Abstract
Background:
The Definitive Endoderm (DE) differentiation using the undefined media and non-human feeders can cause contaminations in the generated cells for therapeutic applications. Therefore, generating safer and more appropriate DE cells is needed. This study compared five different methods to establish an appropriate method for inducing an efficient DE differentiation from Human Induced Pluripotent Stem Cells (hiPSCs) on an appropriate feeder in a more defined medium.
Methods:
Human Induced Pluripotent Stem Cells (hiPSCs) were cultured on inactivated feeders. Passaged hiPSCs, without feeder, were incubated for three days with Activin-A and different endodermal differentiation media including 1-FBS, 2-B27, 3-ITS and albumin fraction-V, 4-B27 and ITS and 5-like the third medium. The feeder cells in the first four methods were Mouse Embryonic Fibroblasts (MEFs) and in the fifth method were human adult bone marrow Mesenchymal Stem Cells (hMSCs). DE markers FOXA2, SOX17 and CXCR4 and also pluripotency marker OCT4 were evaluated using qRT-PCR, as well as FOXA2 by the immunocytochemistry.
Results:
QRT-PCR analysis showed that after three days, the expression levels of DE and pluripotency markers in the differentiated hiPSCs among all five groups did not have any significant differences. Similarly, the immunocytochemistry analysis demonstrated that the differentiated hiPSCs expressed FOXA2, with no significant differences.
Conclusion:
Despite this similarity in the results, the third differentiation medium has more defined and cost effective components. Furthermore, hMSC, a human feeder, is safer than MEF. Therefore, the fifth method is preferable among other DE differentiation methods and can serve as a fundamental method helping the development of regenerative medicine.
Keywords: Endoderm, Induced pluripotent stem cells, Mesenchymal stem cells
Introduction
The application of cell culture-derived products is evolving in medical treatment 1. If unlimited numbers of vital in vitro differentiated cells are obtained, the development of cell-based therapies and the study of the early stages of the drug discovery for the diseases will be improved 2. The ability of hiPSCs for self-renewing and the potential to differentiate into three embryonic germ layers, i.e. ectoderm, mesoderm and endoderm, makes it possible to use them as an appropriate source of cells in regenerative medicine 3. Unlike many other stem cells, Embryonic Stem Cells (ESCs) and iPSCs are able to unlimitedly proliferate without losing potency. The advantage of using iPSCs is the source of autologous cells they supply 4. Thus, they are considered as an infinite source for the production of vital matured cells in vitro and might be used as a main component in the cell therapy 5.
Stem cells, such as iPSCs, are usually maintained on a layer of feeder cells. Feeder layers have many advantages for stem cells, such as maintaining their undifferentiated situation and pluripotency. hiPSCs are usually maintained on inactivated Mouse Embryonic Fibroblasts (MEFs) as a feeder, but applying non-human feeder cells can produce biological contaminants such as exogenous antigens, viruses and pathogens. To avoid these contaminations, diverse human cells, such as fibroblasts from neonatal foreskin, ESC-derived fibroblast-like cells, autologous fibroblasts, immortalized human skin fibroblasts, and human Mesenchymal Stem Cells (hMSCs) were used as feeder layers to maintain ESCs, although their ability was different from each other 6–9. The feeder-free culture techniques for human ESCs have also been reported, but they have many problems such as chromosomal instabilities of human stem cells 8. HMSCs generate several necessary elements for stem cells, for instance TGF-beta. They have been extensively applied for clinical applications. HMSCs are a highly developed feeder for the stem cell cultures and could be derived from healthy adult donors and proliferated multifold before applying as a feeder layer 5,10.
