Abstract
Current guidelines on secondary prevention of cardiovascular disease recommend nurse-coordinated care (NCC) as an effective intervention. However, NCC programmes differ widely and the efficacy of NCC components has not been studied. To investigate the efficacy of NCC and its components in secondary prevention of coronary heart disease by means of a systematic review and meta-analysis of randomised controlled trials. 18 randomised trials (11 195 patients in total) using 15 components of NCC met the predefined inclusion criteria. These components were placed into three main intervention strategies: (1) risk factor management (13 studies); (2) multidisciplinary consultation (11 studies) and (3) shared decision making (10 studies). Six trials combined NCC components from all three strategies. In total, 30 outcomes were observed. We summarised observed outcomes in four outcome categories: (1) risk factor levels (16 studies); (2) clinical events (7 studies); (3) patient-perceived health (7 studies) and (4) guideline adherence (3 studies). Compared with usual care, NCC lowered systolic blood pressure (weighted mean difference (WMD) 2.96 mm Hg; 95% CI 1.53 to 4.40 mm Hg) and low-density lipoprotein cholesterol (WMD 0.23 mmol/L; 95% CI 0.10 to 0.36 mmol/L). NCC also improved smoking cessation rates by 25% (risk ratio 1.25; 95% CI 1.08 to 1.43). NCC demonstrated to have an effect on a small number of outcomes. NCC that incorporated blood pressure monitoring, cholesterol control and smoking cessation has an impact on the improvement of secondary prevention. Additionally, NCC is a heterogeneous concept. A shared definition of NCC may facilitate better comparisons of NCC content and outcomes.
Introduction
Coronary heart disease (CHD) remains a major cause of morbidity and mortality worldwide. Important determinants are the ageing of populations and unhealthy lifestyles.1 2 Patients with established CHD are at very high risk for recurrent cardiovascular events and mortality and are therefore considered the first priority in secondary prevention.3 Although adequate risk factor control to guideline-recommended target levels is highly effective in the secondary prevention setting, recent surveys have shown that risk factor control in clinical practice is far from ideal, leaving substantial room for improvement.4–6 Secondary prevention provided and coordinated by nurses, that is, nurse-coordinated care (NCC), has the potential to improve patient compliance and risk factor control in patients with CHD, although previous reports on the effect of NCC have not shown clear and convincing results.7 8 A previous review concluded that NCC in secondary prevention has a beneficial effect on quality of life.9 However, no consistent relationships were observed between NCC interventions and other outcomes; in another review, almost half of the interventions had no significant effect on study outcomes.10 Heterogeneity in intervention strategies and outcomes hinders comparison between the various studies.10 The European guidelines on cardiovascular disease prevention state that NCC prevention programmes are effective, based on two trials.11 12 Available research is, however, more extensive and the overall findings appeared less conclusive. In the present study, we therefore systematically reviewed the available evidence on the efficacy of NCC in secondary prevention of CHD.
Methods
Search strategy and selection
Using a comprehensive search strategy, we searched MEDLINE, the Cochrane Central Register of Controlled Trials and CINAHL from 1990 up to January 2015, with no language restriction. Since evidence for NCC has evolved after the 1990s, the review was limited to studies published after 1990. The following search terms were entered as independent terms, text words or medical subject headings (MESH) terms: (1) coronary heart disease or cardiovascular patient or cardiovascular diseases and (2) nurse led or case manage* or nurse practitioner or managed care programs/organization and administration. In addition, reference lists of existing reviews were manually searched to identify additional relevant studies. Our MEDLINE search strategy is described in detail in online supplement 1.
Two reviewers independently screened all titles and abstracts identified by the search. Studies that were classified as possibly relevant by at least one reviewer were retrieved in full text and assessed for inclusion using a standardised inclusion form. Multiple publications reporting on the same study were included only when additional relevant outcomes were presented; they were counted as one study. Disagreements were solved by discussion between the two reviewing authors. We conducted our systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.13
Selection criteria
Studies were included only if (a) they were designed as a randomised controlled trial (RCT); (b) patients were hospitalised or being treated by a general practitioner (GP) for secondary prevention of CHD; (c) Trials were included as at least 70% of their included study population had cardiovascular disease (CVD) or reported data separately on a secondary prevention group; (d) a registered nurse was involved as a ‘nurse coordinator’, using Krumholz's description of coordinated care: the development and implementation of a therapeutic plan to integrate the efforts of multiple health professionals14 and (e) the outcomes reported included risk factors, health behaviours, clinical events, patient-perceived health or guideline adherence. For studies meeting these criteria, all other outcomes, except costs, were taken into account in our analysis.
