Abstract
Evidence accumulated over recent years has shown that genetic neurological channelopathies can cause many different neurological diseases. Presentations relating to the brain, spinal cord, peripheral nerve or muscle mean that channelopathies can impact on almost any area of neurological practice. Typically, neurological channelopathies are inherited in an autosomal dominant fashion and cause paroxysmal disturbances of neurological function, although the impairment of function can become fixed with time. These disorders are individually rare, but an accurate diagnosis is important as it has genetic counselling and often treatment implications. Furthermore, the study of less common ion channel mutation-related diseases has increased our understanding of pathomechanisms that is relevant to common neurological diseases such as migraine and epilepsy. Here, we review the molecular genetic and clinical features of inherited neurological channelopathies.
Keywords: CHANNELS, GENETICS, MOLECULAR BIOLOGY
Introduction
Inherited disorders of ion channel function—the ‘genetic channelopathies’ are a rapidly expanding group of neurological disorders and are implicated in many areas of neurological practice. Although the inherited channelopathies are individually rare, the study of these conditions is contributing to our understanding of pathomechanisms of neurological disease in general.
Ion channels are specialised pore-forming proteins that allow the passage of certain ions across the lipid bilayer of the cell membrane. They are typically divided into two broad categories according to their method of activation—voltage or ligand gated. The ‘gating’ of ion channels by transmembrane voltage changes or specific receptor ligands, such as acetylcholine (ACh), together with their selectivity for distinct ion species, underlies the coordination of ion fluxes during action potentials or following neurotransmitter release.1
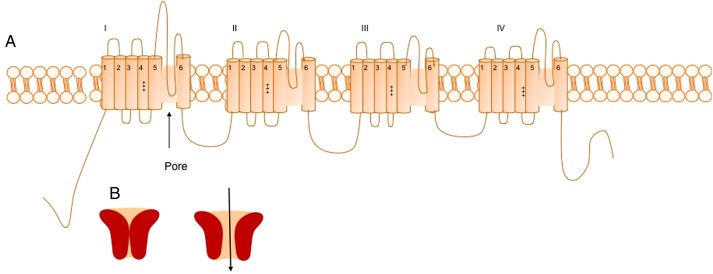
Most ion channels have a similar basic structure—for example, all voltage-gated ion channels have a large pore-forming subunit—the α subunit, composed of four homologous domains (I–IV)—each composed of six transmembrane segments (S1–S6). In all cation channels, the S4 segments contain between four and eight positively charged residues conferring voltage dependence, and the S5-S6 loops form the ion pore. Ion channels are also composed of several accessory subunits that may be cytoplasmic or extracellular that have roles in channel kinetics and membrane stabilisation2 (figure 1).
Figure 1.
(A) Structure of the α subunit of the voltage-gated sodium channel NaV1.1 encoded by SCN1A. The subunit is composed of four domains (I–IV), which are each composed of six transmembrane subunits (S1–S6)—the positive gating charges in the S4 subunit are marked as is the pore region (between S5 and S6). (B) Schematic representation of ion channel in open and closed states.
Although ion channels are essential for the normal function of all eukaryotic cells, they are particularly important in the nervous system for the generation, repression and propagation of action potentials. Ion channels are often highly selective for a particular ionic species, for example, sodium, potassium or calcium. The opening of sodium channels leads to depolarisation of neurons whereas potassium channel opening leads to hyperpolarisation, as does the opening of chloride channels in adult neurons. The opening of calcium channels causes membrane depolarisation, but calcium ions also have more important roles as second messengers.3 Hence, loss of function mutations in potassium or chloride channels or gain of function mutations should lead to disorders characterised by hyperexcitability, such as epilepsy. However, the effect of a mutation depends on the specific neuronal circuitry involved. For example, a mutation that causes a gain of function effect in inhibitory interneurons can decrease excitability.3
Given their importance in neuronal excitability and synaptic transmission through the central and peripheral nervous systems, it is not surprising that mutations in ion channel genes can lead to disease. Many of the mutations that have been associated with ion channel disorders are missense mutations that affect channel kinetics. However, inherited mutations and chromosomal rearrangements can affect any stage of ion channel biogenesis, including transcription, mRNA processing, splicing, translation, folding and trafficking, as well as subunit assembly.
Inherited disorders of ion channels are typically inherited in an autosomal dominant fashion, although there are exceptions, and can cause a variety of neurological syndromes. Typically, symptoms begin relatively early in life and are paroxysmal or episodic, although a fixed deficit may develop with time. These attacks or paroxysms are often precipitated by various triggers. Stress, of some form, is a frequent trigger, whereas certain triggers are disease specific, such as heat in primary erythromelalgia (PE), or rest after exercise or a carbohydrate load in periodic paralysis. Mutations in ion channels can alter channel function such that homoeostasis cannot be maintained in the presence of certain stimuli that would usually be innocuous.4
In this review, we describe the clinical characteristics and genetics of inherited channelopathies of the brain, spinal cord, peripheral nerve and muscle (see online supplemental figure S1).
Channelopathies of the central nervous system
Epilepsy
Although rare, inherited channelopathies account for a substantial fraction of Mendelian epilepsy syndromes and can cause a variety of epilepsy types ranging from severe infantile encephalopathies to relatively benign focal seizures (table 1).
Table 1.
Epilepsy syndromes caused by inherited mutations in ion channel genes
| Channel | Gene | Channel | Epilepsy syndrome(s) |
|---|---|---|---|
| Sodium | SCN1A | α subunit of NaV1.1 | Severe myoclonic epilepsy of infancy (SMEI) Intractable epilepsy with generalised tonic-clonic seizures (IEGTC) Migrating partial seizures of infancy (MPSI) Generalised epilepsy with febrile seizures (GEFS+) |
| SCN1B | β subunit of NaV1.1 | SMEI GEFS+ |
|
| SCN2A | α2 subunit of NaV1.2 | SMEI Ohtahara syndrome Benign familial neonatal infantile seizures (BFNIS) West syndrome Infantile spasms GEFS+ |
|
| SCN3A | α3 of NaV1.3 | Partial epilepsy | |
| SCN8A | α8 subunit of NaV1.6 | Infantile epileptic encephalopathy | |
| Potassium | KCNQ2 | KV7.2 | Benign familial neonatal convulsions Infantile encephalopathy Myokymia associated with neonatal or early infantile epilepsy |
| KCNQ3 | KV7.3 | Benign familial neonatal convulsions | |
| KCNMA1 | Calcium-activated potassium BK (Big Potassium) channel | Generalised epilepsy with paroxysmal movement disorder | |
| KCNA1 | KV1.1 | Epilepsy with episodic ataxia | |
| KCNA2 | KV1.2 | Myoclonic epilepsy and ataxia | |
| KCNJII | Kir6.2 | Developmental delay, epilepsy and neonatal diabetes mellitus (DEND syndrome) | |
| KCNT1 | Sodium-activated potassium channel | MPSI | |
| Calcium | CACNA1H | α subunit of t-type calcium channels | Childhood absence epilepsy |
| CACNA1A | CaV2.1 channel α subunit | Episodic ataxia and childhood absence epilepsy | |
| Acetylcholine receptor (AChR) | CHRNA4, CHRNB2 CHRNA2 | Subunits of nicotinic AChr receptor | Autosomal dominant familial nocturnal frontal lobe epilepsy |
| GABA | GABRA1 | α subunit of GABA receptor | Childhood absence epilepsy Idiopathic generalised epilepsy (IGE) Juvenile myoclonic epilepsy (JME) Infantile spasms, Lennox-Gastaut |
| GABRB2 | B2 subunit of the GABA receptor | Infantile spasms, Lennox-Gastaut | |
| GABRB3 | β3 subunit of GABA receptor | Absence epilepsy Infantile spasms, Lennox-Gastaut |
|
| GABRD | δ subunit of GABA receptor | GEFS+ JME |
|
| GABRG2 | γ2 subunit of GABA receptor | GEFS+ SMEI Childhood absence epilepsy IGE |
Channelopathies associated with epileptic encephalopathies
Early onset epileptic encephalopathies are generally severe epilepsy syndromes that often have a poor neurodevelopmental outcome.
