Abstract
Background:
The presence of rennin-angiotensin components in mammalian ovaries and their involvement in ovarian physiology have been established. In the present study, effects of angiotensin II (Ang II) on sodium-potassium adenosine triphosphatase (Na+/K+/ATPase) expression and development of sheep embryos was evaluated.
Methods:
The abattoir-derived Cumulus Oocyte Complexes (COC) were randomly allocated into three experimental groups; group I) in vitro Maturation (IVM) of oocytes in the presence of Ang II followed by in vitro fertilization (IVF)/in vitro Culture (IVC) (IVM group), group II) IVM/IVF of oocytes followed by IVC wherein the embryos were exposed to Ang II on day 4 of IVC (D4 group), and group III) IVM/IVF and IVC of oocytes without any angiotensin (Control). The blastocyst and hatching rates were recorded on days 6 to 8. Day 8 embryos were immunostained with primary and secondary antibodies against Na+/K+/ATPase α1 and β1 subunits.
Results:
Addition of Ang II during IVM and IVC significantly increased the hatching rate of blastocysts on day 8 compared to the control. The trophectoderm and total blastocyst cells’ numbers were significantly increased by addition of Ang II to the IVM and IVC media, though the expression of Na+/K+/ATPase α1 and β1 subunits were positively influenced by the addition of Ang II on day 4 (D4 group).
Conclusion:
In conclusion, it seems Ang II through positive effects on embryos, expressed as the greater hatching rate and blastocyst cell number, could increase the sheep embryo developmental rate. These improvements might be partly related to the greater expression of Na+/K+/ATPase α1 and β1 subunits when Ang II was added during IVC.
Keywords: Angiotensin II, Development, Embryo, Expression, Na+/K+/ATPase, Sheep
Introduction
Many studies have reported the presence of members of the Rennin-Angiotensin System (RAS) in the ovary, such as prorenin, angiotensinogen, angiotensin-converting enzyme (ACE), Angiotensin II (Ang II) and Ang II receptors (AT1 and AT2), suggesting the involvement of the RAS in ovarian physiology 1. Angiotensin II, an active octapeptide of the RAS, was present in high concentrations in mammalian ovaries, suggesting that it has a role in ovarian function. Concentrations of Ang II increased in human and bovine follicular fluid at the time of ovulation and after exposure to Luteinizing Hormone (LH) or human Chorionic Gonadotropin (hCG) 2. There is also evidence that Ang II has a direct role in ovarian steroidogenesis, follicular atresia, oocyte maturation and ovulation. Furthermore, hCG-induced oocyte maturation was inhibited by a concomitant addition of saralasin (an Ang II antagonist) 3.
Several genes were identified as being directly or indirectly regulated by Ang II including Na+/K+/ATPase 4, nicotinamide adenine dinucleotide phosphate (NADPH), mitogen-activated protein kinases (MAPKs), nuclear factor (NF)-κB, activator protein (AP)-1, cycloxygenase-2 (COX-2) and cell cycle regulators (retinoblastoma susceptibility protein and p53) 5.
The research on mechanisms directing blastocyst formation has demonstrated that (a) Na+/K+/ATPase has a polarized distribution confined to the trophectoderm basolateral membrane regions just before the onset of cavitation, (b) expression of Na+/K+/ATPase subunit genes are up-regulated during the morula to blastocyst transition, (c) treatment with ouabain (a potent and specific inhibitor of the Na+/K+/ATPase) affects cavitation and blastocyst formation in a number of mammalian species, and (d) Na+/K+/ATPase regulates the formation and function of trophectoderm tight junctions 6.
Na+/K+/ATPase subunits are characterized by multiple isoforms so that in mammalian cells there are four and three different isoforms of α (α1, α2, α3, and α4) and β (β1, β2, and β3) subunits, respectively. The α-subunit is responsible for the catalytic and transport properties of the enzyme and contains the binding sites for the cations and ATP 7. The β-subunit is essential for the normal activity of the enzyme, and it appears to be involved in the occlusion of K+ and the modulation of the K+ and Na+ affinity of the enzyme. In addition, in vertebrate cells, the β-subunit may act as a chaperone, stabilizing the correct folding of the α-polypeptide to facilitate its delivery to the plasma membrane. A third protein, termed the γ-subunit, has also been identified in purified preparations of the enzyme 7.