Diverse serum and serum-free culture media for the induction of differentiation of stem cell-derived endoderm cells have been reported in previous studies 11,12. Classifying the animal cell culture media into subcategories according to the rate of defined media, classified from low to high definition, gives us serum-containing medium, reduced-serum medium, serum-free medium, protein-free medium, chemically-defined medium and protein-free chemically-defined medium 1. Generating safer and more efficient cells using more appropriate culture media and feeder layers provides more adequate cells for cell transplantation therapies. To the best of our knowledge, no study has been conducted on the comparison of different differentiation media and feeder layers in endodermal differentiation of hiPSCs. DE differentiation is the first important step to obtain endodermal organs, such as the liver and pancreas 13. Forkhead box A2 (FOXA2), SRY-box containing gene 17 (SOX17) and C-X-C chemokine receptor type 4 (CXCR4) are DE marker genes 14. Octamer-binding Transcription factor 4 (OCT4) is a pluripotency and stem cell specific marker 15. In this study, an attempt was made to focus on the comparison of endodermal differentiation from hiPSCs, on a novel hMSCs feeder and on a typically used MEFs feeder, in different serum and serum-free endodermal differentiation media, regarding their efficiency and safety, and the establishment of an appropriate method to generate adequate cells for cell transplantation therapies.
Materials and Methods
Feeder cell culture
MEFs and human adult bone marrow-derived MSCs were used as feeder layers in this study. For producing MEFs, pregnant female mice were used. The mice were obtained from Stem Cells Technology Research Center (Tehran, Iran) and all animal experiments were performed under control and supervision of its ethical committee. On the 13th day of female mice pregnancy, MEFs were taken from their embryos. The dissecting of the embryos from the womb was done considering sterile conditions and afterwards they were washed with Phosphate-Buffered Saline (PBS). The red organs and embryos' heads were detached, and the rest of the tissue was chopped up and put in 0.25% trypsin at 37°C for 20 min to be digested. The cell suspensions were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin and incubated in a standard gas atmosphere with 95% humidity and 5% CO2 at 37°C. The cells (Passage 3) were moved into 6-well cell culture plates. When they reached 90% confluency, they were mitotically inactivated by mitomycin-C (10 μg/ml, Santa Cruz Biotechnology, USA). Human adult bone marrow-derived MSCs (Passage 5) were obtained from Stem Cells Technology Research Center (Tehran, Iran), under control and supervision of its ethical committee. They were plated and inactivated under the same conditions like MEFs 7,16,17.
hiPSC culture
hiPSCs were obtained from Stem Cells Technology Research Center (Tehran, Iran). The number of passages used, ranged from 8 to 14 7. They were cultured on a layer of inactivated feeder cells in 6-well cell culture plates in DMEM/F12 with 10% knockout serum replacement, 10% FBS stem cell qualified replacement, 1% glutamax (all from Gibco, USA), 1% non-essential amino acids (PAA, Austria), 10 ng/ml basic fibroblast growth factor (Peprotech, USA) and 1% penicillin/streptomycin in a standard gas atmosphere with 95% humidity and 5% CO2 at 37°C 18.
Endodermal differentiation of the hiPSCs
After hiPSC colonies grew up and reached about 70% confluency, hiPSCs were passaged by collagenase type IV (Gibco, USA) treatment and transferred into 0.1% gelatin-coated 6-well cell culture plates at a density of 15×105 cells per well, without feeder cells. They were differentiated into the endoderm cells directly without Embryoid Bodies (EBs) formation, using five protocols coming in the next paragraph. hiPSCs cultured on the MEFs feeder were differentiated using the following four protocols and hiPSCs cultured on the hMSCs feeder were differentiated using only protocol 5. These five protocols include:
hiPSCs were incubated for 3 days with RPMI-1640 medium (Gibco, USA) containing 100 ng/ml Activin A (R&D, USA) and FBS (Gibco, USA). The concentration of FBS was 0% on the first day, 0.1% on the second day and 1.0% on the third day 2,11.
hiPSCs were incubated for 3 days with RPMI-1640 medium (Gibco, USA) containing 100 ng/ml Activin A (R&D, USA) and 1.0% B27 (Gibco, USA) 12,19.
hiPSCs were incubated for 3 days with RPMI-1640 medium (Gibco, USA) containing 100 ng/ml Activin A (R&D, USA), 0.5 mg/ml albumin fraction V (Sigma-Aldrich, USA) and insulin-transferrin-selenite (ITS, Gibco, USA). The concentration of ITS was 0% on the first day, 0.1% on the second day and 1.0% on the third day 18,20.
hiPSCs were incubated for 3 days with RPMI-1640 medium (Gibco, USA) containing 100 ng/ml Activin A (R&D, USA), 1.0% B27 (Gibco, USA) and insulin-transferrin-selenite (ITS, Gibco, USA). The concentration of ITS was 0% on the first day, 0.1% on the second day, and 1.0% on the third day 5.