Quality assessment
Two reviewers independently assessed the risk of bias in the included studies using the Cochrane Collaboration's risk of bias tool, which requires critical evaluation of the following domains: sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other source of bias.15 After this evaluation, each domain of the studies was classified as having low, high or unclear risk of bias.
Data extraction
Data were extracted about the setting and study population, NCC intervention components and both primary and secondary outcomes of included studies. Two reviewers independently extracted all relevant information using a data extraction form. Due to heterogeneity of the data, a descriptive approach was used to summarise components of NCC and their effect on outcomes. Based on consensus, we distinguished three intervention strategies: (1) risk factor management, (2) multidisciplinary consultation and (3) shared decision making. We rated the intensity of the intervention as high (>4 visits plus more than one NCC strategy used), intermediate (3–4 visits) or low (1–2 visits). We defined a multidisciplinary team as a team with >2 disciplines. Furthermore, we classified the observed outcomes into four categories: (1) risk factor levels, (2) clinical events, (3) patient-perceived health and (4) guideline adherence. In our meta-analysis, we pooled the sufficiently homogeneous outcomes to determine the effectiveness of the NCC intervention.
Statistical analysis
We used forest plots to visualise the effects of NCC on systolic blood pressure (SBP), low-density lipoprotein (LDL) cholesterol and smoking cessation compared with usual care, stratified for treatment intensity (high, intermediate, low, unknown). To indicate the differences between these methods, random effects and fixed effects models were used to pool treatment effects. Mantel–Haenszel fixed effect pooling assumes a single true treatment effect and ignores between-study heterogeneity. DerSimonian–Laird random effects pooling takes between-study heterogeneity into account and leads to wider CIs. However, in random effects pooling, small studies receive more weight and this may affect the pooled treatment estimates. If no between-study heterogeneity exists, both methods yield identical results. Heterogeneity was expressed using the I2 statistic. (Pooled) risk ratios were calculated from 2×2 tables, which were derived from the publications, using the metan command (V.3.04, 21 September 2010) in Stata V.13.1.
Results
Study selection
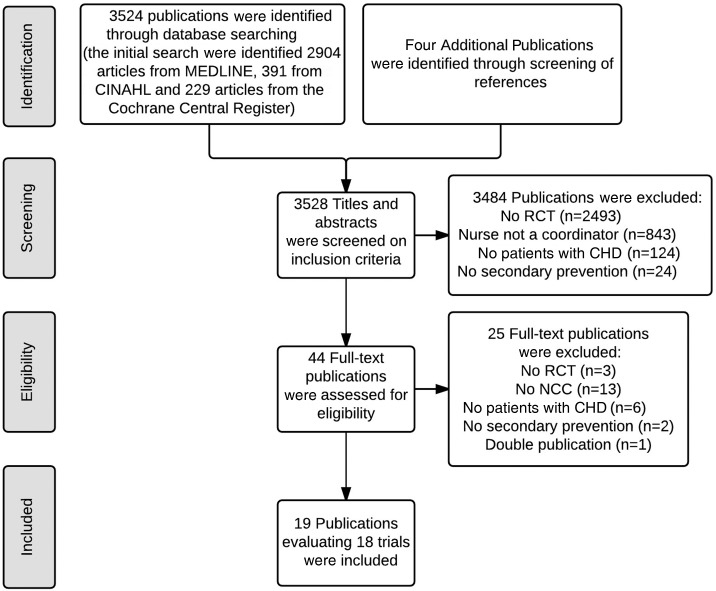
A total of 3524 publications were initially identified (figure 1). Screening the references in these publications yielded another four potentially relevant studies. After two reviewers reviewed titles and abstracts, 44 publications were retrieved in full text. We excluded 25 of these publications after reading the full text (see online supplement 2). To prevent double counting, only Voogdt-Pruis’ primary care study (2010) was included, as it matched our review purpose best.16 Campbell et al reported different outcomes of the same study in two publications. We counted these as one study.17 18 In total, we included 18 studies in our systematic review.
Figure 1.
Flow diagram of selection of trials. CHD, coronary heart disease; NCC, nurse-coordinated care; RCT, randomised controlled trial.