According to the presence of high concentration of Ang II in the follicular fluid at the time of ovulation and the significant role of Na+/K+/ATPase during blastocyst formation and regarding to embryonic genome activation at the 8 to 16-cell stage 8, especially up-regulation of Na+/K+/ATPase gene at this time 6, it was hypothesized that the addition of Ang II to the IVM and IVC media may play a significant role in sheep oocyte and embryo developmental competence.
Materials and Methods
All experimental procedures were reviewed and approved by Avicenna Research Institute, the Bioethics Committee. All chemicals were purchased from Sigma Chemical Company (St. Louis, MO, USA), unless otherwise indicated in the text.
Oocyte collection
Adult sheep ovaries were collected during the breeding season (September to November) from a local slaughterhouse and transported to the laboratory in saline (30–35°C), within 2 to 3 hr following the collection. Ovaries were washed three times with pre-warmed fresh saline (37°C), and all visible follicles with a diameter of 2 to 6 mm were aspirated using gentle vacuum (30 mmHg) via a 20 gauge short beveled needle connected to a vacuum pump. Prior to aspiration, 2 ml pre-incubated hepes-modified tissue culture medium (H-TCM199), supplemented with 50 IU/ml heparin was added to the collecting tube.
In vitro maturation
After aspiration, only oocytes with evenly granulated cytoplasm surrounded by more than three layers of unexpanded cumulus cells (COCs) were selected for In Vitro Maturation (IVM). Before culturing, oocytes were washed in H-TCM supplemented with 10% FBS (Fetal bovine serum, Gibco BRL, Grand Island, NY, USA; v/v), and 2 mM L-glutamine. The oocyte culture medium was consisted of bicarbonate-buffered TCM 199 with 2 mM L-glutamine supplemented with 0.05 U/ml Follicle Stimulating Hormone (FSH), 100 IU/ml penicillin, 100 μg/ml streptomycin, 0.2 mM Na- pyruvate and 10% FBS (v/v), and 10−10 M Ang II in IVM group. The medium was adjusted to 275 mOsm. The selected COCs were pooled and randomly distributed in three experimental groups. In each experimental group, the selected COCs were placed in maturation droplets (10 oocytes/50 μl) and covered by sterile paraffin oil in a 60 mm Petri dish (Falcon 3004; Becton & Dickinson, Franklin Lakes, NJ, USA) and were then incubated under an atmosphere of 5% CO2 and 95% air with 100% humidity at 39°C for 24 hr.
Experimental groups
The selected immature COCs were randomly allocated into three groups: I) IVM of oocytes in the presence of 10−10 M Ang II followed by IVF/IVC (IVM group); II) IVM/IVF of oocytes followed by IVC wherein the embryos were exposed to 10−10 M Ang II on day 4 of IVC (D4 group) and III) IVM/IVF and IVC of oocytes without angiotensin (Control).
The zygotes were then cultured in SOF medium at 39°C under condition of 7% O2, 5% CO2 and 88% N2 in humidified air for 8 days. The cleavage and blastocyst/hatching rates were recorded on days 3 and 6 to 8, respectively (day 0 was defined as day of fertilization). Each treatment was consisted of at least four replicates. To evaluate the effects of Ang II on Na+/K+/ATPase subunits expression, the morula and blastocysts on day 8 were immunostained with specific primary and a common fluorescein isothiocyanate (FITC)-conjugated secondary antibody. The mean fluorescence intensity of the subunits was measured with ImageJ 1.37v software (National Institutes of Health, Bethesda, MD, USA). In each group, the rest of resulting blastocysts were then subjected to differential cell staining.
Preparation of sperm and in vitro fertilization
After IVM, the oocytes were washed four times in HSOF [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-synthetic oviductal fluid] and once in fertilization medium and were then transferred into the fertilization droplets. A frozen semen pool from a single batch of Shaal breed ram, with approved fertility, was used in all experiments. Semen was fractionated on discontinuous percoll (Amersham Biosciences AB, Uppsala, Sweden) gradients as previously described 2. Briefly, 700 μl of each percoll 90% (v/v) and percoll 40% (v/v) were poured at a bottom of a 15 ml Falcon tube and 350 μl of thawed semen was slowly added on the top and tube was then centrifuged at 700×g for 10 min. The fertilization medium was Synthetic Oviductal Fluid (SOF), as originally described by Tervit et al 9, enriched with 20% (v/v) heat inactivated estrous sheep serum and 10 mg/ml heparin. A 5 μl aliquot of sperm suspension, 1.0×106 sperm/ml, was added into the fertilization droplets (45 μl) containing 10 oocytes. Fertilization was carried out by co-incubation of sperm and oocytes in an atmosphere of 5% CO2 in humidified air at 39°C for 18 hr.