The same protocol as protocol 3 was used, but hiPSC colonies grew up on the hMSCs feeder instead of the MEFs feeder.
Evaluation of differentiated cells
Immunocytochemistry:
The cells were washed with PBS, fixed in 4% paraformaldehyde (Sigma-Aldrich, USA) in PBS at 4°C for 20 min and then at Room Temperature (RT) for 5 min. They were washed with PBS twice and then incubated with 0.2% Triton X-100 at RT for 30 min. After washing with PBS, the cells were blocked with 10% goat serum (Gibco, USA) at RT for 30 min and then removed. They were incubated with a primary antibody at 4°C overnight. On the next day, the cells were washed with PBS 3 times. After that, they were incubated with a secondary antibody at RT for 1 hr and then washed with PBS 3 times. The nuclei of cells were stained with DAPI (1 μg/ml) for 30 s at RT, and then washed with PBS. The cells were examined using a fluorescent microscope (Nikon, Japan). The primary antibody was rabbit anti-FOXA2 antibody (Santa Cruz Biotechnology, USA) diluted at 1:200. The secondary antibody was goat anti-rabbit IgG-FITC antibody (Santa Cruz Biotechnology, USA), diluted at 1:200.
Quantitative real-time PCR (qRT-PCR)
Total RNA from the undifferentiated and differentiated hiPSCs was extracted using Trizol reagent (Gibco, USA). Next, 2 μg of total RNA was used with cDNA synthesis kit (Fermentas, USA) and random hexamer primers, according to the manufacturer's protocol. QRT-PCR was done by using SYBR Premix ExTaq kit (Takara, Japan) and a Rotor Gene 6000 real-time PCR system (Corbett, Australia). Data was normalized to a housekeeping gene (Beta2M) and analyzed by the comparative CT method. The primer sequences are shown in the table 1. They were obtained from Stem Cells Technology Research Center (Tehran, Iran) 5. The PCR reaction was composed of 6.5 μl of SYBR Green PCR Master Mix, 0.5 μl 10 μM of forward primer, 0.5 μl 10 μM of reverse primer, 1 μl of template cDNA, and 4.5 μl of water in a total volume of 13 μl. The reaction was done at 95°C for 2 min, followed by 40 cycles of 95°C for 5 s and 60°C for 45 s.
Table 1.
Primer sequences and conditions used in qRT-PCR analysis
| Gene | Sequences (forward and reverse) | Tm(°C) | Product size (bp) |
|---|---|---|---|
| OCT4 | 5′-TTC GCA AGC CCT CAT TTC AC-3′ 5′-CCA TCA CCT CCA CCA CCT G-3′ |
60 | 114 |
| FOXA2 | 5′-AGC GAG TTA AAG TAT GCT GG-3′ 5′-GTA GCT GCT CCA GTC GGA-3′ |
58 | 93 |
| SOX17 | 5′-CAA GAT GCT GGG CAA GTC-3′ 5′-TGG TCC TGC ATG TGC TG-3′ |
60 | 93 |
| CXCR4 | 5′-CGC CAC CAA CAG TCA GAG-3′ 5′-AAC ACA ACC ACC CAC AAG TC-3′ |
58 | 176 |
| Beta2M | 5′-ATG CCT GCC GTG TGA AC-3′ 5′-ATC TTC AAA CCT CCA TGA TG-3′ |
56 | 91 |
Statistical analysis
All experiments were repeated in triplicate. The information was represented as mean±standard deviation (SD). The statistical analyses were done using One-way ANOVA. The p<0.05 (marked*) were considered statistically significant.