Severe myoclonic epilepsy of infancy, also known as Dravet syndrome, manifests as intractable seizures that begin in the first year of life associated with developmental regression and cognitive impairment.5 6 Missense or nonsense mutations in the SCNA1 gene which encodes the pore-forming unit of the fast sodium channel NaV1.1 are present in over 80% of cases and are typically de novo, leading to haploinsufficiency.7 8 More rarely, mutations in other genes including SCN1B and SCN2A have been found, as well as mutations in the GABAA receptor subunit gene GABRG2.9–11 For some time, it was not understood how a mutation in a sodium channel leading to haploinsufficiency and reduced function could cause hyperexcitability. However, it was subsequently found that NaV1.1 channels have an important role in GABAergic inhibitory neurons, thus loss of function of these channels leads to hypoexcitability of inhibitory networks and consequently hyperexcitability of neuronal networks and in turn, epilepsy.12
SCN2A mutations have also been associated with other infantile encephalopathies including infantile spasms, acute encephalitis with refractory repetitive partial seizures, Ohtahara syndrome and recurrent encephalopathy.13 14 Intractable childhood epilepsy with generalised tonic-clinic seizures is a similar disorder to Dravet syndrome and is also associated with mutations in SCN1A.15 Recently, mutations of GABRA1, GABRB2 and GABRB3 were associated with infantile spasms and Lennox-Gastaut syndrome.16 17
Migrating partial seizures of infancy is a rare infantile encephalopathy that presents with focal seizures in the first 6 months of life, associated with acquired microcephaly and developmental stagnation or delay. This condition is genetically heterogeneous, with mutations in the KCNT1 gene that codes for a sodium-activated potassium channel and mutations in SCN1A both described.18 19
Mutations in SCN8A as well as KNCQ2 have been associated with severe epileptic encephalopathies.20–22 Some cases of DEND syndrome, (developmental delay, epilepsy and neonatal diabetes) are caused by mutations in KCNJ11 which encodes the Kir 6.2 subunit of the ATP-sensitive potassium channel.23 24
Generalised epilepsy syndromes
Generalised epilepsy with febrile seizures plus is a genetically and clinically heterogeneous familial epilepsy syndrome.25 Individuals develop febrile seizures early in life that persist beyond the age of 6 years. Numerous different genes have been implicated; namely the sodium channel genes SCN1A, SCN1B, SCN2A and the GABAA receptor subunit genes GABRG2 and GABRD.26–30
Benign familial neonatal infantile seizures is an epilepsy syndrome characterised by sudden onset and subsequent remission of seizures in infancy.31 32 It is caused by missense mutations in the SCN2A gene.8 Benign familial neonatalconvulsions (BFNC) is a similar syndrome characterised by brief seizures, occurring on the second or third day after birth that usually terminate within 6 weeks with normal neurological development.33 34 It can be caused by loss of function mutations in two potassium channel genes, KCNQ2 and KCNQ3, which code for the potassium channel subunits KV7.2 and 7.3, respectively.35–38 Proteins encoded by these genes co-assemble to form a slowly activating and deactivating potassium channel that plays a critical role in regulating the excitability of neurons.39
A syndrome of generalised epilepsy with paroxysmal movement disorders has been shown to be caused in one kindred by a dominant missense mutation in the calcium-activated potassium channel gene KCNMA1.40
Absence epilepsy has been reported in association with mutations in a number of different genes that code for ion channels. Variants in CACNA1H which codes for the α1H pore-forming subunit of T-type calcium channels have been reported, in a subset of patients with childhood absence epilepsy.41 However, mutations have not been found to fully segregate with disease, and the significance of these variants remains unclear.42 Missense mutations of GABRA1, GABRA6, GABARB3 and GABARG2 which encode various GABAA receptor subunits have also been implicated in childhood absence epilepsy.43–45 Missense mutations of GABRA1 and GABRD have been described in familial juvenile myoclonic epilepsy.30 46 Likewise, mutations in GABRA1 and GABRG2 have been associated with idiopathic generalised epilepsy (IGE).47
Recently exome sequencing revealed a mutation in KCNA2 which encodes the potassium voltage-gated channel subfamily A member 2 in a young boy who presented in infancy with ataxia and myoclonic epilepsy.48
Focal epilepsy syndromes
Autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) is a rare syndrome characterised by frequent short-lived motor seizures that typically occur during sleep or on waking.33 49 50 Mutations in three genes encoding subunits of the nicotinic acetylcholine receptor (AChR), CHRNA4, CHRNB2 CHRNA2, have been described in ADNFLE.49–56 Most mutations of the AChR channel gene are located in the pore-forming domain and are associated with a gain of function effect.57 58
A missense mutation in SCN3A, which encodes the α subunit of NaV1.3, has been described in one patient with complex partial seizures. Functional analysis showed that the mutated channel results in prolonged action potentials in neurons expressing Nav1.3.59
Epileptic channelopathies: remaining questions
Epilepsy is a very common condition but monogenic channelopathies only account for a small fraction of the epilepsy seen in clinical practice. Although most epilepsies are not inherited in a Mendelian fashion, it is estimated that about 70% of an individual's risk of developing a disorder such as epilepsy is accounted for by genetic risk factors.60
Large-scale exome screening for ion channel variants in epilepsy has led to the identification of mutations/targets in genes that were previously unexpected to have a role in epilepsy. Single nucleotide polymorphisms in the chloride channel genes CLCN1 and CLCN2 were found in three times as many patients with epilepsy compared with controls.61 CLCN1 was previously thought of as the ‘skeletal muscle chloride channel’ and was not thought to be expressed in the brain. However, molecular localisation revealed widespread presence of the ClC-1 subunit protein in the mouse and human brain, indicating that it may contribute to the regulation of brain excitability and hence may be implicated in epilepsy syndromes.61 Further large-scale genetic studies are likely to lead to the identification of other candidate genes.
However, to date, exome sequencing and large-scale genotyping studies of IGE have been disappointing.62 A possible explanation for the genetics of sporadic epilepsy is that many cases arise from polygenic inheritance, where several variants interact to lower the seizure threshold. Modelling the possible effect of combinations of ion channel mutations in preclinical systems to demonstrate possible pathogenicity is complex and is a major research challenge.
Treatment of epileptic channelopathies
A complete analysis of treatment in all the various epilepsy syndromes caused by channelopathies is beyond the scope of this review. It is clear however that increased understanding of channel dysfunction in various epilepsy syndromes can lead to an individualised approach to treatment. For example, functional work on mutations in KCNQ2 have shown that the functional changes (decreased voltage sensitivity) can be restored by retigabine, a neuronal KV7 activator.63 It has also been recognised for some time that medications that block sodium channel function can worsen seizures in SMEI.8 With further research, there is potential for precision medicine in which drugs target specific channels or even target the mechanism by which a channel becomes dysfunctional.
Cerebellar dysfunction and ataxia
Mutations in ion channels can be associated with both episodic and progressive ataxia syndromes—namely the episodic ataxia syndromes and the spinocerebellar ataxias (SCAs; see online supplemental table S2).
Episodic ataxias
There are two main forms of episodic ataxia—EA1 and EA2. Both are dominantly inherited. Other rarer forms have been reported in individual families.
EA1 is characterised by brief episodes of ataxia that last seconds to minutes. The attacks begin in early childhood and can be provoked by startle, vigorous activity, illness, hunger and emotion.64 65 Cerebellar function is normal in between attacks, but there may be persistent neuromyotonia of skeletal muscles which can be confirmed on electromyography (EMG).65 66 There is an increased incidence of epilepsy associated with EA164 67 68 and there also have been reports of an increased risk of hearing impairment.64 Recently, it has been found that up to 20% of patients accumulate a persistent cerebellar syndrome.65 EA1 is caused by heterozygous, usually missense mutations in the neuronal voltage-gated delayed rectifier potassium channel (KV1.1) gene KCNA1.64 69 KV1.1 channels are fast potassium channels widely expressed in the central nervous system and in peripheral nerve where they regulate axonal excitability.70 Different EA1-associated mutations of KCNA1 affect channel function via diverse effects.68 71 Non-invasive excitability studies on motor nerves in patients with EA1 can detect changes specific to loss of fast potassium channel function in vivo with high sensitivity and specificity.72 Recently, a novel phenotype characterised by long-lasting attacks of jerking muscle contractions associated with hyperthermia, migraine and a short sleep phenotype was described in a patient with a single nucleotide change in KCNA1.73
EA2 also presents with episodes of ataxia but these attacks typically last longer than in EA1, lasting hours to days.74 Approximately 30–50% of patients develop a mild progressive cerebellar ataxia and more than half report migrainous symptoms.74 75 One kindred with episodic ataxia has been shown to also have absence epilepsy and dystonia has also been reported.76 77 EA2 is caused by non-sense, frame shift, splice site and missense mutations in the CACNA1A gene, which encodes the pore-forming α1A subunit of CaV2.1—the P/Q-type calcium channel.78 P/Q-type calcium channels are widely expressed at synapses throughout the central and peripheral nervous systems, and have an important role in triggering neurotransmitter release.79 Functional analysis has revealed different effects of EA2 mutations including altered channel function with reduced calcium current as well as effects on protein folding and trafficking.80–82
Treatment of episodic ataxia
Acetazolamide is often effective in EA2 and can be tried in EA1, although in our experience it is less effective in EA1.83 4- Aminopyridine, a potassium channel blocker, has also been reported in a double-blind randomised trial to have a prophylactic effect on ataxia in EA2.84 The mechanism of action is incompletely understood, but in animal studies, it was shown to restore the diminished precision of pacemaking in Purkinje cells of EA2 mutant mice by prolonging the duration of the action potential.85
Spinocerebellar ataxias
At least three different ion channel genes have been implicated in various forms of SCA including the calcium channel gene CACNA1A and two potassium channel genes, KCNC3 and KCND3. In contrast to many of the other inherited channelopathies, the symptoms of cerebellar dysfunction in SCAs seem to be predominantly progressive, rather than episodic.
SCA6 is allelic with EA2 and FHM type 1 and is caused by expansions of the CAG repeat sequence in the 3′ end of CACNA1A.86 This is a late onset progressive cerebellar syndrome. Extracerebellar features are less prominent than in other forms of SCA.87 The pathogenic mechanism of the polyglutamine repeat expansion in SCA6 is poorly understood. The basic function of the P/Q channels are not affected in SCA6 knock in mice, suggesting that the pathogenesis is related to an accumulation of mutant CaV2.1 channels.88
Some patients with EA2 have also been found to have small CAG expansions in CACNA1A, thus leading to suggestions that SCA6 and EA2 are a clinical continuum.89 Recently, mutations in CACNA1A have been reported in three patients with paroxysmal tonic upgaze in association with motor and language delay and cerebellar ataxia, thus widening the phenotype.90
Two voltage-gated potassium channel genes have been implicated in other forms of SCA. Missense mutations in KCNC3, which encodes KV3.3 have been found in patients with the phenotype of SCA13, which may present as a neurodevelopmental disorder in infancy or an adult onset progressive cerebellar syndrome depending on the causative mutation.91 92 KV3.3 channels are expressed in the cerebellum and have an important role in fast repolarisation of neurons during high frequency repetitive firing.93
Mutations in the gene that codes for KV4.3, KCND3, have been found in patients diagnosed with SCA19 and SCA22. Most of the patients studied developed cerebellar symptoms around middle age with a variable proportion developing extracerebellar features such as cognitive impairment. Initial functional studies suggest that mutations alter trafficking of channels to the cell membrane and also reduced channel function.94 95
No specific treatments have been demonstrated to be effective in patients with progressive SCA.
Migraine
Familial Hemiplegic Migraine (FHM) is a subtype of severe migraine inherited in an autosomal dominant fashion. Patients have severe auras that include unilateral weakness, as well as visual, somatosensory or dysphasic symptoms, typically followed or accompanied by migrainous headache.96 97 FHM is genetically heterogeneous and is classified into three types98 (see online supplemental table S3).
FHM1 accounts for 75% of genetically confirmed cases and is caused by missense mutations in CACNA1A, the same gene that is implicated in EA2 and SCA6.99 Functional expression studies have shown that FHM mutations result in various gain of function effects, including increased CaV2.1 current density in cerebellar neurons and enhanced neurotransmitter release.96 FHM2 is caused by loss of function mutations in the ATP1A2 gene. This gene encodes the α2 subunit of Na+/K+ pumps, which contribute to maintaining transmembrane ion gradients.100 FHM3 is associated with heterozygous mutations in the sodium channel gene SCN1A.101 This is the same gene that is associated with seizure disorders. Why some mutations manifest as migraine while others as epilepsy is not understood.