In vitro culture
After IVF, presumptive zygotes were vortexed for 2 to 3 min to remove the cumulus cells and then washed in H-SOF to remove spermatozoa and cellular debris. They were then allocated to the 20 μl culture drops containing SOF supplemented with 2% (v/v) BME-essential amino acids, 1% (v/v) MEM-non essential amino acids, 1 mM glutamine and 8 mg/ml fatty acid free Bovine Serum Albumin (BSA) and 10−10 M Ang II on day 4 of D4 group. They were then cultured at 39°C under conditions of 7% O2, 5% CO2 and 88% N2 in humidified air. On the third and fifth day of culture, 10% (v/v) charcoal stripped FBS was added to the medium. The osmolarity was maintained at 270 to 285 mOsmol. The percentage of cleaved embryos on day 3 and the percentage of total blastocysts on days 6, 7 and 8 were expressed on the basis of the number of oocytes at the onset of culture, and the percentage of expanded and hatched blastocysts on days 6, 7 and 8 was proportional to the total number of blastocysts on the same day.
Differential staining
The blastocysts were incubated in Triton X-100 prepared in the base medium for 15 s. The blastocysts were then stained in the base medium containing 30 μg/ml Propidium Iodide (PI) for 1 min and after two washes in base medium were then transferred into ice-cold ethanol containing 10 μg/ml Hoechst for 15 min. The blastocysts were then directly mounted into a small droplet of glycerol on a glass slide and examined under a Nikon TE 300 epifluorescent microscope (Nikon Corporation, Melville, USA). Inner Cell Mass (ICM) nuclei appeared blue because of DNA labeling with Hoechst, while Trophectoderm (TE) cells appeared red due to staining of nuclear DNA with the membrane impermeable PI.
Immunostaining and quantification of fluorescence intensity
As previously described 10 the embryos at morula and blastocyst stage were washed 3 times with base medium (PBS supplemented with 0.1% (w/v) Polyvinyl alcohol (PVA) and then fixed with 4% (w/v) paraformaldehyde for 10 min at 37°C. After rinsing with base medium, the embryos were blocked in PBS supplemented with 1% (w/v) BSA and 10% (v/v) sheep serum for 30 min at room temperature. Blocking solution was removed and the embryos were transferred to primary antibody solution at 37°C for 4 hr and then kept overnight at 4°C. The embryos were washed several times and transferred to the secondary antibody solution at 37°C and maintained for 4 hr. The primary antibodies against Na+/K+/ATPase subunits (Na+/K+/ATPase anti-α1 Monoclonal Antibody, Thermo scientific, Rockford, USA and Na+/K+/ATPase anti-β1 Monoclonal Antibody, Abcam, Cambridge) were prepared in antibody diluent (PBS supplemented with 1% (w/v) BSA and 3% (v/v) sheep serum). The Na+/K+/ATPase antibodies against α1 and β1 subunits were diluted at 1:50 (v/v) and 1:100 (v/v), respectively and the common secondary antibody was diluted at 1:50 (v/v) with antibody diluents. In negative controls, the primary antibodies were omitted. The secondary antibody was sheep anti-mouse antibody conjugated with fluorescein isothiocyanate (Avicenna Research Institute, Tehran, Iran). After immunostaining, the embryos were directly mounted into a small droplet of glycerol on a glass slide and examined under an epifluorescence microscope. Observations were performed by a Nikon TE 300 (Nikon Corporation, Melville, USA) epifluorescence microscope with excitation wavelengths of 488 nm (for FITC). All images were recorded digitally with a high-resolution Charge-Coupled Device (CCD) (Nikon Corporation) and duration of exposure for acquiring each type of fluorescence was kept constant. After subtracting the background, mean fluorescence intensity was measured by manually outlining all embryos with ImageJ 1.37v software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were collected over at least four replicates. Differences among groups were analyzed by one-way ANOVA followed by post hoc Fisher LSD test using SigmaPlot (version 11.0). When equal variance test failed, the treatments were compared by Student-Newman-Keuls Method. When normality test failed, the Kruskal-Wallis ANOVA was applied. The comparison in expression of Na+/K+/ATPase α1 and β1 subunits between corresponding embryos was performed using t-test. Differences were considered significant when p≤0.01. Data were expressed as mean±SEM.