Trial characteristics
Total sample sizes ranged from 138 to 2142 participants in 12 countries of four continents (see online supplement 3). Patients with CHD were recruited during hospital admission11 19–26 or at outpatient clinics,27 28 a community health clinic,29 a secondary prevention unit30 or general practices.16 18 31 32 The study participants’ mean age ranged from 54 to 75 years.22 29 ‘Usual care’ generally consisted of routine aftercare by a GP or cardiologist (see online supplement 3). In six of the trials, routine care was more intensive and included a cardiac rehabilitation programme.23 25 26 28 30 33
Risk of bias in included studies
Online supplement 4 presents the risk of bias across the included studies; 13 of 18 studies (72%) were considered to have a high risk of bias for one or more domains. In general, there was a low risk of selection bias; all studies, except two,30 33 used a valid method for random sequence generation and 4 of 18 trials (22%) used non-individual randomisation methods.11 24 31 32 Allocation concealment was unsatisfactory or not reported in five trials (28%).11 18 24 30 33 In one trial, ‘the patients were randomised by the researchers’,18 which resulted in a high risk of bias. Blinding of intervention is not possible in this type of studies, which increases the possibility of performance bias. Four trials (22%) blinded the outcome assessors using an independent research assistant to carry out the clinical assessments,21 24 28 32 and in three additional trials, outcome data were independently retrieved from hospital records.22 23 25 The risk of detection bias in the other trials was classified as either unclear or high. Six trials collected outcome data incompletely,11 16 21 24 27 30 had many missing values16 or unclear exclusions from the analysis.11 Seven studies (39%) did not report prespecified outcomes19–21 26 27 30 33 in the primary publication or in a trial registry or design paper, if available. Of 18 trials in total, five recent trials (28%) were registered in a trial registry.11 22 25 28 29 Eleven studies (61%) used one or more self-reported outcomes for lifestyle-related risk factors, which may have introduced bias.34
Description of the intervention by strategy
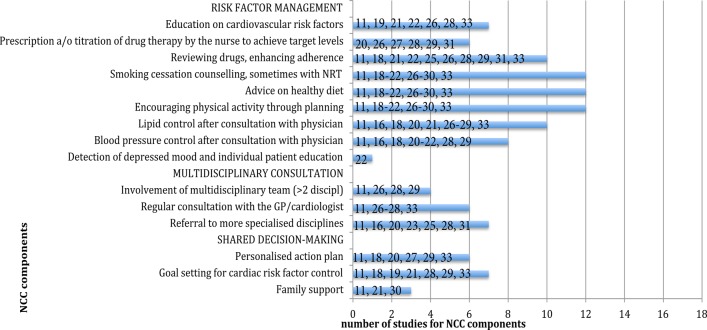
The NCC programmes varied in components and intensity (see online supplement 3). We identified 15 components of the NCC intervention and grouped them into three strategies (figure 2): (1) risk factor management, for example, lifestyle counselling, blood pressure and lipid control; (2) multidisciplinary consultation, for example, consultation and referral and (3) shared decision making, for example, goal setting and family support.
Figure 2.
Components of nurse-coordinated care (NCC) by strategy in 18 studies. Presented numbers in the figure are study references. a/o, and/or; GP, general practitioner; NRT, nicotine replacement therapy.
Risk factor management
Risk factor management was the most commonly used NCC strategy and was reported in 13 studies (72%). In six studies (33%), nurses were authorised to prescribe or titrate medication.20 26–29 31 In two of these studies, this was done according to prespecified algorithms.26 29 To encourage a more active lifestyle, NCC interventions consisted of ‘instruction to participate in a home-based exercise programme’,29 ‘Stepping Out’ programmes to promote physical activity,18 starting a physical training programme in the first 3 months of the intervention,30 recommendation to walk briskly for 20 min daily26 or referral to a physiotherapist.11
Multidisciplinary consultation
The second strategy, multidisciplinary consultation, was assessed in 11 studies (61%). ‘Involvement of a multidisciplinary team’ was part of this strategy in four trials (22%).11 26 28 29 Seven trials11 16 20 23 25 28 31 (39%) incorporated ‘referral to more specialised disciplines’ as needed.
Shared decision making
The third strategy, ‘shared decision making’, was incorporated in 10 studies (56%). This strategy refers to implementing family support,11 21 30 goal setting for cardiac risk factor control11 18 19 21 28 29 33 and a personalised action plan.11 18 20 27 29 33
The included studies varied in terms of the duration of the intervention (2–24 months), frequency of visits (3–14 contacts) and follow-up time (3–24 months). The majority used a 12-month follow-up period (see online supplement 6). In eight studies (44%), telephone follow-up was used,19 21 22 25–27 29 33 and in six studies (33%) home visits were part of the intervention (see online supplement 3).19 21–23 25 27 Six trials included four or more visits plus more than one NCC strategy (high intensity)11 18 26–29; six trials were rated as intermediate intensity,16 19–21 30 33 three trials were rated as low intensity22 25 31 and three studies were rated as unclear intensity (see online supplement 3).23 24 32
Description of outcomes by category
Outcomes of NCC varied considerably (see online supplement 5a,b). In total, 30 NCC outcomes were measured. We grouped observed outcomes into four categories: (1) risk factor levels; (2) clinical events; (3) patient-perceived health and (4) guideline adherence.