Knowledge of the molecular mechanisms of the different forms of FHM have led to the suggestion that they can be treated with acetazolamide or other agents that target ion channels such as verapamil and flunarizine, which act on some calcium channels, and lamotrigine, which acts on both sodium and calcium channels.102 103 However, randomised evidence for the efficacy of any particular treatment is lacking.98
Familial hyperekplexia
Familial hyperekplexia—also known as hereditary startle disease is characterised by neonatal hypertonia, hyper-reflexia, myoclonic jerks and an exaggerated startle response to sensory stimuli. The hypertonicity and hyper-reflexia typically improve during infancy but the exaggerated startle response continues into adulthood.104
Mutations in GLRA1 account for 80% of hereditary hyperekplexia and are most commonly inherited in an autosomal dominant fashion, although recessive and compound heterozygous cases also occur.104 Missense, nonsense, frameshift and splice site mutations, and large deletions have all been described.50 105 GLRA1 encodes the α subunit of the postsynaptic glycine receptor chloride channel which mediates fast inhibition in the brainstem and spinal cord.104 Mutations impair glycine receptor function, resulting in increased excitability in pontomedullary reticular neurons and abnormal spinal reciprocal inhibition.104 106 Hyperekplexia can also be caused by mutations in the GLRB gene, which encodes the β subunit of the glycine receptor, and in SLC6A5, which encodes the presynaptic glycine transporter type 2.107–110 Clonazepam is the drug of choice as it enhances GABAA receptor-mediated inhibition and was shown in a randomised trial to significantly reduce startle activity.104 111
Inherited channelopathies of peripheral nerves
Ion channel disorders have implicated in various inherited diseases of peripheral nerve including pain syndromes and neuropathies (see online supplemental table S4).
Pain syndromes
Gene mutations in ion channels have been associated with increased pain perception whereas other mutations cause insensitivity to pain. Both ligand-gated and voltage-gated ion channels have a pivotal role in the detection and transmission of stimuli from nociceptors.
Point mutations in the sodium channel gene SCN9A which codes for the α subunit of NaV1.7 channels have been associated with two different pain syndromes associated with gain of function—primary erythromelalgia and paroxysmal extreme pain disorder.112 Nonsense mutations in the same gene have been associated with a syndrome causing congenital insensitivity to pain. NaV1.7 channels are expressed in dorsal root ganglion (DRG) neurons where they regulate excitability of pain fibres. A different phenotype resulting from nonsense mutations in SCN9A was described in two Japanese kindreds—hereditary sensory and autonomic neuropathy type IID.113 These patients had adolescent or congenital onset of loss of pain and temperature sensation and autonomic dysfunction with evidence of reduction in sensory nerve action potentials on nerve conduction studies.
Primary erythromelalgia
PE is a rare syndrome characterised by intense burning pain, usually of the extremities, with marked erythema and increased skin temperature.114 Symptoms usually begin in the first two decades. Precipitating factors for pain include heat, exercise, tight clothing and certain foods. The pain is initially episodic but sometimes can become constant with fluctuations.115 Mutations in the gene SCN9A which encodes the NaV1.7 sodium channel are typically inherited in an autosomal dominant fashion and lower the voltage threshold for a sodium current in dorsal root ganglia neurons, increasing their firing frequency in response to stimulation, slowing their activation and increasing their response to slow ramp-like stimuli.116–119
Paroxsymal extreme pain disorder
Paroxsymal extreme pain disorder (PEPD) is a distinct syndrome, previously known as familial rectal pain syndrome.120 The characteristic feature is severe frequently visceral pain that affects various parts of the body including the rectum and genitalia, although the face and limbs can also be involved.121 The pain can be associated with autonomic features including flushing, lacrimation, rhinorrhea and tonic attacks with apnoea and bradycardia.122 123 Physical factors such as defecation and eating can trigger attacks, as can emotion.115
In contrast to gain of function mutations in PE, functional studies have shown that SCN9A mutations in PEPD impair the fast inactivation of sodium channels leading to a persistent sodium current.121
Treatment of painful channelopathies
Treatment of the painful channelopathies can be difficult, as patients do not respond to standard analgesics. Oral mexiletine and topical lidocaine, both sodium channel blockers can be effective in PE.124 Mexiletine is a non-selective sodium channel blocker and has been shown to have a normalising effect on the hyperpolarised channels seen in gain of function NaV1.7 mutations.125 Patients often also use physical measures such as immersing feet in cold water. Patients with PEPD may obtain relief from carbamazepine which can help block the abnormal persistent sodium currents due to impaired inactivation of NaV1.7 seen in this disorder.121
Congenital insensitivity to pain
In contrast to the above disorders which are autosomal dominant, recessive, loss of function mutations of SCN9A result in congenital insensitivity to pain.126 Patients develop repeated painless fractures and injuries, which although painless can be crippling.126 127 A mutation in the gene SCN11A which encodes NaV1.9, a voltage-gated sodium channel primarily expressed in nociceptors, has also been found in patients with congenital insensitivity to pain; however in contrast to loss of function SCN9A mutations in this condition, SCN11A mutations are associated with a gain of function with sustained depolarisation of nociceptors impeding the generation of action potentials.128
Small fibre neuropathy
The above disorders are rare diseases but recently gain of function missense variants in SCN9A that encodes the NaV1.7 channel have been found in approximately 30% of a cohort of patients with idiopathic small fibre neuropathy.129 Mutations in the SCN10A and SCN11A genes which encode NaV1.8 and NaV1.9, respectively, have also been described in a small number of patients with painful peripheral neuropathy, suggesting that inherited channelopathies may play a role in commonly encountered clinical syndromes.130 131
Familial episodic pain syndrome
A different channel type is affected in familial episodic pain syndrome (FEPS), a rare dominantly inherited disorder132 characterised by episodes of severe pain, triggered by cold and hunger, localised principally to the upper body. It is caused by a gain of function missense mutation in the TRPA1 gene, which encodes TRPV1. TRPA1 is part of a family of transient receptor potential (TRP) channels, a large superfamily of cation channels. TRPA1 is expressed in primary afferent nociceptors and plays an important role in response to environmental irritants.133 A distinct type of FEPS has been described in two large Chinese kindreds who were found to have mutations in SCN11A.134 Mutations in FEPS cause a gain of function with hyperexcitability of the cells of the DRG.115
Motor and sensory neuropathies
Several inherited neuropathies that present mainly with motor dysfunction are known to be due to ion channel dysfunction.
Three allelic disorders, Charcot-Marie-Tooth disease type IIC (HSMNIIC), scapuloperoneal spinal muscular atrophy (SPSMA) and congenital distal spinal muscular atrophy (SMA) are caused by mutations in another class of TRP channel—the TRPV4 channels.135 136 HSMNIIC is an autosomal dominant axonal neuropathy characterised by progressive distal limb weakness and weakness of the diaphragm laryngeal muscles and vocal cords.137 SPSMA manifests as progressive weakness of scapular and peroneal muscles, laryngeal palsy and skeletal abnormalities.138 Congenital distal SMA affects lower motor neurons with variable disease severity ranging from congenital weakness restricted to the distal lower limbs to more severe forms with involvement of pelvic girdle and trunk muscles and arthrogryposis.135 TRPV4 encodes a channel that is broadly permeable to cations including calcium, and can be activated by mechanical stimuli, heat and endogenous and synthetic agonists. TRPV4 is widely expressed in the brain and spinal cord.136 There is a lack of consensus regarding the disease mechanism of TRPV mutations causing neurological disease with both gain of function and loss of function effects reported.137 139 140
Peripheral nerve hyperexcitability
Peripheral nerve hyperexcitability comprises a heterogeneous group of diseases characterised by spontaneous and continuous muscle activity (myokymia), muscle cramps, stiffness and fasciculations.141 It is commonly seen as part of the EA1 phenotype resulting from mutations in KV1.1 potassium channels.69 142 Mutations in the KV7.2 potassium channel gene (KCNQ2) associated with BFNC have also been found to cause a genetic form of peripheral nerve hyperexcitabililty.141 143
Congenital myasthenic syndromes
Congenital myasthenic syndromes (CMS) are a heterogeneous group of genetic disorders that affect the neuromuscular junction. They are typically inherited in a recessive fashion and can be caused by mutations in proteins of the neuromuscular junction that are presynaptic, synaptic or postsynaptic. Mutations in over 15 different genes have been identified to date.144 Of relevance to inherited channelopathies, the most common type of CMS is caused by mutations in various genes encoding the subunits of muscle AChRs. Mutations in any one of the adult subunits of the AChR channel can result in deficiency or kinetic abnormality of the AChR145 (see online supplemental table S5).
Recessive mutations of CHRNA1, CHRNB1, CHRND and CHRNE, which code for α1, β1, δ and ε subunits, respectively, have all been implicated in primary AChR deficiency syndromes. Mutations in the ε (epsilon) subunit are most frequently encountered.144 Most patients with AChR deficiency syndromes present with feeding problems, ptosis and ophthalmoplegia in early infancy. Patients tend to respond well to pyridostigmine and/or 3, 4-diaminopyridine (DAP), a potassium channel blocker that prolongs the presynaptic action potential, thereby enhancing ACh release.144 145
Other mutations can affect the kinetics of AChRs. Slow channel syndrome is the only dominantly inherited CMS, and can be caused by mutations in any of the AChR subunit genes. The underlying pathology is a gain of function with sustained activation of the AChR with either delayed channel closure or enhanced ACh affinity. This prolonged channel opening in turn can result in an end-plate myopathy.146 Symptoms usually present in childhood with delayed motor milestones and ocular signs such as ptosis and ophthalmoplegia. It is important to recognise this syndrome as conventional treatment with pyridostigmine or 3, 4-DAP can worsen symptoms. Treatment is with fluoxetine or quinidine, both act as open channel blockers.147
Fast channel syndrome is a rare and severe form of CMS, in which AChRs open for a shorter time than normal. It is associated with loss of function mutations of AChR α1, δ and ε subunits. Children are typically affected from birth with respiratory failure, feeding difficulties with ptosis and ophthalmoplegia. Pyridostigmine and 3, 4-DAP may be beneficial.144 146
Skeletal muscle channelopathies
The skeletal muscle channelopathies are a heterogeneous group of disorders whose clinical manifestations range from flaccid paralysis to myotonia. They are divided into the non-dystrophic myotonias (NDMs) and the periodic paralyses and are caused by mutations in skeletal muscle ion channels that affect muscle excitability (see online supplemental table S6).
Non-dystrophic myotonias
The NDMs are a group of skeletal muscle channelopathies that present with myotonia (delayed muscle relaxation following voluntary contraction) without systemic features. This group of conditions includes myotonia congenita (MC), paramyotonia congenita (PMC) and the sodium channel myotonias (SCMs).