Results
Effect of Ang II supplementation on embryo development in vitro
Addition of 10−10 M Ang II to the IVM media could significantly increase the rate of total blastocysts on day 6 compared with control (p=0.03). Additionally, supplementation of IVM or IVC media with Ang II significantly increased the blastocyst hatching rate on day 8 of culture compared with control (Table 1; p=0.03). There was no significant difference in cleavage, expanded and hatched blastocyst rates on days 6 and 7 as well as blastocyst rate on day 8 of culture between groups in the presence or absence of Ang II (Table 1).
Table 1.
The effects of angiotensin on culture media during IVM or Day 4 of IVC on embryo development
| Group | Oocyte N | Cleavage N (%) | Blastocyst % (Mean±SEM) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 6 | Day 7 | Day 8 | |||||||
| Expanded | Total | Expanded | Hatched | Total | Hatched | Total | |||
| Control | 152 | 130(85.2±7) | 38.9±14.2 | 9.9±1 a | 57.3±6.6 | 21.6±5.8 | 23±5 | 36.2±2.3 a | 35.4±4.3 |
| IVM | 188 | 178(94.6±2.7) | 49.4±9.1 | 21.4±3.9 b | 47.6±8.5 | 35.4±2.7 | 35.2±3.6 | 66.9±5 b | 38.8±2.7 |
| D4 | 204 | 174(83.8 ±4.9) | 33.3±23.6 | 12.9±2.6 a.b | 53.6±13.8 | 27.6±9.8 | 31±5.2 | 54.8±4 b | 37.8±5.8 |
| P-value | -- | p≥0.05 | p≥0.05 | p=0.03 | p≥0.05 | p≥0.05 | p≥0.05 | p=0.001 | p≥0.05 |
Numbers with different lowercase superscript letters in the same column differ significantly. Total blastocyst includes early, expanded, and hatched blastocysts. The percentage of blastocysts at Days 6 to 8 were expressed based on the number of oocytes at the onset of culture, and the percentages of expanded and hatched blastocysts at Days 7 and 8 were expressed based on the total number of blastocysts at the same day.
Effect of Ang II supplementation on blastocyst cells number
The number of ICM in blastocysts was not influenced by IVM or IVC media supplemented with Ang II (p>0.05). The TE and total cells numbers, however, significantly increased by the addition of Ang II in IVM and D4 groups (Figure 1; Table 2; p=0.001). Furthermore, the ICM/Total ratio was significantly greater in control group (p=0.01)
Figure 1.
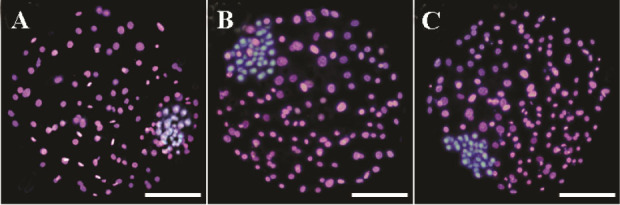
Differential staining of resulting blastocysts supplemented with (images B and C, respectively) and without aldosterone (image A) during IVM and day 4 of IVC. The inner cell mass nuclei and trophectoderm cells were stained blue and pink, due to staining with Hoechst and PI (Propidium iodide), respectively.
Table 2.
The effects of angiotensin on culture media during IVM or Day 4 of IVC on blastocyst cells number
| Group | Blastocyst N | Blastocyst cell numbers | Ratio %±SEM | ||
|---|---|---|---|---|---|
| ICM Mean±SEM | TE Mean±SEM | Total Mean±SEM | |||
| Control | 32 | 23.7±1.7 | 112.8±2.7 a | 136.6±1.7 a | 17.4±1.3 a |
| IVM | 26 | 21.4±2.2 | 150.4±1.9 b | 171.9±2.2 b | 12.3±1.2 b |
| D4 | 28 | 20.1±2.7 | 144.5±1.7 b | 164.6±2.2 b | 12±1.4 b |
| P-value | -- | p≥0.05 | p<0.001 | p<0.001 | p=0.015 |
Numbers with different lowercase superscript letters in the same column differ significantly (p < 0.05).