Risk factor levels
In 14 studies (78%), outcomes of NCC studies were measured as improvement of risk factor levels with heterogeneous treatment effects (see online supplement 6). One study used SCORE, a comprehensive cardiovascular risk algorithm designed for the primary prevention setting, as the study outcome.28 Figures 3–5 present our meta-analyses of weighted mean differences and relative risk (RR) calculations of trials reporting on SBP, LDL cholesterol and smoking cessation, respectively.
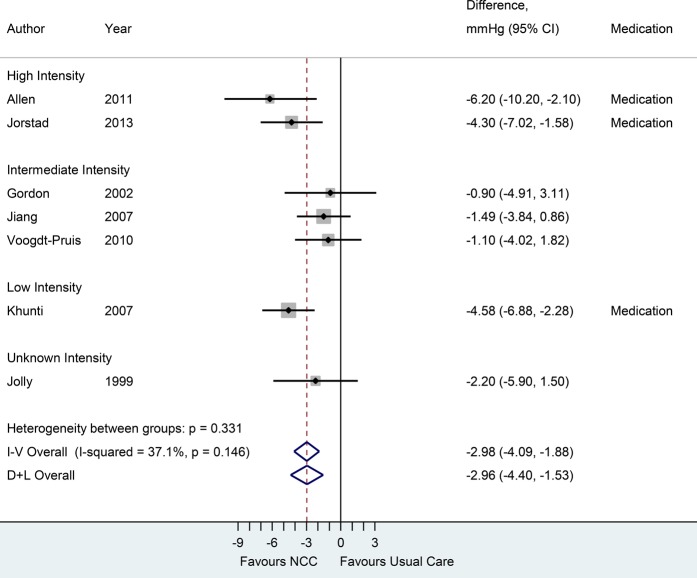
Figure 3.
Forest plot of seven randomised trials on the effect of nurse-coordinated care (NCC) on systolic blood pressure. Trials are ordered by treatment intensity and year. Medication indicates trials using medication-titration; I–V, inverse-variance (fixed effects); D+L, DerSimonian–Laird (random effects). Random effects estimates in the subgroups are identical to the fixed effects estimates, no between-trial heterogeneity. Except for two trials (Gorden et al, Jiang et al), all trials used a 12-month follow-up period.
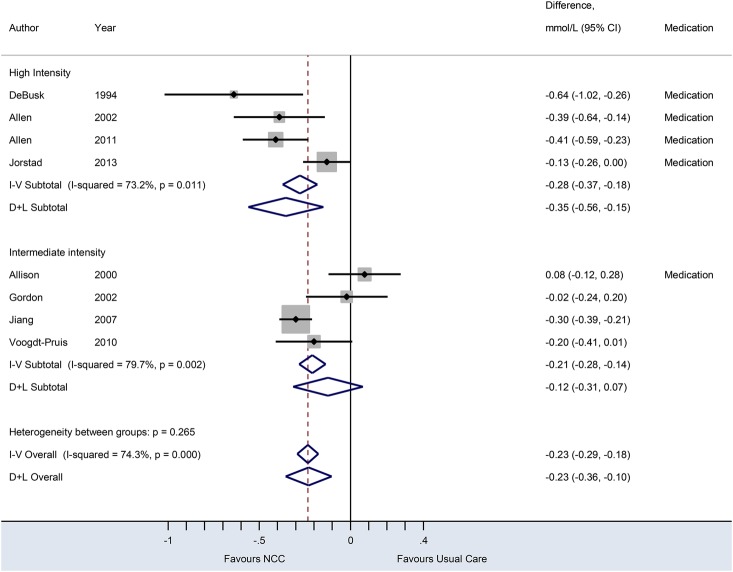
Figure 4.
Forest plot of eight randomised trials on the effect of nurse-coordinated care (NCC) on serum low-density lipoprotein cholesterol concentrations. Trials are ordered by treatment intensity and year. Medication indicates trials using medication-titration; I–V, inverse-variance (fixed effects); D+L, DerSimonian–Laird (random effects). Except for three trials (Allison et al, Gorden et al, Jiang et al), all trials used a 12-month follow-up period.
Figure 5.