Myotonia congenita
MC is the commonest of the skeletal muscle channelopathies148 and can be inherited in an autosomal dominant (Thomsen disease) or recessive fashion (Becker disease). It is characterised by muscle stiffness that predominantly affects the limbs. Symptoms may be worsened by rest, infection or stress, and can be accompanied by muscle hypertrophy. Patients often exhibit a warm-up phenomenon when muscle stiffness improves with repeated activity. Patients with recessive MC may also have transient weakness on the initiation of a movement.
MC is caused by mutations in the skeletal muscle chloride channel CLCN1, which encodes the channel ClC-1.149 ClC-1 underlies the majority of the resting conductance of skeletal muscle. Functional expression studies show that pathogenic mutations can reduce the macroscopic chloride current, predisposing to muscle fibre depolarisation and after-discharges.150 Typically, nonsense, missense and frame shift mutations that do not affect the functional properties for the wild-type subunits in the channel dimer are recessively inherited. Missense mutations that shift the voltage dependence of activation out of the physiological range are often dominantly inherited.151–154 Recessive mutations generally result in more severe symptoms. Recently, it was found that up to 6% of patients with a recessive family history but only one mutation in CLCN1 carry whole exons or duplications in the CLCN1 gene, thus revealing a novel genetic cause for recessive MC.155
PMC and SCMs
Two other groups of disorders characterised clinically by myotonia are associated with sodium channel mutations—PMC and the SCMs.
PMC presents as muscle stiffness early in life. However, in contrast with MC, symptoms are worsened by exertion (paramyotonia) and cold.156 PMC is also associated with episodes of weakness, which can last for hours or days. In contrast, SCMs are a subgroup of myotonic disorders that are characterised clinically by pure myotonia without weakness. The severity of SCMs is highly variable varying from a severe form with onset in infancy to mild forms that only cause isolated eyelid myotonia.157 The infantile forms can be associated with potentially fatal laryngospasm highlighting the importance of genetic counselling in affected adult patients with these disorders.156 157 The presence of eyelid closure myotonia is specific for mutations in SCN4A and can help to clinically differentiate this disorder from MC.158
Both PMC and the SCMs are caused by dominantly inherited mutations in the SCN4A, which encodes the skeletal muscle voltage-gated sodium channel, NaV1.4. The same mutation has been shown to cause either condition in different pedigrees.159 SCN4A mutations cause a gain of function effect on the encoded α4 subunit of the muscle sodium channel NaV1.4. They disrupt fast inactivation or cause a hyperpolarising shift in the voltage dependence of activation.150 Recently, a small group of patients with myotonia with heterozygous SCN4A mutations and single CLCN1 mutations were described, widening the genetic spectrum.160
Treatment of myotonia has improved considerably in recent years. In vitro and animal studies have shown that the sodium channel blocker mexiletine reduces muscle fibre excitability caused by common NDM mutation.161 162 A recent double-blind, placebo-controlled crossover study of patients with NDM confirmed its efficacy.163 Acetazolamide, which has been shown to stabilise membrane excitability through a direct effect on the chloride channel, has also been used and is particularly helpful if there are concerns regarding the proarrhythmogenic side effects of mexiletine.164 165 Experimental studies have suggested that lacosamide and ranolazine, drugs that are used for epilepsy and angina respectively, enhance slow in activation of sodium channels and may be an alternative to mexiletine in patients with MC.166 Other options include carbamazepine and phenytoin, although good-quality evidence is lacking.
Periodic paralyses
The inherited periodic paralyses are a group of disorders comprised of three conditions; hypokalaemic periodic paralysis (Hypo PP), hyperkalaemic periodic paralysis (Hyper PP) and Andersen-Tawil syndrome (ATS).
Hypokalemic periodic paralysis
Hypo PP is the most common form of periodic paralysis and is characterised by episodes of flaccid muscle weakness that occur in association with a low serum potassium level. Attacks last hours to days and typically affect the limbs; respiratory involvement is rare. Precipitants include carbohydrate meals and rest after exercise.156 With time, the frequency of attacks may diminish and a fixed proximal weakness may develop.167 Hypo PP is inherited in an autosomal dominant fashion but has a reduced penetrance in women, a feature seen in several muscle channelopathies. Causal mutations were first identified in CACNA1S, which encodes the α1S subunit of the skeletal muscle calcium channel CaV1.1.168 169 These account for approximately 80% of cases. Mutations in the sodium channel gene SCN4A, also associated with the SCMs, account for approximately 10% of cases but up to 10–20% of cases remain genetically undefined.170
The overwhelming majority of mutations in Hypo PP, whether in calcium or in sodium channels, occur in the voltage-sensing region of the channel.171 How these lead to attacks of paralysis has been a puzzle for many years. Recently, it has emerged that the mutations open an abnormal cation leak pathway through the voltage sensor itself, separate from the main pore of the channel—the gating pore current.172 173 The association of attacks with hypokalaemia is thought to reflect the tendency for the inwardly rectifying potassium channel Kir2.1 to fail to conduct when the extracellular potassium concentration is low.173–175
Recently, bumetanide, an inhibitor of the Na-K-2Cl co-transporter was shown to prevent this paradoxical depolarisation in hypokalaemic conditions in animal studies and was also shown to prevent attacks in mouse models of sodium and calcium channel mutations.173 176 Clinical trials of bumetanide for Hypo PP are starting.
Hyperkalaemic periodic paralysis
Hyper PP is characterised by episodes of muscle weakness in association with elevated serum potassium. In addition to paralysis, myotonia may also be a feature.156 170 The attacks of paralysis are typically shorter than Hypo PP lasting minutes to hours but can become prolonged with age, lasting up to 2 days.156 170 177 Hyper PP is caused by mutations in the sodium channel gene SCN4A. The mutations in Hyper PP tend to impair inactivation of the NaV1.4 sodium channel, leading to persistent sodium influx, depolarisation and inexcitability.178 179 Some Hyper PP mutations have also been shown to shift the voltage dependence of activation in the negative direction, allowing channels to open sooner.180 The association with hyperkalaemia probably reflects in part a positive feedback loop, whereby depolarisation leads to potassium efflux, which results in a further depolarisation.181
Andersen-Tawil syndrome
ATS is a rare disorder characterised by a triad of periodic paralysis, cardiac defects and skeletal abnormalities, although not every patient will have all three features.182 The periodic paralysis is typically associated with low levels of potassium but can be associated with normokalaemia or hyperkalaemia. Cardiac abnormalities seen include enlarged U waves, a prolonged QUC interval and ventricular arrythymias.183 Cardiac arrest occurs in approximately 10% of patients with ATS and cardiac screening is mandatory.184 185 Distinctive facial features seen in ATS include micrognathia, low set ears, hypertelorism, clindactyly and syndactyly.185 ATS is caused by mutations in the coding exon 2 of the KCNJ2 gene which encodes the inward rectifying potassium channel Kir2.1.186 These channels contribute to the resting membrane potential in the heart, brain and skeletal muscle.
No current through Kir2.1 channels is seen when mutant KCNJ2 channels are expressed in vitro. Co-expression of wild-type channels with mutant channels results in reduction in inward rectifying currents indicating a dominant negative effect of the mutation.186 187 Up to 10% or 20% of patients will not have a mutations in KCNJ2.184 Recently, mutations in KCNJ5, the gene encoding Kir 3.4 was found to cause ATS in a patient with typical muscle and cardiac features but without dysmorphism.188
Treatment of the periodic paralyses
Management of periodic paralysis rests on trigger avoidance. Oral potassium can speed attack resolution in Hypo PP whereas ingestion of sweets and mild exercise can hasten attack resolution in Hyper PP. Inhaled salbutamol has also been shown to be effective in treating attacks of Hyper PP.189 Occasionally, prophylactic treatment is required. Acetazolamide is often a first-line treatment for both Hyper PP and Hypo PP. It has been shown to increase muscle strength and endurance in a small randomised controlled trial.190 Dichlorphenamide was shown to reduce attack frequency in a double-blind, placebo-controlled trial in Hyper PP and Hypo PP.191 An additional option in Hypo PP includes potassium-sparing diuretics such as spironolactone or amiloride.156 Pinacidil, a potassium channel agonist, was found to improve muscle strength in a small randomised controlled trial.192
Electrophysiology in skeletal muscle channelopathies
The functional consequences of ion channel mutations in skeletal muscle can be examined by electrophysiology. Myotonia on needle EMG is seen in all forms of NDM but severity can vary and the duration of myotonic discharges on EMG can be used to distinguish sodium and chloride channel myotonias.193 194 Measurement of compound action potential amplitudes before and after exercise, the short and long exercise test, helps to distinguish between the different skeletal muscle channelopathies.
MRI in skeletal muscle channelopathies
MRI has recently been developed for diagnosis and monitoring use in skeletal muscle channelopathies. A hyperintense central stripe in the medial gastrocnemius muscle appears to be specific for NDM, particularly MC.195 Fatty infiltration of muscles can also be seen on MRI which is consistent with the clinical observation of fixed weakness in some patients over time.177 Patients with Hypo PP who have permanent weakness are also found to have fatty muscle replacement on MRI. An increase in 23Na+ MRI signal intensity can seen in patients with Hypo PP suggesting muscle oedema which can be reduced by acetazolamide treatment, indicating that muscle imaging is likely to play an increasing role in therapy monitoring in the future.175
Thyrotoxic periodic paralysis
Thyrotoxic periodic paralysis is a rare condition causing attacks indistinguishable from Hypo PP but in the presence of thyrotoxicosis. The disorder is most common in young Asian and Latin American men in whom 10% of thyrotoxic males develop episodic weakness.190 Candidate gene sequencing has revealed mutations in KCNJ18 which encodes Kir 2.6, an inwardly rectifying potassium channel that is expressed in skeletal muscle and transcriptionally regulated by thyroid hormones in one-third of patients.196
Conclusion
Although individually rare, the inherited channelopathies can be accurately diagnosed by careful clinical assessment and DNA-based diagnosis. An accurate diagnosis is important for genetic counselling and to direct treatment options. Recent molecular genetic advances have provided insights into pathophysiological mechanisms that are potentially relevant to more common paroxysmal disorders such as epilepsy and migraine. Ion channels are an attractive target for investigation of these common diseases with polygenic inheritance. However, to date, genetic association studies have not revealed clear mechanistic understanding, possibly because of the complexity of elucidating the effect of multiple genetic channel variation interactions. The increased use of whole genome sequencing is generating very large amounts of genetic data including variations in ion channel genes. However, extensive biophysical characterisation in representative model systems will be required to determine the contribution of different variants to common paroxysmal neurological diseases.