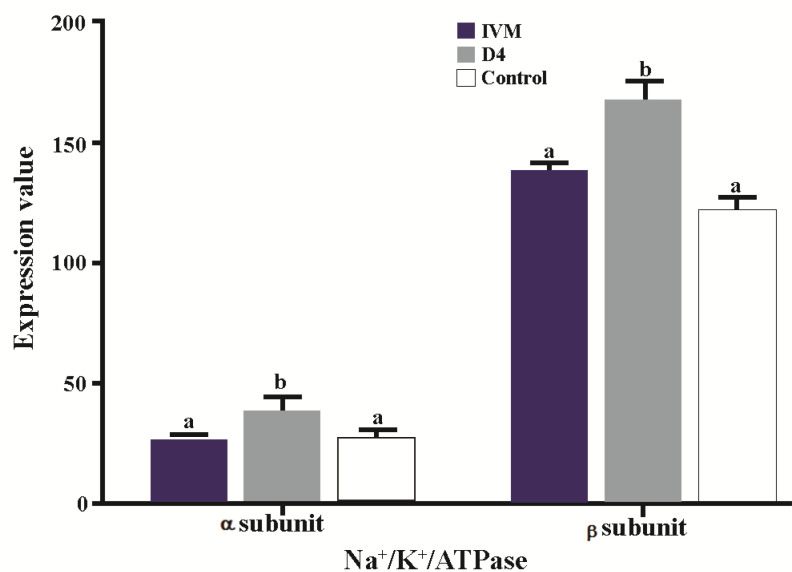
Effect of Ang II supplementation on Na+/K+/ATPase subunits expression in embryos
There was a significant difference in Na+/K+/ATPase subunits expression among treatment groups (Figure 2). The expression of Na+/K+/ATPase α1 and β1 subunits when IVC medium was supplemented with Ang II (Figure 3; p=0.02 and p<0.001, respectively) significantly increased compared to the control. Media supplementation with Ang II during IVM, however, had no influence on expression of Na+/K+/ATPase α1 and β1 subunits.
Figure 2.
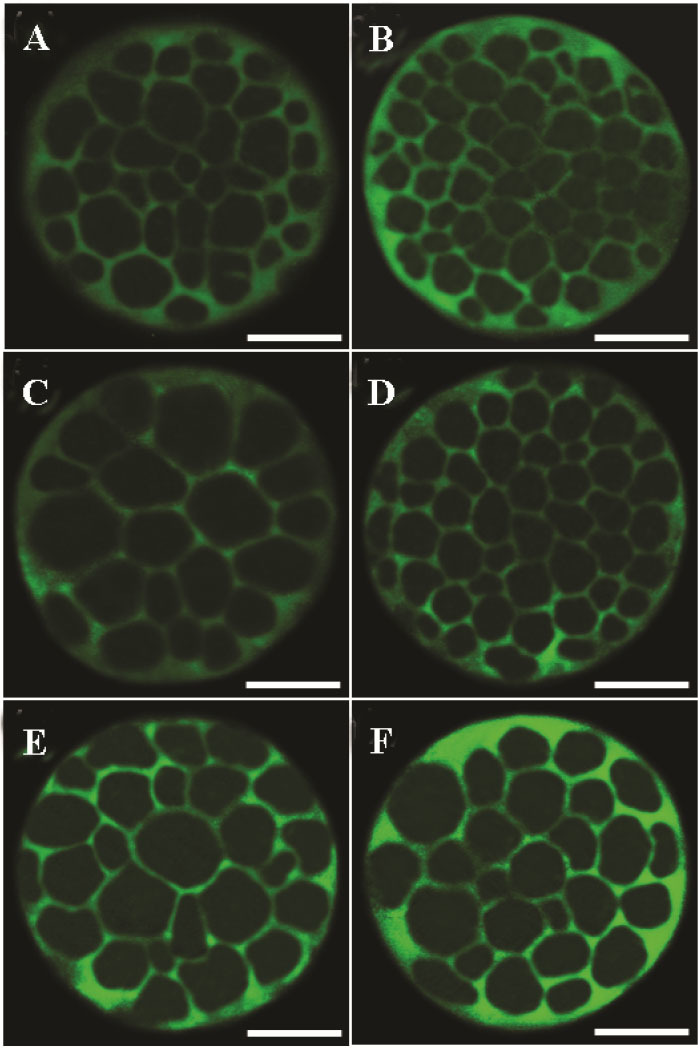
Angiotensin II supplementation during IVM and IVC and its effect on Na+/K+/ATPase α1 and β1 subunits expression in derived embryos. Bright green color in each micrograph indicates the presence of α1 and β1 Na+/K+/ATPase subunits probed by respective primary antibodies (anti-α1 and anti-β1 Na+/K+/ATPase) which have been subsequently identified by secondary antibody conjugated with fluorescein isothiocyanate. Bars, 40 μm. The intensity of Na+/K+/ATPase α1 and β1 subunits in embryos of control group (A and B, respectively). Expression of Na+/K+/ATPase α1 subunit in embryos that were exposed to Ang II during IVM (C). Expression of Na+/K+/ATPase β1 subunit in embryos that were exposed to Ang II during IVM (D). Expression of Na+/K+/ATPase α1 subunit in embryos that were exposed to Ang II during day 4 of IVC (E). Expression of Na+/K+/ATPase β1 subunit in embryos that were exposed to AngII during day 4 of IVC (F).