Results
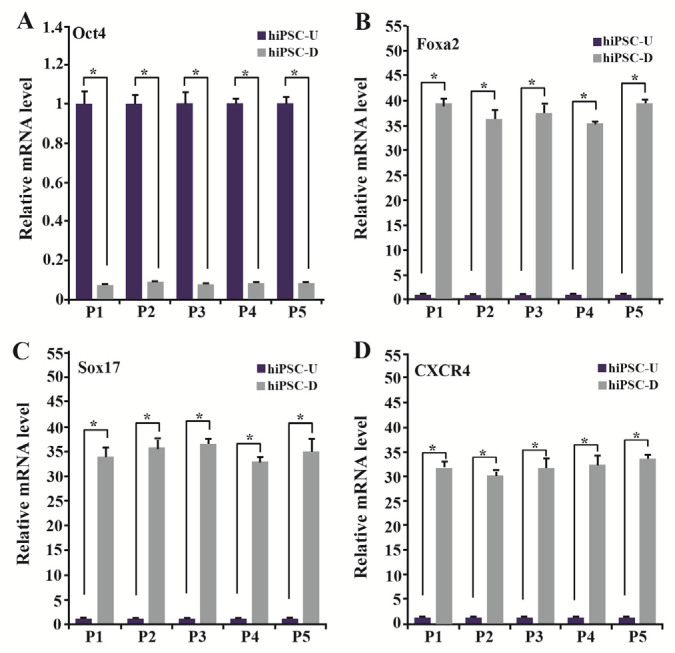
MEFs and HMSCs displayed spindle-shaped and fibroblastic morphology (Figures 1A and 1B). hiPSCs colonies were cultured on a layer of mitotically inactivated MEFs or hMSCs as a feeder. hiPSCs had morphologically large nuclei and little cytoplasm. Colonies of hiPSCs were flat and tightly packed (Figures 1C and 1D). For the endodermal differentiation, hiPSCs were passaged and transferred into the cell culture plates without feeder cells. hiPSCs cultured on MEFs feeder were differentiated using protocols 1 to 4 and those on hMSCs feeder were differentiated using protocol 5 which is like protocol 3 in used materials (Figures 1E and 1F). After three days of growth factor treatment, the mRNA expression levels of DE markers FOXA2, SOX17 and CXCR4 and also the pluripotency marker OCT4 were evaluated in the differentiated cells using qRT-PCR to determine the in vitro differentiation efficiency. Also, DE and pluripotency markers in the undifferentiated hiPSCs were evaluated as well. QRTPCR analysis showed that the undifferentiated hiPSCs expressed OCT4 in a high level, while the expression levels of FOXA2, SOX17 and CXCR4 were remarkably low. After 3 days of growth factor treatment, the expression levels of DE markers were increased significantly (p<0.05), whereas, the expression level of OCT4 was reduced significantly (p<0.05) in the differentiated cells using different DE differentiation media and feeder layers (Figure 2). These qRT-PCR results showed that hiPSCs could be differentiated into DE cells efficiently in vitro. These data also demonstrated that mRNA expression levels of DE and pluripotency markers did not have any significant differences in the generated cells using listed DE differentiation media and two feeder layers, MEFs and hMSCs. The expression level of FOXA2 was increased 39.47±0.84 times in the differentiated hiPSCs using protocol 1(P1), compared to the undifferentiated hiPSCs, 36.12±2.01 using P2, 37.34±2.03 using P3, 35.12±0.72 using P4 and 39.41±0.83 using P5 (Figure 2B). The expression level of other DE markers, SOX17 and CXCR4, and also the pluripotency marker OCT4, in the differentiated hiPSCs compared to the undifferentiated hiPSCs, are present in figure 2.
Figure 1.
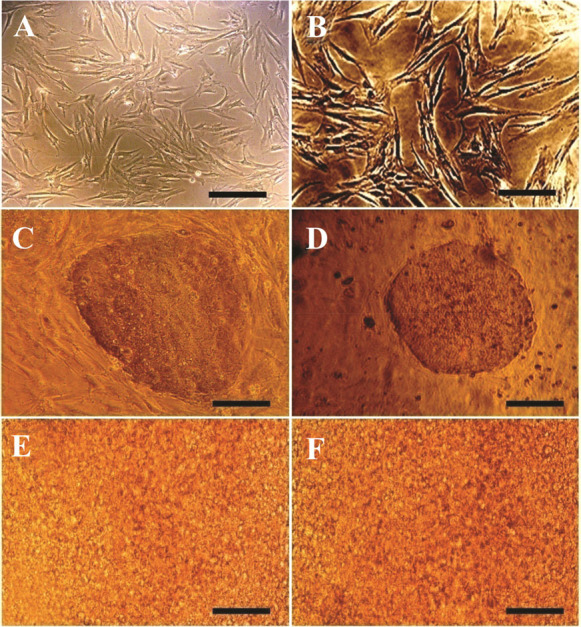
Phase contrast microscopy images of A) MEFs (Scale bar= 200 μm); B) hMSCs (Scale bar=100 μm); C) hiPSC colones on MEFs feeder (Scale bar=200 μm); D) hiPSC colonies on hMSCs feeder (Scale bar=200 μm); E) differentiated hiPSCs using protocol 3 and MEFs feeder (Scale bar=800 μm); F) differentiated hiPSCs using protocol 5 and hMSCs feeder (Scale bar=800 μm).