Supplementary Material
Acknowledgments
JS was the John Newsom-Davis Research fellow funded by the Myasthenia Gravis Association. The authors' research is supported by an MRC Centre grant award and a Wellcome Trust Strategic award. The authors’ research is also supported by the NIHR Biomedical Research Centre at UCLH NHS Foundation Trust. More information about channelopathies genetic diagnosis is available from MGH m.hanna@ucl.ac.uk.
Footnotes
Contributors: JS was involved in concept and design of paper, literature review, drafting and revision of manuscript. DMK was involved in revision of manuscript. MGH was involved in concept and design of paper and revision of manuscript.
Funding: Wellcome Trust; MRC Centre; Myasthenia Gravis Association; National Institute for Health Research.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Felix R. Channelopathies: ion channel defects linked to heritable clinical disorders. J Med Genet 2000;37:729–40. 10.1136/jmg.37.10.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graves TD, Hanna MG. Neurological channelopathies. Postgrad Med J 2005;81:20–32. 10.1136/pgmj.2004.022012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hübner CA, Jentsch TJ. Ion channel diseases. Hum Mol Genet 2002;11:2435–45. 10.1093/hmg/11.20.2435 [DOI] [PubMed] [Google Scholar]
- 4.Ryan DP, Ptácek LJ. Episodic neurological channelopathies. Neuron 2010;68:282–92. 10.1016/j.neuron.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 5.Claes L, Del-Favero J, Ceulemans B, et al. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet 2001;68:1327–32. 10.1086/320609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 2010;51:1650–8. 10.1111/j.1528-1167.2010.02640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogiwara I, Miyamoto H, Morita N, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 2007;27:5903–14. 10.1523/JNEUROSCI.5270-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenhouse SA, Ellis R, Zuberi S. SCN1A genetic test for Dravet syndrome (severe myoclonic epilepsy of infancy and its clinical subtypes) for use in the diagnosis, prognosis, treatment and management of Dravet syndrome. PLoS Curr 2013;5 pii: ecurrents.eogt.c553b83d745dd79bfb61eaf35e522b0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patino GA, Claes LR, Lopez-Santiago LF, et al. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci 2009;29:10764–78. 10.1523/JNEUROSCI.2475-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi X, Yasumoto S, Kurahashi H, et al. Clinical spectrum of SCN2A mutations. Brain Dev 2012;34:541–5. 10.1016/j.braindev.2011.09.016 [DOI] [PubMed] [Google Scholar]
- 11.Ishii A, Kanaumi T, Sohda M, et al. Association of nonsense mutation in GABRG2 with abnormal trafficking of GABAA receptors in severe epilepsy. Epilepsy Res 2014;108:420–32. 10.1016/j.eplepsyres.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 12.Yu FH, Mantegazza M, Westenbroek RE, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 2006;9:1142–9. 10.1038/nn1754 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Kato M, Osaka H, et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology 2013;81:992–8. 10.1212/WNL.0b013e3182a43e57 [DOI] [PubMed] [Google Scholar]
- 14.Fukasawa T, Kubota T, Negoro T, et al. A case of recurrent encephalopathy with SCN2A missense mutation. Brain Dev 2015;37:631–4. 10.1016/j.braindev.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara T, Sugawara T, Mazaki-Miyazaki E, et al. Mutations of sodium channel alpha subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain 2003;126(Pt 3):531–46. 10.1093/brain/awg053 [DOI] [PubMed] [Google Scholar]
- 16.Hirose S. Mutant GABA(A) receptor subunits in genetic (idiopathic) epilepsy. Prog Brain Res 2014;213:55–85. 10.1016/B978-0-444-63326-2.00003-X [DOI] [PubMed] [Google Scholar]
- 17.Allen AS, Berkovic SF, Cossette P, et al. , Epi4K Consortium; Epilepsy Phenome/Genome Project. De novo mutations in epileptic encephalopathies. Nature 2013;501:217–21. 10.1038/nature12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McTague A, Appleton R, Avula S, et al. Migrating partial seizures of infancy: expansion of the electroclinical, radiological and pathological disease spectrum. Brain 2013;136:1578–91. 10.1093/brain/awt073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcia G, Fleming MR, Deligniere A, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet 2012;44:1255–9. 10.1038/ng.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veeramah KR, O'Brien JE, Meisler MH, et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet 2012;90:502–10. 10.1016/j.ajhg.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol 2012;71:15–25. 10.1002/ana.22644 [DOI] [PubMed] [Google Scholar]
- 22.Milh M, Boutry-Kryza N, Sutera-Sardo J, et al. Similar early characteristics but variable neurological outcome of patients with a de novo mutation of KCNQ2. Orphanet J Rare Dis 2013;8:80 10.1186/1750-1172-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slingerland AS, Hattersley AT. Mutations in the Kir6.2 subunit of the K ATP channel and permanent neonatal diabetes: new insights and new treatment. Ann Med 2005;37:186–95. 10.1080/07853890510007287 [DOI] [PubMed] [Google Scholar]
- 24.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004;350:1838–49. 10.1056/NEJMoa032922 [DOI] [PubMed] [Google Scholar]
- 25.Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus. A genetic disorder with heterogeneous clinical phenotypes. Brain 1997;120(Pt 3):479–90. [DOI] [PubMed] [Google Scholar]
- 26.Sugawara T, Tsurubuchi Y, Agarwala KL, et al. A missense mutation of the Na+ channel alpha II subunit gene Na(v)1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci USA 2001;98:6384–9. 10.1073/pnas.111065098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace RH, Wang DW, Singh R, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel β1 subunit gene SCN1B. Nat Genet 1998;19:366–70. 10.1038/1252 [DOI] [PubMed] [Google Scholar]
- 28.Escayg A, Heils A, MacDonald BT, et al. A novel SCN1A mutation associated with generalized epilepsy with febrile seizures plus—and prevalence of variants in patients with epilepsy. Am J Hum Genet 2001;68:866–73. 10.1086/319524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baulac S, Huberfeld G, Gourfinkel-An I, et al. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 2001;28:46–8. 10.1038/88254 [DOI] [PubMed] [Google Scholar]
- 30.Dibbens LM, Feng HJ, Richards MC, et al. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet 2004;13:1315–19. 10.1093/hmg/ddh146 [DOI] [PubMed] [Google Scholar]
- 31.Herlenius E, Heron SE, Grinton BE, et al. SCN2A mutations and benign familial neonatal-infantile seizures: the phenotypic spectrum. Epilepsia 2007;48:1138–42. 10.1111/j.1528-1167.2007.01049.x [DOI] [PubMed] [Google Scholar]
- 32.Heron SE, Crossland KM, Andermann E, et al. Sodium-channel defects in benign familial neonatal-infantile seizures. Lancet 2002;360:851–2. 10.1016/S0140-6736(02)09968-3 [DOI] [PubMed] [Google Scholar]
- 33.Gourfinkel-An I, Baulac S, Nabbout R, et al. Monogenic idiopathic epilepsies. Lancet Neurol 2004;3:209–18. 10.1016/S1474-4422(04)00706-9 [DOI] [PubMed] [Google Scholar]
- 34.Ronen GM, Rosales TO, Connolly M, et al. Seizure characteristics in chromosome 20 benign familial neonatal convulsions. Neurology 1993;43:1355–60. 10.1212/WNL.43.7.1355 [DOI] [PubMed] [Google Scholar]
- 35.Singh NA, Charlier C, Stauffer D, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet 1998;18:25–9. 10.1038/ng0198-25 [DOI] [PubMed] [Google Scholar]
- 36.Charlier C, Singh NA, Ryan SG, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 1998;18:53–5. 10.1038/ng0198-53 [DOI] [PubMed] [Google Scholar]
- 37.Biervert C, Schroeder BC, Kubisch C, et al. A potassium channel mutation in neonatal human epilepsy. Science 1998;279:403–6. 10.1126/science.279.5349.403 [DOI] [PubMed] [Google Scholar]
- 38.Hirose S, Zenri F, Akiyoshi H, et al. A novel mutation of KCNQ3 (c.925T—>C) in a Japanese family with benign familial neonatal convulsions. Ann Neurol 2000;47:822–6. [DOI] [PubMed] [Google Scholar]
- 39.Wang HS, Pan Z, Shi W, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 1998;282:1890–3. 10.1126/science.282.5395.1890 [DOI] [PubMed] [Google Scholar]
- 40.Du W, Bautista JF, Yang H, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 2005;37:733–8. 10.1038/ng1585 [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Lu J, Pan H, et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol 2003;54:239–43. 10.1002/ana.10607 [DOI] [PubMed] [Google Scholar]
- 42.Heron SE, Phillips HA, Mulley JC, et al. Genetic variation of CACNA1H in idiopathic generalized epilepsy. Ann Neurol 2004;55:595–6. 10.1002/ana.20028 [DOI] [PubMed] [Google Scholar]
- 43.Maljevic S, Krampfl K, Cobilanschi J, et al. A mutation in the GABA(A) receptor alpha(1)-subunit is associated with absence epilepsy. Ann Neurol 2006;59:983–7. 10.1002/ana.20874 [DOI] [PubMed] [Google Scholar]
- 44.Tanaka M, Olsen RW, Medina MT, et al. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am J Hum Genet 2008;82:1249–61. 10.1016/j.ajhg.2008.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurba KN, Hernandez CC, Hu N, et al. GABRB3 mutation, G32R, associated with childhood absence epilepsy alters α1β3γ2L γ-aminobutyric acid type A (GABAA) receptor expression and channel gating. J Biol Chem 2012;287:12083–97. 10.1074/jbc.M111.332528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cossette P, Liu L, Brisebois K, et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet 2002;31:184–9. 10.1038/ng885 [DOI] [PubMed] [Google Scholar]
- 47.Lachance-Touchette P, Brown P, Meloche C, et al. Novel α1 and γ2 GABAA receptor subunit mutations in families with idiopathic generalized epilepsy. Eur J Neurosci 2011;34:237–49. 10.1111/j.1460-9568.2011.07767.x [DOI] [PubMed] [Google Scholar]
- 48.Pena SD, Coimbra RL. Ataxia and myoclonic epilepsy due to a heterozygous new mutation in KCNA2: proposal for a new channelopathy. Clin Genet 2015;87:e1–3. 10.1111/cge.12542 [DOI] [PubMed] [Google Scholar]