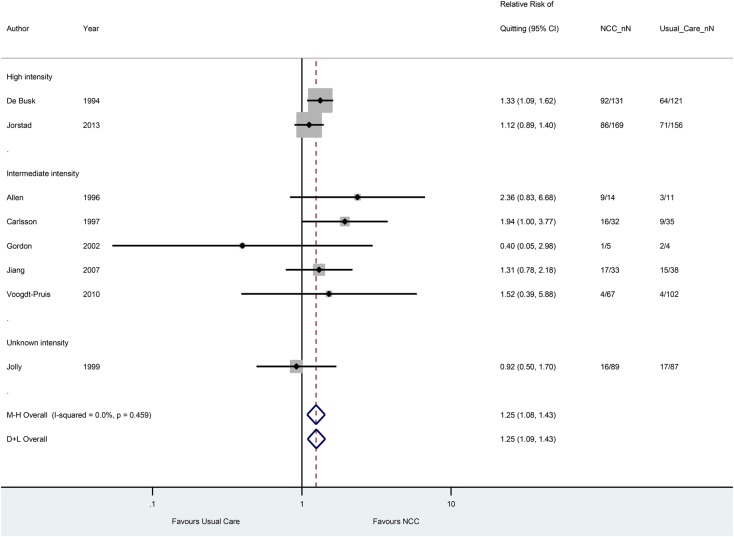
Forest plot of eight randomised trials on the effect of nurse-coordinated care (NCC) on smoking cessation rates. Trials are ordered by treatment intensity and year. M–H indicates Mantel–Haenszel (fixed effects), D+L indicates DerSimonian–Laird (random effects). The trial by Wood et al11 was excluded since only the absolute cessation risk difference (of 10.4% (−0.30 to 21.20) in favour of NCC) was reported and pooling of absolute risk differences caused much heterogeneity in the stratum with the intermediate intensity trials. NCC_nN and Usual_Care_nN denote the number of quitters (n) of the total number of smokers at baseline (N) in the NCC intervention groups and usual care groups, respectively. Except for one trial (Jiang et al), all trials used a 12-month follow-up period.
Seven studies reported on SBP outcomes. The NCC intervention decreased SBP by 2.96 mm Hg (95% CI 1.53 to 4.40 mm Hg) compared with usual care with low-to-moderate between-study heterogeneity (I2=37.1%). Eight trials reported on LDL cholesterol outcomes. The effect of NCC compared with usual care on LDL cholesterol was −0.23 mmol/L (95% CI −0.36 to −0.10 mmol/L), with substantial heterogeneity (I2=74.3%). Trials incorporating prescription and/or titration of drug therapy by nurses were associated with a significant reduction in LDL cholesterol and SBP, compared with usual care. Meta-analysis of eight trials comparing smoking cessation rates, generally self-reported (75%), between NCC and usual care yielded a pooled RR of 1.25 (95% CI 1.08 to 1.43). Random effects and fixed effects models showed no between-study heterogeneity in treatment effects (I2=0.0%). Six studies reported smoking cessation rates at 12 months,16 19 24 26 28 30 one study at 6 months21 and one study at 12 weeks of follow-up.33
Clinical events
In total, seven studies reported on clinical events (see online supplement 5b) and five studies reported on recurrent events and the duration of hospitalisation17 23 25 or readmission rates17 20 25 28 at assessment time >6 months. In four of these studies, a reduction was shown for all-cause and cardiovascular readmission rates or the duration of hospitalisation and other CVD rates or recurrent coronary events.17 18 20 23 28 A disease management programme23 significantly reduced the secondary outcome emergency department encounters (incidence density ratio –2.08, p<0.001), claims for diagnostic or therapeutic services (830 vs 1208 claims, p=0.012) and the use of laboratory services (1481 vs 2401, p=0.007) in favour of the NCC intervention. The trials that assessed the outcomes all-cause mortality,20 25 time to readmission or death22 or event-free survival25 all showed no effect of NCC versus usual care on these outcomes.