Figure 2.
QRT-PCR analysis of DE and pluripotency markers. Beta2M was considered as the housekeeping gene. The values of undifferentiated hiPSCs were set at 1. Experimental values were expressed as mean±standard deviation (SD); * p<0.05, hiPSC-U: undifferentiated hiPSCs, hiPSC-D: differentiated hiPSCs, P1: Protocol 1, P2: Protocol 2, P3: Protocol 3, P4: Protocol 4, P5: Protocol 5.
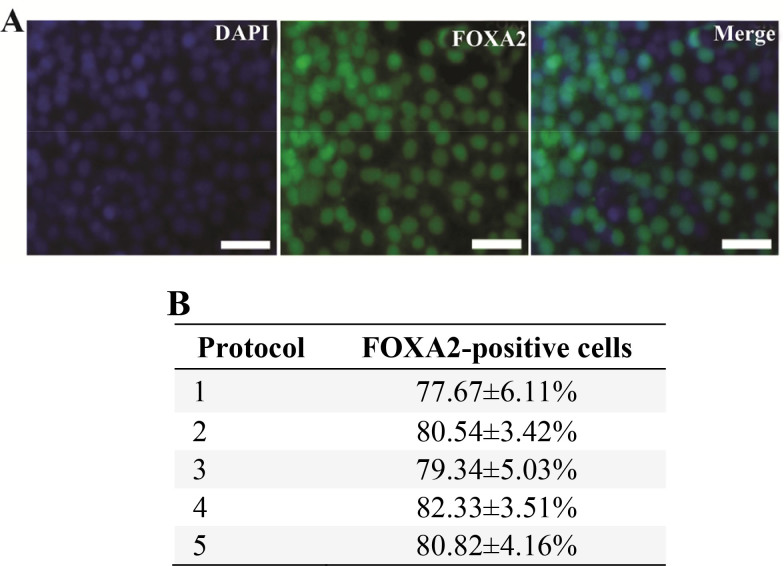
Also, DE marker FOXA2 expression was evaluated at the protein level using immunocytochemical analysis. The number of positively stained cells with DE marker FOXA2 was determined and it was divided into the total number of the cells, determined by counting the number of their stained nuclei. Based on this number, the percentage of differentiated cells was obtained 21. After treatment with different endodermal differentiation media for three days, immunocytochemical analysis showed that 77.67–82.33% of the cells generated using five methods expressed DE marker FOXA2 (Figures 3A and 3B). These results demonstrated that the generated cells expressed DE marker FOXA2 in a high level using different endodermal differentiation media and feeder layers and no significant differences in the percentages of the differentiated cells of the five groups were detected in the positive DE marker FOXA2.
Figure 3.
A: Immunocytochemistry staining of expression of DE marker FOXA2 in differentiated hiPSCs. After treatment with different endodermal differentiation media for three days, the differentiated hiPSCs were stained with rabbit anti-FOXA2 antibody and goat anti-rabbit IgG-FITC antibody. Nuclei were stained with DAPI. Scale bar=100 μm; B: The percentage of the cells expressing FOXA2 on the third day of the differentiation. Data represent the proportion of FOXA2-positive cells to the total cells in percentage. No significant differences in the percentages of the FOXA2-positive cells of five groups were detected. Data show the mean±standard deviation (SD) of three independent experiments.