- 49.Oldani A, Zucconi M, Asselta R, et al. Autosomal dominant nocturnal frontal lobe epilepsy. A video-polysomnographic and genetic appraisal of 40 patients and delineation of the epileptic syndrome. Brain 1998;121(Pt 2):205–23. [DOI] [PubMed] [Google Scholar]
- 50.Lemoine D, Jiang R, Taly A, et al. Ligand-gated ion channels: new insights into neurological disorders and ligand recognition. Chem Rev 2012;112:6285–318. 10.1021/cr3000829 [DOI] [PubMed] [Google Scholar]
- 51.Steinlein OK, Mulley JC, Propping P, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 1995;11:201–3. 10.1038/ng1095-201 [DOI] [PubMed] [Google Scholar]
- 52.Leniger T, Kananura C, Hufnagel A, et al. A new Chrna4 mutation with low penetrance in nocturnal frontal lobe epilepsy. Epilepsia 2003;44:981–5. 10.1046/j.1528-1157.2003.61102.x [DOI] [PubMed] [Google Scholar]
- 53.De Fusco M, Becchetti A, Patrignani A, et al. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet 2000;26:275–6. 10.1038/81566 [DOI] [PubMed] [Google Scholar]
- 54.Kuryatov A, Gerzanich V, Nelson M, et al. Mutation causing autosomal dominant nocturnal frontal lobe epilepsy alters Ca2+ permeability, conductance, and gating of human alpha4beta2 nicotinic acetylcholine receptors. J Neurosci 1997;17:9035–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sáenz A, Galán J, Caloustian C, et al. Autosomal dominant nocturnal frontal lobe epilepsy in a Spanish family with a Ser252Phe mutation in the CHRNA4 gene. Arch Neurol 1999;56:1004–9. 10.1001/archneur.56.8.1004 [DOI] [PubMed] [Google Scholar]
- 56.Rózycka A, Trzeciak WH. Genetic basis of autosomal dominant nocturnal frontal lobe epilepsy. J Appl Genet 2003;44:197–207. [PubMed] [Google Scholar]
- 57.Hoda JC, Gu W, Friedli M, et al. Human nocturnal frontal lobe epilepsy: pharmocogenomic profiles of pathogenic nicotinic acetylcholine receptor beta-subunit mutations outside the ion channel pore. Mol Pharmacol 2008;74:379–91. 10.1124/mol.107.044545 [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues-Pinguet NO, Pinguet TJ, Figl A, et al. Mutations linked to autosomal dominant nocturnal frontal lobe epilepsy affect allosteric Ca2+ activation of the alpha 4 beta 2 nicotinic acetylcholine receptor. Mol Pharmacol 2005;68:487–501. 10.1124/mol.105.011155 [DOI] [PubMed] [Google Scholar]
- 59.Holland KD, Kearney JA, Glauser TA, et al. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett 2008;433:65–70. 10.1016/j.neulet.2007.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kullmann DM, Waxman SG. Neurological channelopathies: new insights into disease mechanisms and ion channel function. J Physiol 2010;588(Pt 11):1823–7. 10.1113/jphysiol.2010.190652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen TT, Klassen TL, Goldman AM, et al. Novel brain expression of ClC-1 chloride channels and enrichment of CLCN1 variants in epilepsy. Neurology 2013;80:1078–85. 10.1212/WNL.0b013e31828868e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heinzen EL, Depondt C, Cavalleri GL, et al. Exome sequencing followed by large-scale genotyping fails to identify single rare variants of large effect in idiopathic generalized epilepsy. Am J Hum Genet 2012;91:293–302. 10.1016/j.ajhg.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miceli F, Soldovieri MV, Ambrosino P, et al. Genotype–phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of Kv7.2 potassium channel subunits. Proc Natl Acad Sci 2013;110:4386–91. 10.1073/pnas.1216867110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomlinson SE, Rajakulendran S, Tan SV, et al. Clinical, genetic, neurophysiological and functional study of new mutations in episodic ataxia type 1. J Neurol Neurosurg Psychiatry 2013;84:1107–12. 10.1136/jnnp-2012-304131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graves TD, Cha YH, Hahn AF, et al. , CINCH Investigators. Episodic ataxia type 1: clinical characterization, quality of life and genotype-phenotype correlation. Brain 2014;137(Pt 4):1009–18. 10.1093/brain/awu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomlinson SE, Hanna MG, Kullmann DM, et al. Clinical neurophysiology of the episodic ataxias: insights into ion channel dysfunction in vivo. Clin Neurophysiol 2009;120:1768–76. 10.1016/j.clinph.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 67.Zuberi SM, Eunson LH, Spauschus A, et al. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain 1999;122(Pt 5):817–25. 10.1093/brain/122.5.817 [DOI] [PubMed] [Google Scholar]
- 68.Eunson LH, Rea R, Zuberi SM, et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol 2000;48:647–56. [DOI] [PubMed] [Google Scholar]
- 69.Browne DL, Gancher ST, Nutt JG, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet 1994;8:136–40. 10.1038/ng1094-136 [DOI] [PubMed] [Google Scholar]
- 70.Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol 2001;112:1575–85. 10.1016/S1388-2457(01)00595-8 [DOI] [PubMed] [Google Scholar]
- 71.Adelman JP, Bond CT, Pessia M, et al. Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron 1995;15:1449–54. 10.1016/0896-6273(95)90022-5 [DOI] [PubMed] [Google Scholar]
- 72.Tomlinson SE, Tan SV, Kullmann DM, et al. Nerve excitability studies characterize Kv1.1 fast potassium channel dysfunction in patients with episodic ataxia type 1. Brain 2010;133(Pt 12):3530–40. 10.1093/brain/awq318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D'Adamo MC, Gallenmüller C, Servettini I, et al. Novel phenotype associated with a mutation in the KCNA1(Kv1.1) gene. Front Physiol 2014;5:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jen JC, Graves TD, Hess EJ, et al. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain 2007;130:2484–93. 10.1093/brain/awm126 [DOI] [PubMed] [Google Scholar]
- 75.Spacey SD, Materek LA, Szczygielski BI, et al. Two novel CACNA1A gene mutations associated with episodic ataxia type 2 and interictal dystonia. Arch Neurol 2005;62:314–16. 10.1001/archneur.62.2.314 [DOI] [PubMed] [Google Scholar]
- 76.Imbrici P, Jaffe SL, Eunson LH, et al. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain 2004;127(Pt 12):2682–92. 10.1093/brain/awh301 [DOI] [PubMed] [Google Scholar]
- 77.Kinder S, Ossig C, Wienecke M, et al. Novel frameshift mutation in the CACNA1A gene causing a mixed phenotype of episodic ataxia and familiar hemiplegic migraine. Eur J Paediatr Neurol 2015;19:72–4. 10.1016/j.ejpn.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 78.Jen J, Kim GW, Baloh RW. Clinical spectrum of episodic ataxia type 2. Neurology 2004;62:17–22. 10.1212/01.WNL.0000101675.61074.50 [DOI] [PubMed] [Google Scholar]
- 79.Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol 2002;25:31–50. 10.1385/MN:25:1:031 [DOI] [PubMed] [Google Scholar]
- 80.Guida S, Trettel F, Pagnutti S, et al. Complete loss of P/Q calcium channel activity caused by a CACNA1A missense mutation carried by patients with episodic ataxia type 2. Am J Hum Genet 2001;68:759–64. 10.1086/318804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spacey SD, Hildebrand ME, Materek LA, et al. Functional implications of a novel EA2 mutation in the P/Q-type calcium channel. Ann Neurol 2004;56:213–20. 10.1002/ana.20169 [DOI] [PubMed] [Google Scholar]
- 82.Wan J, Khanna R, Sandusky M, et al. CACNA1A mutations causing episodic and progressive ataxia alter channel trafficking and kinetics. Neurology 2005;64:2090–7. 10.1212/01.WNL.0000167409.59089.C0 [DOI] [PubMed] [Google Scholar]
- 83.Baloh RW. Episodic ataxias 1 and 2. Handb Clin 2012;103:595–602. [DOI] [PubMed] [Google Scholar]
- 84.Strupp M, Kalla R, Claassen J, et al. A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias. Neurology 2011;77:269–75. 10.1212/WNL.0b013e318225ab07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alviña K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci 2010;30:7258–68. 10.1523/JNEUROSCI.3582-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet 1997;15:62–9. 10.1038/ng0197-62 [DOI] [PubMed] [Google Scholar]
- 87.Schöls L, Krüger R, Amoiridis G, et al. Spinocerebellar ataxia type 6: genotype and phenotype in German kindreds. J Neurol Neurosurg Psychiatry 1998;64:67–73. 10.1136/jnnp.64.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watase K, Barrett CF, Miyazaki T, et al. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci USA 2008;105:11987–92. 10.1073/pnas.0804350105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jodice C, Mantuano E, Veneziano L, et al. Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum Mol Genet 1997;6:1973–8. 10.1093/hmg/6.11.1973 [DOI] [PubMed] [Google Scholar]
- 90.Blumkin L, Leshinsky-Silver E, Michelson M, et al. Paroxysmal tonic upward gaze as a presentation of de-novo mutations in CACNA1A. Eur J Paediatr Neurol 2015;19:292–7. 10.1016/j.ejpn.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 91.Waters MF, Minassian NA, Stevanin G, et al. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet 2006;38:447–51. 10.1038/ng1758 [DOI] [PubMed] [Google Scholar]
- 92.Minassian NA, Lin MC, Papazian DM. Altered Kv3.3 channel gating in early-onset spinocerebellar ataxia type 13. J Physiol 2012;590(Pt 7):1599–614. 10.1113/jphysiol.2012.228205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 2001;24:517–26. 10.1016/S0166-2236(00)01892-0 [DOI] [PubMed] [Google Scholar]
- 94.Lee YC, Durr A, Majczenko K, et al. Mutations in KCND3 cause spinocerebellar ataxia type 22. Ann Neurol 2012;72:859–69. 10.1002/ana.23701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duarri A, Jezierska J, Fokkens M, et al. Mutations in potassium channel kcnd3 cause spinocerebellar ataxia type 19. Ann Neurol 2012;72:870–80. 10.1002/ana.23700 [DOI] [PubMed] [Google Scholar]
- 96.Ducros A, Tournier-Lasserve E, Bousser MG. The genetics of migraine. Lancet Neurol 2002;1:285–93. 10.1016/S1474-4422(02)00134-5 [DOI] [PubMed] [Google Scholar]
- 97.Terwindt GM, Ophoff RA, Haan J, et al. Familial hemiplegic migraine: a clinical comparison of families linked and unlinked to chromosome 19.DMG RG. Cephalalgia 1996;16:153–5. 10.1046/j.1468-2982.1996.1603153.x [DOI] [PubMed] [Google Scholar]