Figure 3.
Effect of Ang II supplementation in culture medium during IVM and IVC (Day 4) on expression of α1 and β1 Na+/K+/ATPase subunits in embryos. The values are expressed as mean±SEM. For each Na+/K+/ATPase subunit, bars with different letters indicate significant differences between experimental groups (IVM, D4, and control; p<0.05).
Discussion
It has been demonstrated that expression of Na+/K+/ATPase genes increased during the morula to blastocyst transition and hatching process 6. Therefore, considering the increased concentration of Ang II at the time of ovulation in follicular fluid and with regard to bovine embryonic genome activation at the 8 to 16-cell stage 8, especially up-regulation of Na+/K+/ATPase gene at this time 6, it could be inferred that addition of Ang II to the IVM and IVC media may play a significant role in sheep oocyte and embryo developmental competence.
In the present study, the addition of Ang II to the IVM medium (IVM group) significantly increased the rate of blastocyst on day 6 of culture which was indicative of higher speed of embryo development compared to the control. Though, this persuasion effect was gradually eliminated as such no difference was observed on day 8 of culture. Furthermore, media supplementation with Ang II (in both IVM and D4 groups) significantly increased the hatching rate of blastocysts on day 8.
Previous studies have indicated that Ang II has significantly increased the expression of Na+/K+/ATPase α1 and β1 subunits in the number of cultured cells such as rat vascular smooth muscle 11, astrocyte 4, proximal tubules of pig kidney 12 and canine kidney distal tubules 13. It has been shown that Na+/K+/ATPase has a fundamental role in regulating trophectoderm tight junction formation and function 6. Inhibition of Na+/K+/ATPase by ouabain is associated with the failure to constitute an efficient trophectoderm tight junctional seal that is required for expansion of the blastocyst cavity and hatching 6. On the other hand, blastocyst expansion only occurs when the tight junction permeability seal is fully formed to restrict the leakage of fluid via paracellular routes, thus ensuring the expansion of the cavity as fluid accumulates 14. Therefore, considering the inductive role of Ang II in Na+/K+/ATPase expression 4, it might be hypothesized that at least in fresh oocytes, Ang II could exert its inductive effect during morula to blastocyst and blastocyst to hatched blastocyst transition which in turn led to the greater day 6 blastocyst rate in IVM group as well as greater hatching rate in IVM and D4 groups compared to the control.
Apart from the inductive role of Ang II in expression of Na+/K+/ATPase and the subsequent embryo development, this question that whether this compound can exert its effect via other pathway (s) remains to be elucidated. The crucial role of cyclooxygenase-2 (COX-2) as one of the important regulators of mammalian hatching process has been indicated in many species 15–18. The COX-2 can modulate hatching process via regulation of zonalytic proteases which rather exclusively occur in Trophectodermal Projections (TEPs) at the abembryonic pole of the blastocyst 19. In hamster, the expression of COX-2 mRNA and its protein has been observed in 8-cell through hatched blastocyst stages 19. It has also been indicated that Ang II can induce expression of several pro-inflammatory genes, including COX-2 5,20. From above, it is hypothesized that greater hatching rate in Ang II supplemented groups might be induced by COX-2 pathway.
In between, the positive effect of AngII on expression of Na+/K+/ATPase α1 and β1 subunits was only evident when media supplementation was done during IVC (Figure 2). One possibility for low influence of Ang II supplementation during IVM on Na+/K+/ATPase subunits expression might be related to the time interval between Ang II supplementation (during IVM) and time of Na+/K+/ATPase α1 and β1 expression assessment (blastocyst stage on day 8). An increase in Na+/K+/ATPase genes expression during morula to blastocyst transition and hatching process 6 may bring up the point that the addition of Ang II on day 4 of IVC has a greater effect on Na+/K+/ATPase subunits expression compared to IVM supplementation (Figure 2).