Discussion
Choosing a suitable endodermal differentiation medium and feeder layer is an important step for obtaining safe and efficient endoderm cells, especially for their use in the differentiation into the cells used for cell replacement therapy. In this report, DE and pluripotency markers of the cells differentiated from hiPSCs in three days using four different endodermal differentiation media and two different feeders were compared. hiPSCs were cultured on hMSCs or MEFs feeder. The hiPSCs colonies cultured on hMSCs feeder were more typical and with more defined borders than colonies on MEFs feeder (Figure 1). After three days of growth factors treatment, qRT-PCR results showed that in the differentiated cells using different differentiation media and feeder layers, the expression levels of DE markers FOXA2, SOX17 and CXCR4 were increased significantly, whereas, the expression level of OCT4 was reduced significantly (Figure 2). Immunocytochemical analysis showed that after three days, 77.67–82.33% of the generated cells using five methods expressed FOXA2 (Figure 3). These data were consistent with the existing reports in the endodermal differentiation of hiPSCs 5,12,18,22. In our study, these qRT-PCR and immunocytochemical results showed that hiPSCs were differentiated into DE cells efficiently using different differentiation media and feeder layers. These results also demonstrated that the cells differentiated from hiPSCs, using different listed differentiation media and feeder layers, were similar and showed no significant differences. Therefore, they were differentiated with similar efficiencies. However, the materials used in these four differentiation media were not similar in terms of the level of defined media and also the sources of their feeder cells were not identical. By using hMSCs feeder, which is a human feeder, biological contaminations of non-human feeders can be prevented.
In previous reports, the differentiation was done through EBs formation that imitates embryo development 22 or without EBs formation 4. Although EBs formation is helpful for an efficient differentiation of stem cells, the derived cells are a mixture belonging to three germ layers; consequently, it is better to differentiate stem cells into the endoderm cells directly without EBs formation by culturing a monolayer of stem cells 23. Therefore, in this report, hiPSCs were moved into the cell culture plates straightly without EBs formation.
In an existing study 5, it has been indicated that hiPSCs could be efficiently differentiated into the endoderm cells through EBs formation in the differentiation media, including Activin A, ITS and B27 supplements using hMSCs feeder. Furthermore, in the fifth method of this study, it was indicated that hiPSCs could be efficiently differentiated into the endoderm cells in a more defined medium without B27 supplement, only using Activin A, ITS and albumin fraction V, without EBs formation, and using hMSCs feeder. Bovine Serum Albumin is also one of the ingredients of B27 supplement. Therefore, the differences of our fifth method with previously published methods 5 are the use of a non-EB formation method and also a more defined and cost effective medium with fewer components.
In other studies it has been indicated that hiPSCs and hESCs could be efficiently differentiated into the endoderm cells without EBs formation in the differentiation media, including Activin A, ITS and albumin fraction V using MEFs feeder 18,20. Therefore, in comparison to previously published methods 18,20, in our fifth method of this study, it was shown that hiPSCs could be efficiently differentiated into the endoderm cells in the same medium, but by using hMSCs feeder.
Similar to other existing reports 11,12, our data showed that Activin A can induce efficient endodermal differentiation in the serum-free and in serum containing media. Various concentrations of Activin will induce different fates in cells. High dose of Activin induces endoderm efficiently, while mesoderm is induced at low and intermediate concentrations of Activin 24. The treatment with only Activin is not sufficient to induce the endoderm efficiently 25. Therefore, some supplements were used in our differentiation media beside a high concentration of Activin A. hiPSCs were differentiated into the endoderm cells efficiently using all the listed endodermal differentiation media.
In the first differentiation medium, FBS was used in addition to Activin A 2,11. The application of the animal serum in culture media has some disadvantages. Serum is an ill-defined ambiguous supplement, containing unknown and undesirable factors; it has different batch to batch compositions (both quantitatively and qualitatively). These can cause transferring a number of different undefined variables and contaminations to the cell culture and derived products. Therefore, it may hinder the therapeutic applications of the generated cells 20,26.
In the second and fourth differentiation media, B27 supplement was used in addition to Activin A 4,12. B27 supplement is a serum-free supplement that contains twenty components. B27 contains five ingredients of animal/human origin, including Catalase, Superoxide Dismutase, Human Transferrin, Bovine Serum Albumin (BSA) and D-Galactose and additionally two components in which animal/human-derived ingredients are used in the manufacturing process, including Human Recombinant Insulin and Progesterone 27.