- 98.Pelzer N, Stam AH, Haan J, et al. Familial and sporadic hemiplegic migraine: diagnosis and treatment. Curr Treat Options Neurol 2013;15:13–27. 10.1007/s11940-012-0208-3 [DOI] [PubMed] [Google Scholar]
- 99.Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996;87:543–52. 10.1016/S0092-8674(00)81373-2 [DOI] [PubMed] [Google Scholar]
- 100.De Fusco M, Marconi R, Silvestri L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet 2003;33:192–6. 10.1038/ng1081 [DOI] [PubMed] [Google Scholar]
- 101.Dichgans M, Freilinger T, Eckstein G, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005;366:371–7. 10.1016/S0140-6736(05)66786-4 [DOI] [PubMed] [Google Scholar]
- 102.Athwal BS, Lennox GG, Elliott MA, et al. Acetazolamide responsiveness in familial hemiplegic migraine. Ann Neurol 1996;40:820–1. 10.1002/ana.410400525 [DOI] [PubMed] [Google Scholar]
- 103.Jen JC. Familial Hemiplegic Migraine. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews®. Seattle, WA: University of Washington, Seattle, 1993–2015. http://www.ncbi.nlm.nih.gov/books/NBK1388 [Google Scholar]
- 104.Zhou L, Chillag KL, Nigro MA. Hyperekplexia: a treatable neurogenetic disease. Brain Dev 2002;24:669–74. 10.1016/S0387-7604(02)00095-5 [DOI] [PubMed] [Google Scholar]
- 105.Shiang R, Ryan SG, Zhu YZ, et al. Mutations in the alpha 1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat Genet 1993;5:351–8. 10.1038/ng1293-351 [DOI] [PubMed] [Google Scholar]
- 106.Langosch D, Laube B, Rundström N, et al. Decreased agonist affinity and chloride conductance of mutant glycine receptors associated with human hereditary hyperekplexia. EMBO J 1994;13:4223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chung SK, Bode A, Cushion TD, et al. GLRB is the third major gene of effect in hyperekplexia. Hum Mol Genet 2013;22:927–40. 10.1093/hmg/dds498 [DOI] [PubMed] [Google Scholar]
- 108.Carta E, Chung SK, James VM, et al. Mutations in the GlyT2 gene (SLC6A5) are a second major cause of startle disease. J Biol Chem 2012;287:28975–85. 10.1074/jbc.M112.372094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eulenburg V, Becker K, Gomeza J, et al. Mutations within the human GLYT2 (SLC6A5) gene associated with hyperekplexia. Biochem Biophys Res Commun 2006;348:400–5. 10.1016/j.bbrc.2006.07.080 [DOI] [PubMed] [Google Scholar]
- 110.Rees MI, Harvey K, Pearce BR, et al. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat Genet 2006;38:801–6. 10.1038/ng1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tijssen MA, Schoemaker HC, Edelbroek PJ, et al. The effects of clonazepam and vigabatrin in hyperekplexia. J Neurol Sci 1997;149:63–7. 10.1016/S0022-510X(97)05378-1 [DOI] [PubMed] [Google Scholar]
- 112.Fischer TZ, Waxman SG. Familial pain syndromes from mutations of the NaV1.7 sodium channel. Ann N Y Acad Sci 2010;1184:196–207. 10.1111/j.1749-6632.2009.05110.x [DOI] [PubMed] [Google Scholar]
- 113.Yuan J, Matsuura E, Higuchi Y, et al. Hereditary sensory and autonomic neuropathy type IID caused by an SCN9A mutation. Neurology 2013;80:1641–9. 10.1212/WNL.0b013e3182904fdd [DOI] [PubMed] [Google Scholar]
- 114.Van Genderen PJ, Michiels JJ, Drenth JP. Hereditary erythromelalgia and acquired erythromelalgia. Am J Med Genet 1993;45:530–1. 10.1002/ajmg.1320450426 [DOI] [PubMed] [Google Scholar]
- 115.Bennett DL, Woods CG. Painful and painless channelopathies. Lancet Neurol 2014;13:587–99. 10.1016/S1474-4422(14)70024-9 [DOI] [PubMed] [Google Scholar]
- 116.Yang Y, Wang Y, Li S, et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythromelalgia. J Med Genet 2004;41:171–4. 10.1136/jmg.2003.012153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dib-Hajj SD, Rush AM, Cummins TR, et al. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain 2005;128(Pt 8):1847–54. 10.1093/brain/awh514 [DOI] [PubMed] [Google Scholar]
- 118.Cummins TR, Dib-Hajj SD, Waxman SG. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci 2004;24:8232–6. 10.1523/JNEUROSCI.2695-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waxman SG, Merkies IS, Gerrits MM, et al. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. Lancet Neurol 2014;13:1152–60. 10.1016/S1474-4422(14)70150-4 [DOI] [PubMed] [Google Scholar]
- 120.Fertleman CR, Ferrie CD. What's in a name—familial rectal pain syndrome becomes paroxysmal extreme pain disorder. J Neurol Neurosurg Psychiatry 2006;77:1294–5. 10.1136/jnnp.2006.089664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fertleman CR, Baker MD, Parker KA, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 2006;52:767–74. 10.1016/j.neuron.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 122.Fertleman CR, Ferrie CD, Aicardi J, et al. Paroxysmal extreme pain disorder (previously familial rectal pain syndrome). Neurology 2007;69:586–95. 10.1212/01.wnl.0000268065.16865.5f [DOI] [PubMed] [Google Scholar]
- 123.Choi JS, Boralevi F, Brissaud O, et al. Paroxysmal extreme pain disorder: a molecular lesion of peripheral neurons. Nat Rev Neurol 2011;7:51–5. 10.1038/nrneurol.2010.162 [DOI] [PubMed] [Google Scholar]
- 124.Nathan A, Rose JB, Guite JW, et al. Primary erythromelalgia in a child responding to intravenous lidocaine and oral mexiletine treatment. Pediatrics 2005;115:e504–7. 10.1542/peds.2004-1395 [DOI] [PubMed] [Google Scholar]
- 125.Cregg R, Cox JJ, Bennett DL, et al. Mexiletine as a treatment for primary erythromelalgia: normalization of biophysical properties of mutant L858F NaV 1.7 sodium channels. Br J Pharmacol 2014;171:4455–63. 10.1111/bph.12788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cox JJ, Reimann F, Nicholas AK, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006;444:894–8. 10.1038/nature05413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goldberg YP, MacFarlane J, MacDonald ML, et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet 2007;71:311–19. 10.1111/j.1399-0004.2007.00790.x [DOI] [PubMed] [Google Scholar]
- 128.Leipold E, Liebmann L, Korenke GC, et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet 2013;45:1399–404. 10.1038/ng.2767 [DOI] [PubMed] [Google Scholar]
- 129.Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012;71:26–39. 10.1002/ana.22485 [DOI] [PubMed] [Google Scholar]
- 130.Faber CG, Lauria G, Merkies IS, et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci USA 2012;109:19444–9. 10.1073/pnas.1216080109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang J, Han C, Estacion M, et al. , PROPANE Study Group. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 2014;137(Pt 6):1627–42. 10.1093/brain/awu079 [DOI] [PubMed] [Google Scholar]
- 132.Kremeyer B, Lopera F, Cox JJ, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010;66:671–80. 10.1016/j.neuron.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bautista DM, Jordt SE, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006;124:1269–82. 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 134.Zhang XY, Wen J, Yang W, et al. Gain-of-function mutations in SCN11A cause familial episodic pain. Am J Hum Genet 2013;93:957–66. 10.1016/j.ajhg.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zimoń M, Baets J, Auer-Grumbach M, et al. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 2010;133(Pt 6):1798–809. 10.1093/brain/awq109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nilius B, Owsianik G. Channelopathies converge on TRPV4. Nat Genet 2010;42:98–100. 10.1038/ng0210-98 [DOI] [PubMed] [Google Scholar]
- 137.Landouré G, Zdebik AA, Martinez TL, et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet 2010;42:170–4. 10.1038/ng.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeLong R, Siddique T. A large New England kindred with autosomal dominant neurogenic scapuloperoneal amyotrophy with unique features. Arch Neurol 1992;49:905–8. 10.1001/archneur.1992.00530330027010 [DOI] [PubMed] [Google Scholar]
- 139.Deng HX, Klein CJ, Yan J, et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet 2010;42:165–9. 10.1038/ng.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Auer-Grumbach M, Olschewski A, Papić L, et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet 2010;42:160–4. 10.1038/ng.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wuttke TV, Jurkat-Rott K, Paulus W, et al. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology 2007;69:2045–53. 10.1212/01.wnl.0000275523.95103.36 [DOI] [PubMed] [Google Scholar]
- 142.Gancher ST, Nutt JG. Autosomal dominant episodic ataxia: a heterogeneous syndrome. Mov Disord 1986;1:239–53. 10.1002/mds.870010404 [DOI] [PubMed] [Google Scholar]
- 143.Dedek K, Kunath B, Kananura C, et al. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc Natl Acad Sci USA 2001;98:12272–7. 10.1073/pnas.211431298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Finlayson S, Beeson D, Palace J. Congenital myasthenic syndromes: an update. Pract Neurol 2013;13:80–91. 10.1136/practneurol-2012-000404 [DOI] [PubMed] [Google Scholar]
- 145.Burke G, Cossins J, Maxwell S, et al. Distinct phenotypes of congenital acetylcholine receptor deficiency. Neuromuscul Disord 2004;14:356–64. 10.1016/j.nmd.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 146.Barišić N, Chaouch A, Müller JS, et al. Genetic heterogeneity and pathophysiological mechanisms in congenital myasthenic syndromes. Eur J Paediatr Neurol 2011;15:189–96. 10.1016/j.ejpn.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 147.Chaouch A, Müller JS, Guergueltcheva V, et al. A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol 2012;259:474–81. 10.1007/s00415-011-6204-9 [DOI] [PubMed] [Google Scholar]
- 148.Horga A, Raja Rayan DL, Matthews E, et al. Prevalence study of genetically defined skeletal muscle channelopathies in England. Neurology 2013;80:1472–5. 10.1212/WNL.0b013e31828cf8d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koch MC, Steinmeyer K, Lorenz C, et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 1992;257:797–800. 10.1126/science.1379744 [DOI] [PubMed] [Google Scholar]