Patient-perceived health
Six publications reported patient-perceived health outcomes with different instruments and showed small effects (see online supplement 5b and 6).18 20 24 25 29 31 Three studies showed a statistically significant improvement on the following questionnaires (or one of their subscales): the short form 36 (SF-36),18 chest pain,18 perception of chronic illness care29 and the Seattle Angina Questionnaire.31
Guideline adherence
Three trials reported better results for the NCC intervention compared with the usual care group on the outcome category ‘guideline adherence’, which implies assessment of risk factors according to secondary prevention guidelines.18 31 32
Summary of effective interventions and their NCC components
We found that interventions that include independent prescription and/or titration of drug therapy by nurses and a high-intensity strategy appeared to be effective in reducing SBP and LDL cholesterol (figures 3 and 4).20 26–29 31 Effective components regarding behavioural interventions were goal setting for cardiac risk factor control plus identification of barriers, an approach that positively affected the risk factor profile in several studies.11 18 19 21 29
Of 11 trials with prespecified primary outcomes, eight trials demonstrated positive outcomes for NCC compared with usual care: for the outcome category risk factor levels: total cholesterol,16 29 31 LDL cholesterol,29 triglyceride,29 pharmacological treatment,31 SCORE,28 blood pressure28 29 and diet;11 clinical events: all-cause and cardiovascular readmission (days)23 and guideline adherence.18 32 Half of these studies were classified as high intensity, including >4 face-to-face contacts11 18 28 29 and frequent telephone follow-up in one of them.29
Discussion
The evidence summarised in this review suggests that prescription and/or titration of drug therapy by nurses, in combination with a high-intensity strategy, can decrease SBP and LDL cholesterol. NCC also improved smoking cessation substantially by 25%, but, although nurses’ attention for lifestyle-related risk factors was a common component in the reviewed studies, this did not result in weight loss. Evidence from cardiac rehabilitation studies with exercise and multimodal interventions showed an effect on mortality.35 This effect might have been achieved through improved adherence to lifestyle modification and medication, which may be a result of frequent follow-up visits by nurses. The intervention components and outcome measures were very heterogeneous. This indicates that NCC is not yet a clearly defined concept, as well as a complex intervention. Complex interventions, including several components, are made up of various interconnecting parts and it is therefore difficult to evaluate the contribution of individual components. Furthermore, breaking down these complex interventions into separate components does not take into account the synergistic effects of combining these components. In most studies, NCC interventions were multifaceted, broadly structured and therefore lacked focus. As there is a variation in the selection of outcomes in the included studies, it is important to answer the question what should be appropriate goals for NCC. Consensus about NCC content and reporting of outcome measurements for RCTs would facilitate a better evidence base for future. In 2006, the American Heart Association Disease Management Taxonomy Writing Group published a statement about defining and classifying different care models, in particular disease management.14 The interdisciplinary writing group designed a conceptual model and its proposed components to allow comparisons across interventions of disease management trials. This statement forms an ideal starting point to compare diverse disease management programmes and to assess specific components associated with effectiveness. Such an initiative would also be valuable for the development of NCC programmes.
Limitations
We encountered heterogeneity in our meta-analyses. We also observed between-study differences that we could not explain. Although the composition of NCC programmes was heterogeneous, this was not always the case for their relative effects on outcomes. The overall quality of the RCTs in this review was moderate. At the same time, it was encouraging that more recent studies had better methodological quality and clinical trial registration. One older study was deemed to be of low or unclear quality since it did not describe critical components for assessing the risk of bias.30 We nevertheless included this study in the meta-analysis of smoking cessation. Many studies were at risk of selective reporting. In several studies, no prespecified primary and secondary endpoints were stated. Self-reported outcomes were used as well, so the observed effects could be overestimated or underestimated. The results should therefore be interpreted with caution.
Overweight and smoking remained persistent and prevalent risk factors in many of the studies. A recent review on the efficacy of lifestyle modification programmes to support behaviour change in patients with CHD found that comprehensive lifestyle modification programmes reduced mortality by 34% and cardiac readmissions by 35%.36 Interventions incorporating four self-regulation techniques (ie, goal setting, planning, self-monitoring, feedback) were associated with greater lifestyle benefits. This is in line with our finding that goal setting is a successful component for both behavioural counselling and medication-regulated risk factors. Community-based comprehensive lifestyle programmes take this approach and this might be a new opportunity to achieve weight reduction in patients with CHD.37–40
Despite clinical heterogeneity, we conclude that effective NCC interventions consist of these components: (i) prescription and/or titration of drug therapy by nurses26–29 31 particularly with predefined algorithms,26 29 (ii) tailored behavioural counselling with goal setting11 18 19 21 29 33 and (iii) frequent follow-up visits and telephone contacts.26 27 29
Our review shows that when NCC incorporates blood pressure monitoring, cholesterol control and smoking cessation, it may improve secondary prevention. Finding effective interventions to achieve weight reduction in patients with CHD remains an important challenge for future. Additionally, NCC has shown to be a heterogeneous concept. We recommend a shared definition of NCC to facilitate better comparisons of NCC content and outcomes.
Supplementary Material
Acknowledgments
The authors thank Ms Faridi van Etten-Jamaludin, clinical librarian at AMC, University of Amsterdam, for her valuable contribution in developing the search strategy.
Footnotes
Contributors: MS, RJGP, BMB and WJMSoR participated in the design of the systematic review. MS, JD and PJ performed study selection, quality assessment and extraction of data. MS, GtR, PJ and JD were involved in the data analyses. MS, GtR, JD, RJGP, WJMSoR were involved in the interpretation and discussion of results. MS drafted the manuscript. JD, PJ and WJMSoR contributed to the drafting of the review. GtR, SMB, BMB and WJMSoR provided critical revision for important intellectual content. All authors approved the final version of the manuscript.