In the third differentiation medium, albumin fraction V and ITS supplement were used in addition to Activin A 18,20. Also, in the fourth differentiation media, both supplements, B27 and ITS 5, were used. ITS supplement is a serum-free supplement which contains three components. It consists of one ingredient of animal/human origin, which is Human Transferrin and also one component in which animal/human-derived ingredients are used in its manufacturing process, which is Human Recombinant Insulin 28.
Some contaminants like lipid contents of albumin are found in undefined animal-derived components 29. In case the cells which use these undefined animal-derived components are applied for therapeutic use, they may cause problems 4. A troublesome matter of tissue regeneration, using in vitro differentiated cells, is contamination with the undefined factors 25. Lack of using animal-derived components has been increased due to the development of therapeutic applications for products derived from cell culture. This elimination helps to reduce the transfer of unwanted contaminations 1.
B27 and ITS supplements are both serum-free and incompletely defined media, but ITS supplement has fewer animal-derived components; therefore, it has fewer disadvantages according to its animal-derived components 27–29. For example, according to previous reports 4, the repetition capacity of the process is decreased by using undefined factors because of its potential variability whereas variability is reduced by using well-defined components, hence making it easier to carry out comparative studies. In addition, the concentration of ITS ingredients is written in its manual 28, while the concentration of B27 ingredients is not written in its manual and it is confidential 27. This can lead to our inability to analyze the effects of different concentrations of B27 ingredients in the process when B27 supplement is used in it. It seems that the cost of the process can be decreased as well by using albumin fraction V and ITS instead of B27 or instead of B27 and ITS both. Moreover, as the previous studies indicate, using well-defined components makes the analysis of molecular pathways, which regulate differentiation, easier and more accurate 4; however, numerous undefined components may result in unwelcome effects 12.
Conclusion
Altogether, the fifth DE differentiation method, including the combination of Activin A, albumin fraction V and ITS, and hMSCs feeder, appears to be a preferable DE differentiation method among other listed methods for inducing the differentiation of hiPSCs into DE cells. This is an efficient and safe method for direct induction of DE differentiation from hiPSCs. It can contribute as a fundamental method to assist the development of cell transplantation therapies and regenerative medicine.
Acknowledgement
The authors would like to thank the Center for Development of Clinical Research of Namazee Hospital and Dr. Nasrin Shokrpour for editorial assistance. This work was supported by a grant from the Iranian Council of Stem Cell Technology.
Footnotes
Conflict of Interest
All authors declare no conflicts of interest.
References
- 1. Jayme DW, Smith SR. Media formulation options and manufacturing process controls to safeguard against introduction of animal origin contaminants in animal cell culture. Cytotechnology 2000; 33 (1–3): 27– 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basma H, Soto-Gutiérrez A, Yannam GR, Liu L, Ito R, Yamamoto T, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 2009; 136 (3): 990– 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sekine K, Takebe T, Suzuki Y, Kamiya A, Nakauchi H, Taniguchi H. Highly efficient generation of definitive endoderm lineage from human induced pluripotent stem cells. Transplant Proc 2012; 44 (4): 1127– 1129. [DOI] [PubMed] [Google Scholar]
- 4. Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010; 51 (1): 297– 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mobarra N, Soleimani M, Kouhkan F, Hesari Z, Lahmy R, Mossahebi-Mohammadi M, et al. Efficient differentiation of human induced pluripotent stem cell (hiPSC) derived hepatocyte-like cells on hMSCs feeder. Int J Hematol Oncol Stem Cell Res 2014; 8 (4): 20– 29. [PMC free article] [PubMed] [Google Scholar]
- 6. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131 (5): 861– 872. [DOI] [PubMed] [Google Scholar]