- 150.Cannon SC. Voltage-sensor mutations in channelopathies of skeletal muscle. J Physiol 2010;588:1887–95. 10.1113/jphysiol.2010.186874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.George AL Jr, Sloan-Brown K, Fenichel GM, et al. Nonsense and missense mutations of the muscle chloride channel gene in patients with myotonia congenita. Hum Mol Genet 1994;3:2071–2. [PubMed] [Google Scholar]
- 152.Heine R, George AL Jr, Pika U, et al. Proof of a non-functional muscle chloride channel in recessive myotonia congenita (Becker) by detection of a 4 base pair deletion. Hum Mol Genet 1994;3:1123–8. 10.1093/hmg/3.7.1123 [DOI] [PubMed] [Google Scholar]
- 153.Fialho D, Schorge S, Pucovska U, et al. Chloride channel myotonia: exon 8 hot-spot for dominant-negative interactions. Brain 2007;130(Pt 12):3265–74. 10.1093/brain/awm248 [DOI] [PubMed] [Google Scholar]
- 154.George AL Jr, Crackower MA, Abdalla JA, et al. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet 1993;3:305–10. 10.1038/ng0493-305 [DOI] [PubMed] [Google Scholar]
- 155.Raja Rayan DL, Haworth A, Sud R, et al. A new explanation for recessive myotonia congenita: exon deletions and duplications in CLCN1. Neurology 2012;78:1953–8. 10.1212/WNL.0b013e318259e19c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Raja Rayan DL, Hanna MG. Skeletal muscle channelopathies: nondystrophic myotonias and periodic paralysis. Curr Opin Neurol 2010;23:466–76. 10.1097/WCO.0b013e32833cc97e [DOI] [PubMed] [Google Scholar]
- 157.Matthews E, Manzur AY, Sud R, et al. Stridor as a neonatal presentation of skeletal muscle sodium channelopathy. Arch Neurol 2011;68:127–9. 10.1001/archneurol.2010.347 [DOI] [PubMed] [Google Scholar]
- 158.Trivedi JR, Bundy B, Statland J, et al. , CINCH Consortium. Non-dystrophic myotonia: prospective study of objective and patient reported outcomes. Brain 2013;136(Pt 7):2189–200. 10.1093/brain/awt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matthews E, Tan SV, Fialho D, et al. What causes paramyotonia in the United Kingdom? Common and new SCN4A mutations revealed. Neurology 2008;70:50–3. 10.1212/01.wnl.0000287069.21162.94 [DOI] [PubMed] [Google Scholar]
- 160.Furby A, Vicart S, Camdessanché JP, et al. Heterozygous CLCN1 mutations can modulate phenotype in sodium channel myotonia. Neuromuscul Disord 2014;24:953–9. 10.1016/j.nmd.2014.06.439 [DOI] [PubMed] [Google Scholar]
- 161.Desaphy JF, De Luca A, Tortorella P, et al. Gating of myotonic Na channel mutants defines the response to mexiletine and a potent derivative. Neurology 2001;57:1849–57. 10.1212/WNL.57.10.1849 [DOI] [PubMed] [Google Scholar]
- 162.Wang GK, Russell C, Wang SY. Mexiletine block of wild-type and inactivation-deficient human skeletal muscle hNav1.4 Na+ channels. J Physiol 2004;554(Pt 3):621–33. 10.1113/jphysiol.2003.054973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Statland JM, Bundy BN, Wang Y, et al. Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. JAMA 2012;308:1357–65. 10.1001/jama.2012.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Markhorst JM, Stunnenberg BC, Ginjaar IB, et al. Clinical experience with long-term acetazolamide treatment in children with nondystrophic myotonias: a three-case report. Pediatr Neurol 2014;51:537–41. 10.1016/j.pediatrneurol.2014.05.027 [DOI] [PubMed] [Google Scholar]
- 165.Eguchi H, Tsujino A, Kaibara M, et al. Acetazolamide acts directly on the human skeletal muscle chloride channel. Muscle Nerve 2006;34:292–7. 10.1002/mus.20585 [DOI] [PubMed] [Google Scholar]
- 166.Novak KR, Norman J, Mitchell JR, et al. Sodium channel slow inactivation as a therapeutic target for myotonia congenita. Ann Neurol 2015;77:320–32. 10.1002/ana.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Links TP, Zwarts MJ, Wilmink JT, et al. Permanent muscle weakness in familial hypokalaemic periodic paralysis. Clinical, radiological and pathological aspects. Brain 1990;113(Pt 6):1873–89. [DOI] [PubMed] [Google Scholar]
- 168.Jurkat-Rott K, Lehmann-Horn F, Elbaz A, et al. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet 1994;3:1415–19. 10.1093/hmg/3.8.1415 [DOI] [PubMed] [Google Scholar]
- 169.Ptácek LJ, Tawil R, Griggs RC, et al. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell 1994;77:863–8. 10.1016/0092-8674(94)90135-X [DOI] [PubMed] [Google Scholar]
- 170.Miller TM, Dias da Silva MR, Miller HA, et al. Correlating phenotype and genotype in the periodic paralyses. Neurology 2004;63:1647–55. 10.1212/01.WNL.0000143383.91137.00 [DOI] [PubMed] [Google Scholar]
- 171.Matthews E, Labrum R, Sweeney MG, et al. Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology 2009;72:1544–7. 10.1212/01.wnl.0000342387.65477.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature 2007;446:76–8. 10.1038/nature05598 [DOI] [PubMed] [Google Scholar]
- 173.Geukes Foppen RJ, van Mil HG, van Heukelom JS. Effects of chloride transport on bistable behaviour of the membrane potential in mouse skeletal muscle. J Physiol 2002;542(Pt 1):181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Struyk AF, Cannon SC. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J Gen Physiol 2007;130:11–20. 10.1085/jgp.200709755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jurkat-Rott K, Weber MA, Fauler M, et al. K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proc Natl Acad Sci USA 2009;106:4036–41. 10.1073/pnas.0811277106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu F, Mi W, Cannon SC. Bumetanide prevents transient decreases in muscle force in murine hypokalemic periodic paralysis. Neurology 2013;80:1110–16. 10.1212/WNL.0b013e3182886a0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Suetterlin K, Männikkö R, Hanna MG. Muscle channelopathies: recent advances in genetics, pathophysiology and therapy. Curr Opin Neurol 2014;27:583–90. 10.1097/WCO.0000000000000127 [DOI] [PubMed] [Google Scholar]
- 178.Cannon SC, Brown RH Jr, Corey DP. A sodium channel defect in hyperkalemic periodic paralysis: Potassium-induced failure of inactivation. Neuron 1991;6:619–26. 10.1016/0896-6273(91)90064-7 [DOI] [PubMed] [Google Scholar]
- 179.Lehmann-Horn F, Küther G, Ricker K, et al. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve 1987;10:363–74. 10.1002/mus.880100414 [DOI] [PubMed] [Google Scholar]
- 180.Cummins TR, Zhou J, Sigworth FJ, et al. Functional consequences of a Na+ channel mutation causing hyperkalemic periodic paralysis. Neuron 1993;10:667–78. 10.1016/0896-6273(93)90168-Q [DOI] [PubMed] [Google Scholar]
- 181.Cannon SC, Brown RH Jr, Corey DP. Theoretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels. Biophys J 1993;65:270–88. 10.1016/S0006-3495(93)81045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nguyen HL, Pieper GH, Wilders R. Andersen-Tawil syndrome: clinical and molecular aspects. Int J Cardiol 2013;170:1–16. 10.1016/j.ijcard.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 183.Zhang L, Benson DW, Tristani-Firouzi M, et al. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation 2005;111:2720–6. 10.1161/CIRCULATIONAHA.104.472498 [DOI] [PubMed] [Google Scholar]
- 184.Sansone V, Tawil R. Management and treatment of Andersen-Tawil syndrome (ATS). Neurother J Am Soc Exp Neurother 2007;4:233–7. 10.1016/j.nurt.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 185.Tawil R, Ptacek LJ, Pavlakis SG, et al. Andersen's syndrome: potassium-sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann Neurol 1994;35:326–30. 10.1002/ana.410350313 [DOI] [PubMed] [Google Scholar]
- 186.Plaster NM, Tawil R, Tristani-Firouzi M, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 2001;105:511–19. 10.1016/S0092-8674(01)00342-7 [DOI] [PubMed] [Google Scholar]
- 187.Haruna Y, Kobori A, Makiyama T, et al. Genotype-phenotype correlations of KCNJ2 mutations in Japanese patients with Andersen-Tawil syndrome. Hum Mutat 2007;28:208 10.1002/humu.9483 [DOI] [PubMed] [Google Scholar]
- 188.Kokunai Y, Nakata T, Furuta M, et al. A Kir3.4 mutation causes Andersen-Tawil syndrome by an inhibitory effect on Kir2.1. Neurology 2014;82:1058–64. 10.1212/WNL.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 189.Hanna MG, Stewart J, Schapira AH, et al. Salbutamol treatment in a patient with hyperkalaemic periodic paralysis due to a mutation in the skeletal muscle sodium channel gene (SCN4A). J Neurol Neurosurg Psychiatry 1998;65:248–50. 10.1136/jnnp.65.2.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Links TP, Zwarts MJ, Oosterhuis HJ. Improvement of muscle strength in familial hypokalaemic periodic paralysis with acetazolamide. J Neurol Neurosurg Psychiatry 1988;51:1142–5. 10.1136/jnnp.51.9.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tawil R, McDermott MP, Brown R Jr, et al. Randomized trials of dichlorphenamide in the periodic paralyses. Working Group on Periodic Paralysis. Ann Neurol 2000;47:46–53. [DOI] [PubMed] [Google Scholar]
- 192.Ligtenberg JJ, Van Haeften TW, Van Der Kolk LE, et al. Normal insulin release during sustained hyperglycaemia in hypokalaemic periodic paralysis: role of the potassium channel opener pinacidil in impaired muscle strength. Clin Sci (Lond) 1996;91:583–9. 10.1042/cs0910583 [DOI] [PubMed] [Google Scholar]
- 193.Tan SV, Matthews E, Barber M, et al. Refined exercise testing can aid DNA-based diagnosis in muscle channelopathies. Ann Neurol 2011;69:328–40. 10.1002/ana.22238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Drost G, Stunnenberg BC, Trip J, et al. Myotonic discharges discriminate chloride from sodium muscle channelopathies. Neuromuscul Disord 2015;25:73–80. 10.1016/j.nmd.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 195.Morrow JM, Matthews E, Raja Rayan DL, et al. Muscle MRI reveals distinct abnormalities in genetically proven non-dystrophic myotonias. Neuromuscul Disord 2013;23:637–46. 10.1016/j.nmd.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ryan DP, da Silva MRD, Soong TW, et al. Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell 2010;140:88–98. 10.1016/j.cell.2009.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.