left upper part of the figure, by arrows).
Based on the experimental work and the abundant literature documenting the presence and activity of coagulation proteases in the atherosclerotic vessel wall, there is substantial interest in any pleiotropy of (anti)coagulation, certainly in human beings exposed to anticoagulation for decades, like in AF. Is there a reason to suspect effects in the long run? Yes, the vitamin K antagonists have taught us that long-term exposure is associated with increased vascular calcification due to the inhibition of various vessel wall vitamin-K-dependent proteins.8 Direct effects of other, direct-acting anticoagulants on vessel wall proteases like thrombin and factor Xa, therefore, seem likely, also since these new synthetic agents are very small, likely allowing endothelial cell passage.
Returning to the RE-LY data, the investigators (and sponsor) are to be commended for having performed another very important subanalysis. The data suggest that this specific thrombin inhibitor may, unexpectedly, attenuate an important cardiovascular risk factor, and this may theoretically add to the efficacy profile of this drug. One should be careful though since this effect was not aimed for and an effect of a drug beyond the targeted antithrombotic action, on cell metabolic pathways, cannot be regarded as a wanted spin-off effect. Still, given the fact that most people with AF are elderly and prone to suffer from atherosclerosis, an additional reduction in a cardiovascular risk factor burden may be a helpful extra effect in the long run. More important is to extend the experimental findings on protection against atherosclerosis towards human studies. The long-term use of new classes of anticoagulants that may interfere with the many biological effects of thrombin and other serine proteases in complex processes like atherothrombosis deserves attention from the medical community currently embracing new direct oral anticoagulants as practical, effective and safe anticoagulants.
Acknowledgments
Sybren ten Cate, PhD contributed the figure.
Footnotes
Competing interests: The author is chair of the Netherlands Federation of Anticoagulation clinics and is involved in several experimental and clinical studies with different anticoagulants. However, he does not hold any stock or has any other financial interests in the development, or use of any anticoagulant as antithrombotic or antiatherosclerotic agent.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 2.Joseph P, Pare G, Wallentin L, et al. . Dabigatran and reduction in serum apolipoprotein B. Heart 2016;102:57–62.. [DOI] [PubMed] [Google Scholar]
- 3.Spronk HM, Borissoff JI, ten Cate H. New insights into modulation of thrombin formation. Curr Atheroscler Rep 2013;15:363 10.1007/s11883-013-0363-3 [DOI] [PubMed] [Google Scholar]
- 4.Kadoglou NP, Moustardas P, Katsimpoulas M, et al. . The beneficial effects of a direct thrombin inhibitor, dabigatran etexilate, on the development and stability of atherosclerotic lesions in apolipoprotein E-deficient mice: dabigatran etexilate and atherosclerosis. Cardiovasc Drugs Ther 2012;26:367–74. 10.1007/s10557-012-6411-3 [DOI] [PubMed] [Google Scholar]
- 5.Lee IO, Kratz MT, Schirmer SH, et al. . The effects of direct thrombin inhibition with dabigatran on plaque formation and endothelial function in apolipoprotein E-deficient mice. J Pharmacol Exp Ther 2012;343:253–7. 10.1124/jpet.112.194837 [DOI] [PubMed] [Google Scholar]
- 6.Borissoff JI, Otten JJ, Heeneman S, et al. . Genetic and pharmacological modifications of thrombin formation in apolipoprotein e-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner. PLoS ONE 2013;8:e55784 10.1371/journal.pone.0055784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pingel S, Tiyerili V, Mueller J, et al. . Thrombin inhibition by dabigatran attenuates atherosclerosis in ApoE deficient mice. Arch Med Sci 2014;10:154–60. 10.5114/aoms.2014.40742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schurgers LJ, Spronk HM. Differential cellular effects of old and new oral anticoagulants: consequences to the genesis and progression of atherosclerosis. Thromb Haemost 2014;112:909–17. 10.1160/TH14-03-0268 [DOI] [PubMed] [Google Scholar]