It has been indicated that the Na+/K+/ATPase β1 subunit is required to support early development to the morula (16–32 cells) stage of mouse embryos 6. Other studies have demonstrated that Na+/K+/ATPase β1 subunit gene products display a very dramatic up-regulation just before blastocyst formation, suggesting that up-regulation of this gene is required for cavitation and hatching process to occur. Based on what stated about, it could be inferred that the up-regulation of Na+/K+/ATPase β1 subunit should have a positive effect on blastocyst and hatched blastocyst formation in Ang II treated groups (Table 1).
Concerning the Na+/K+/ATPase α1 subunit, its mRNAs were present throughout preimplantation development and displayed a much more gradual increase as the embryo progressed to the blastocyst stage 6. In our study, despite the lower expression of α1 subunit compared to β1 subunit, it seems it may have a role in blastocyst and hatched blastocyst formation in embryos exposed to Ang II during IVC. Therefore, one explanation for the greater hatching rate in IVM and D4 groups might be related to the greater expression of Na+/K+/ATPase α1 and β1 subunits compared to the controls.
In the present study, TE and total cell numbers were significantly increased by the addition of Ang II to IVM and IVC media. In mouse, embryo cell numbers significantly decreased when embryos were treated with ouabain as a Na+/K+/ATPase inhibitor 13,21. It has been shown that Ang II by induction of Na+/K+/ATPase expression may probably reduce cell apoptosis which in turn can increase the total cell number 22,23. Greater ICM/Total ratio in the control compared to Ang II supplemented groups was related to the lower number of TE cells in the former group that superficially raises the ICM/Total ratio in the control compared to the treated groups. On the other hand, greater TE numbers in treated groups provide the better conditions for appropriate placentation and embryo implantation compared to the control.
Conclusion
As shown, addition of Ang II to the IVM and/or day 4 of IVC media significantly increased the hatching rates in derived blastocysts. The expression of Na+/K+/ATPase α1 and β1 subunits in embryos significantly increased when the embryos were exposed to Ang II during IVC. Moreover, the positive effect of AngII on blastocyst cell number in embryos was observed in TE cells.
Acknowledgement
The authors would like to thank Avicenna Research Institute (grant no. 90–32) for financial support and providing the opportunity to conduct the study.
References
- 1. Honorato-Sampaio K, Pereira VM, Santos RA, Reis AM. Evidence that angiotensin-(1–7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp Physiol 2012; 97 (5): 642– 650. [DOI] [PubMed] [Google Scholar]
- 2. Stefanello JR, Barreta MH, Porciuncula PM, Arruda JN, Oliveira JF, Oliveira MA, et al. Effect of angiotensin II with follicle cells and insulin-like growth factor-I or insulin on bovine oocyte maturation and embryo development. Theriogenology 2006; 66 (9): 2068– 2076. [DOI] [PubMed] [Google Scholar]
- 3. Giometti IC, Bertagnolli AC, Ornes RC, da Costa LF, Carambula SF, Reis AM, et al. Angiotensin II reverses the inhibitory action produced by theca cells on bovine oocyte nuclear maturation. Theriogenology 2005; 63 (4): 1014– 1025. [DOI] [PubMed] [Google Scholar]
- 4. Muscella A, Aloisi F, Marsigliante S, Levi G. Angiotensin II modulates the activity of Na+, K+-ATPase in cultured rat astrocytes via the AT1 receptor and protein kinase C-delta activation. J Neurochem 2000; 74 (3): 1325– 1331. [DOI] [PubMed] [Google Scholar]
- 5. Min LJ, Mogi M, Iwai M, Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev 2009; 8 (2): 113– 121. [DOI] [PubMed] [Google Scholar]
- 6. Madan P, Rose K, Watson AJ. Na/K-ATPase beta1 subunit expression is required for blastocyst formation and normal assembly of trophectoderm tight junction-associated proteins. J Biol Chem 2007; 282 (16): 12127– 12134. [DOI] [PubMed] [Google Scholar]