Funding: MS is supported by a research grant from the Netherlands Organisation for Scientific Research (NWO).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.ESC. European cardiovascular disease statistics 2012. http://www.escardio.org/about/what/advocacy/EuroHeart/Pages/2012-CVD-statistics.aspx (accessed 16 Apr 2013).
- 2.WHO World Health Organisation. The leading causes of death in the world, 2000 and 2012. fact sheet N 310. Updated May 2014 2014. http://www.who.int/topics/mortality/en/
- 3.Perk J, De Backer G, Gohlke H, et al. . European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Int J Behav Med 2012;19:403–88. 10.1007/s12529-012-9242-5 [DOI] [PubMed] [Google Scholar]
- 4.Kotseva K, Wood D, De Bacquer D, et al. . EUROASPIRE IV: a European society of cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2015:1–13. [DOI] [PubMed] [Google Scholar]
- 5.Kotseva K, Wood D, De Backer G, et al. . EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil 2009;16:121–37. 10.1097/HJR.0b013e3283294b1d [DOI] [PubMed] [Google Scholar]
- 6.Iestra JA, Kromhout D, van der Schouw YT, et al. . Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: a systematic review. Circulation 2005;112:924–34. 10.1161/CIRCULATIONAHA.104.503995 [DOI] [PubMed] [Google Scholar]
- 7.Berra K. Does nurse case management improve implementation of guidelines for cardiovascular disease risk reduction? J Cardiovasc Nurs 2011;26:145–67. 10.1097/JCN.0b013e3181ec1337 [DOI] [PubMed] [Google Scholar]
- 8.Schadewaldt V, Schultz T. Nurse-led clinics as an effective service for cardiac patients: results from a systematic review. Int J Evid Based Healthc 2011;9:199–214. 10.1111/j.1744-1609.2011.00217.x [DOI] [PubMed] [Google Scholar]
- 9.Page T, Lockwood C, Conroy-Hiller T. Effectiveness of nurse-led cardiac clinics in adult patients with a diagnosis of coronary heart disease. Int J Evid Based Healthc 2005;3:2–26. 10.1111/j.1479-6988.2005.00019.x [DOI] [PubMed] [Google Scholar]
- 10.Allen JK, Dennison CR. Randomized trials of nursing interventions for secondary prevention in patients with coronary artery disease and heart failure: systematic review. J Cardiovasc Nurs 2010;25:207–20. 10.1097/JCN.0b013e3181cc79be [DOI] [PubMed] [Google Scholar]
- 11.Wood DA, Kotseva K, Connolly S, et al. . Nurse-coordinated multidisciplinary, family-based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster-randomised controlled trial. Lancet 2008;371:1999–2012. 10.1016/S0140-6736(08)60868-5 [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Alderman EL, Fair JM, et al. . Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. the Stanford coronary risk intervention project (SCRIP). Circulation 1994;89:975–90. 10.1161/01.CIR.89.3.975 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumholz HM, Currie PM, Riegel B, et al. . A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation 2006;114:1432–45. 10.1161/CIRCULATIONAHA.106.177322 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voogdt-Pruis HR, Beusmans GH, Gorgels AP, et al. . Effectiveness of nurse-delivered cardiovascular risk management in primary care: a randomised trial. Br J Gen Pract 2010;60:40–6. 10.3399/bjgp10X482095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell NC, Thain J, Deans HG, et al. . Secondary prevention clinics for coronary heart disease: randomised trial of effect on health. BMJ 1998;316:1434–7. 10.1136/bmj.316.7142.1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell NC, Ritchie LD, Thain J, et al. . Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart 1998;80:447–52. 10.1136/hrt.80.5.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JK. Coronary risk factor modification in women after coronary artery bypass surgery. Nurs Res 1996;45:260–5. 10.1097/00006199-199609000-00002 [DOI] [PubMed] [Google Scholar]
- 20.Allison TG, Farkouh ME, Smars PA, et al. . Management of coronary risk factors by registered nurses versus usual care in patients with unstable angina pectoris (a chest pain evaluation in the emergency room [CHEER] substudy). Am J Cardiol 2000;86:133–8. 10.1016/S0002-9149(00)00848-1 [DOI] [PubMed] [Google Scholar]
- 21.Jiang X, Sit JW, Wong TK. A nurse-led cardiac rehabilitation programme improves health behaviours and cardiac physiological risk parameters: evidence from Chengdu, china. J Clin Nurs 2007;16:1886–97. 10.1111/j.1365-2702.2007.01838.x [DOI] [PubMed] [Google Scholar]