- 7. Havasi P, Nabioni M, Soleimani M, Bakhshandeh B, Parivar K. Mesenchymal stem cells as an appropriate feeder layer for prolonged in vitro culture of human induced pluripotent stem cells. Mol Biol Rep 2013; 40 (4): 3023– 3031. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi K, Narita M, Yokura M, Ichisaka T, Yamanaka S. Human induced pluripotent stem cells on autologous feeders. PLoS One 2009; 4 (12): e8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unger C, Gao S, Cohen M, Jaconi M, Bergstrom R, Holm F, et al. Immortalized human skin fibroblast feeder cells support growth and maintenance of both human embryonic and induced pluripotent stem cells. Hum Reprod 2009; 24 (10): 2567– 2581. [DOI] [PubMed] [Google Scholar]
- 10. Montes R, Ligero G, Sanchez L, Catalina P, de la Cueva T, Nieto A, et al. Feeder-free maintenance of hESCs in mesenchymal stem cell-conditioned media: distinct requirements for TGF-beta and IGF-II. Cell Res 2009; 19 (6): 698– 709. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells 2008; 26 (5): 1117– 1127. [DOI] [PubMed] [Google Scholar]
- 12. Takata A, Otsuka M, Kogiso T, Kojima K, Yoshikawa T, Tateishi R, et al. Direct differentiation of hepatic cells from human induced pluripotent stem cells using a limited number of cytokines. Hepatol Int 2011; 5 (4): 890– 898. [DOI] [PubMed] [Google Scholar]
- 13. Mathew S, Jaramillo M, Zhang X, Zhang LA, Soto-Gutiérrez A, Banerjee I. Analysis of alternative signaling pathways of endoderm induction of human embryonic stem cells identifies context specific differences. BMC Syst Biol 2012; 6: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McLean AB, D'Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 2007; 25 (1): 29– 38. [DOI] [PubMed] [Google Scholar]
- 15. Chen L, Daley GQ. Molecular basis of pluripotency. Hum Mol Genet 2008; 17 (R1): R23– 27. [DOI] [PubMed] [Google Scholar]
- 16. Adams GN, LaRusch GA, Stavrou E, Zhou Y, Nieman MT, Jacobs GH, et al. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood 2011; 117 (14): 3929– 3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakhshandeh B, Soleimani M, Hafizi M, Ghaemi N. A comparative study on nonviral genetic modifications in cord blood and bone marrow mesenchymal stem cells. Cytotechnology 2012; 64 (5): 523– 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res 2009; 19 (11): 1233– 1242. [DOI] [PubMed] [Google Scholar]
- 19. Duan Y, Ma X, Zou W, Wang C, Bahbahan IS, Ahuja TP, et al. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells 2010; 28 (4): 674– 686. [DOI] [PubMed] [Google Scholar]
- 20. Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 2007; 45 (5): 1229– 1239. [DOI] [PubMed] [Google Scholar]
- 21. Prasajak P, Leeanansaksiri W. Developing a new two-step protocol to generate functional hepatocytes from wharton's jelly-derived mesenchymal stem cells under hypoxic condition. Stem Cells Int 2013; 2013: 762196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghodsizadeh A, Taei A, Totonchi M, Seifinejad A, Gourabi H, Pournasr B, et al. Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem Cell Rev 2010; 6 (4): 622– 632. [DOI] [PubMed] [Google Scholar]
- 23. Katsuda T, Sakai Y, Ochiya T. Induced pluripotent stem cell-derived hepatocytes as an alternative to human adult hepatocytes. J Stem Cells 2012; 7 (1): 1– 17. [PubMed] [Google Scholar]
- 24. Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development 2004; 131 (7): 1651– 1662. [DOI] [PubMed] [Google Scholar]
- 25. Ninomiya H, Mizuno K, Terada R, Miura T, Ohnuma K, Takahashi S, et al. Improved efficiency of definitive endoderm induction from human induced pluripotent stem cells in feeder and serum-free culture system. In Vitro Cell Dev Biol Anim 2015; 51 (1): 1– 8. [DOI] [PubMed] [Google Scholar]
- 26. Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX 2010; 27 (1): 53– 62. [DOI] [PubMed] [Google Scholar]
- 27. Thermo Fisher Scientific Company [Internet]. Available from: http://www.lifetechnologies.com/ir/en/home/technical-resources/media-formulation.250.html.
- 28. Thermo Fisher Scientific Company [Internet]. Available from: http://www.lifetechnologies.com/ir/en/home/technical-resources/media-formulation.334.html.
- 29. Usta SN, Scharer CD, Xu J, Frey TK, Nash RJ. Chemically defined serum-free and xeno-free media for multiple cell lineages. Ann Transl Med 2014; 2 (10): 97. [DOI] [PMC free article] [PubMed] [Google Scholar]