- 7. Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol 1998; 275 (5 Pt 2): F633– 650. [DOI] [PubMed] [Google Scholar]
- 8. Lim JM, Hansel W. Exogeneous substances affecting development of in vitro-derived bovine embryos before and after embryonic genome activation. Theriogenology 2000; 53 (5): 1081– 1091. [DOI] [PubMed] [Google Scholar]
- 9. Tervit HR, Whittingham DG, Rowson LE. Successful culture in vitro of sheep and cattle ova. J Reprod Fertil 1972; 30 (3): 493– 497. [DOI] [PubMed] [Google Scholar]
- 10. Naderi MM, Sarvari A, Saviz A, Naji T, Borjian Boroujeni S, Heidari B, et al. The effect of Aldosterone on Na+/K+/ATPase expression and development of embryos derived from vitrified-warmed sheep oocytes. Small Ruminant Research 2015; 126: 44– 51. [Google Scholar]
- 11. Isenovic ER, Jacobs DB, Kedees MH, Sha Q, Milivojevic N, Kawakami K, et al. Angiotensin II regulation of the Na+ pump involves the phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways in vascular smooth muscle cells. Endocrinology 2004; 145 (3): 1151– 1160. [DOI] [PubMed] [Google Scholar]
- 12. Gomes CP, Leão-Ferreira LR, Caruso-Neves C, Lopes AG. Adenosine reverses the stimulatory effect of angiotensin II on the renal Na+-ATPase activity through the A2 receptor. Regul Pept 2005; 129 (1–3): 9– 15. [DOI] [PubMed] [Google Scholar]
- 13. Lara LS, De Carvalho T, Leão-Ferreira LR, Lopes AG, Caruso-Neves C. Modulation of the (Na(+)+K+)ATPase activity by Angiotensin-(1–7) in MDCK cells. Regul Pept 2005; 129 (1–3): 221– 226. [DOI] [PubMed] [Google Scholar]
- 14. Violette MI, Madan P, Watson AJ. Na+/K+ -ATPase regulates tight junction formation and function during mouse preimplantation development. Dev Biol 2006; 289 (2): 406– 419. [DOI] [PubMed] [Google Scholar]
- 15. Huang JC, Wun WS, Goldsby JS, Egan K, FitzGerald GA, Wu KK. Prostacyclin receptor signaling and early embryo development in the mouse. Hum Reprod 2007; 22 (11): 2851– 2856. [DOI] [PubMed] [Google Scholar]
- 16. Liszewska E, Reinaud P, Billon-Denis E, Dubois O, Robin P, Charpigny G. Lysophosphatidic acid signaling during embryo development in sheep: involvement in prostaglandin synthesis. Endocrinology 2009; 150 (1): 422– 434. [DOI] [PubMed] [Google Scholar]
- 17. Kim JS, Chae JI, Song BS, Lee KS, Choo YK, Chang KT, et al. Iloprost, a prostacyclin analogue, stimulates meiotic maturation and early embryonic development in pigs. Reprod Fertil Dev 2010; 22 (2): 437– 447. [DOI] [PubMed] [Google Scholar]
- 18. Wang H, Wen Y, Mooney S, Behr B, Polan ML. Phospholipase A(2) and cyclooxygenase gene expression in human preimplantation embryos. J Clin Endocrinol Metab 2002; 87 (6): 2629– 2634. [DOI] [PubMed] [Google Scholar]
- 19. Sen Roy S, Seshagiri PB. Expression and function of cyclooxygenase-2 is necessary for hamster blastocyst hatching. Mol Hum Reprod 2013; 19 (12): 838– 851. [DOI] [PubMed] [Google Scholar]
- 20. Sugiyama T, Yoshimoto T, Sato R, Fukai N, Ozawa N, Shichiri M, et al. Endothelin-1 induces cyclooxygenase-2 expression and generation of reactive oxygen species in endothelial cells. J Cardiovasc Pharmacol 2004; 44 Suppl 1: S332– 335. [DOI] [PubMed] [Google Scholar]
- 21. Sarvari A, Naderi MM, Sadeghi MR, Akhondi MM. A technique for facile and precise transfer of mouse embryos. Avicenna J Med Biotech 2012; 5 (1): 62– 65. [PMC free article] [PubMed] [Google Scholar]
- 22. Uhm SJ, Gupta MK, Chung HJ, Kim JH, Park Ch, Lee HT. Relationship between developmental ability and cell number of day 2 porcine embryos produced by parthenogenesis or somatic cell nuclear transfer. Asian-Australas J Anim Sci 2009; 22 (4): 483– 491. [Google Scholar]
- 23. Gupta MK, Uhm SJ, Han DW, Lee HT. Embryo quality and production efficiency of porcine parthenotes is improved by phytohemagglutinin. Mol Reprod Dev 2007; 74 (4): 435– 444. [DOI] [PubMed] [Google Scholar]