- 22.Meisinger C, Stollenwerk B, Kirchberger I, et al. . Effects of a nurse-based case management compared to usual care among patients with myocardial infarction: results from the randomized controlled KORINNA study. BMC Geriatr 2013;13:115 10.1186/1471-2318-13-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young W, Rewa G, Goodman SG, et al. . Evaluation of a community-based inner-city disease management program for postmyocardial infarction patients: a randomized controlled trial. CMAJ 2003;169:905–10. [PMC free article] [PubMed] [Google Scholar]
- 24.Jolly K, Bradley F, Sharp S, et al. . Randomised controlled trial of follow up care in general practice of patients with myocardial infarction and angina: final results of the Southampton heart integrated care project (SHIP). The SHIP Collaborative Group. BMJ 1999;318:706–11. 10.1136/bmj.318.7185.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrington MJ, Carrington MJ, Chan YK, et al. . A multicenter, randomized trial of a nurse-led, home-based intervention for optimal secondary cardiac prevention suggests some benefits for men but not for women: the young at heart study. Circ Cardiovasc Qual Outcomes 2013;6:379–89. 10.1161/CIRCOUTCOMES.111.000006 [DOI] [PubMed] [Google Scholar]
- 26.DeBusk RF, Miller NH, Superko HR, et al. . A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med 1994;120:721–9. 10.7326/0003-4819-120-9-199405010-00001 [DOI] [PubMed] [Google Scholar]
- 27.Allen JK, Blumenthal RS, Margolis S, et al. . Nurse case management of hypercholesterolemia in patients with coronary heart disease: results of a randomized clinical trial. Am Heart J 2002;144:678–86. 10.1016/S0002-8703(02)00141-2 [DOI] [PubMed] [Google Scholar]
- 28.Jorstad HT, von Birgelen C, Alings AM, et al. . Effect of a nurse-coordinated prevention programme on cardiovascular risk after an acute coronary syndrome: main results of the RESPONSE randomised trial. Heart 2013;99:1421–30. 10.1136/heartjnl-2013-303989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen JK, Dennison-Himmelfarb CR, Szanton SL, et al. . Community outreach and cardiovascular health (COACH) trial: a randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circ Cardiovasc Qual Outcomes 2011;4:595–602. 10.1161/CIRCOUTCOMES.111.961573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson R, Lindberg G, Westin L, et al. . Influence of coronary nursing management follow up on lifestyle after acute myocardial infarction. Heart 1997;77:256–9. 10.1136/hrt.77.3.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khunti K, Stone M, Paul S, et al. . Disease management programme for secondary prevention of coronary heart disease and heart failure in primary care: a cluster randomised controlled trial. Heart 2007;93:1398–405. 10.1136/hrt.2006.106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher M, Yudkin P, Wright L, et al. . Cluster randomised controlled trial to compare three methods of promoting secondary prevention of coronary heart disease in primary care. BMJ 2001;322:1338 10.1136/bmj.322.7298.1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon NF, English CD, Contractor AS, et al. . Effectiveness of three models for comprehensive cardiovascular disease risk reduction. Am J Cardiol 2002;89:1263–8. 10.1016/S0002-9149(02)02323-8 [DOI] [PubMed] [Google Scholar]
- 34.Shiely F, Hayes K, Perry IJ, et al. . Height and weight bias: the influence of time. PLoS One 2013;8:e54386 10.1371/journal.pone.0054386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Riemenschneider F, Damm K, Meinhard C, et al. . Evaluation of medical and health economic effectiveness of non-pharmacological secondary prevention of coronary heart disease. GMS Health Technol Assess 2009;5:16 10.3205/hta000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen V, De Gucht V, Dusseldorp E, et al. . Lifestyle modification programmes for patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2013;20:620–40. 10.1177/2047487312462824 [DOI] [PubMed] [Google Scholar]
- 37.McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict Behav 2006;31:1650–60. 10.1016/j.addbeh.2005.12.014 [DOI] [PubMed] [Google Scholar]
- 38.Jebb SA, Ahern AL, Olson AD, et al. . Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–92. 10.1016/S0140-6736(11)61344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jolly K, Lewis A, Beach J, et al. . Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: lighten up randomised controlled trial. BMJ 2011;343:d6500 10.1136/bmj.d6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto AM, Fava JL, Hoffmann DA, et al. . Combining behavioral weight loss treatment and a commercial program: a randomized clinical trial. Obesity (Silver Spring) 2013;21:673–80. 10.1002/oby.20044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.