Abstract
Objective
Innate lymphoid cells (ILCs) are a newly discovered subset of immune cells that promote tissue homeostasis and protect against pathogens. ILCs produce cytokines also produced by T lymphocytes that have been shown to affect atherosclerosis, but the influence of ILCs on atherosclerosis has not been explored.
Approach and Results
We demonstrate that CD25+ ILCs that produce type 2 cytokines (ILC2s) are present in the aorta of atherosclerotic immunodeficient ldlr−/−rag1−/− mice. To investigate the role of ILCs in atherosclerosis, ldlr−/−rag1−/− mice were concurrently fed an atherogenic diet and treated with either ILC-depleting anti-CD90.2 antibodies or with IL-2/anti-IL-2 complexes that expand CD25+ ILCs. Lesion development was not affected by anti-CD90.2 treatment, but was reduced in IL-2/anti-IL-2 -treated mice. These IL-2 treated mice had reduced VLDL cholesterol and increased triglycerides compared to controls and reduced apolipoprotein B100 gene expression in the liver. IL-2/anti-IL-2 treatment caused expansion of ILC2s in aorta and other tissues, elevated levels of IL-5, systemic eosinophila and hepatic eosinophilic inflammation. Blockade of IL-5 reversed the IL-2-complex-induced eosinophilia but did not change lesion size.
Conclusions
This study demonstrates that expansion of CD25-expressing ILCs by IL-2/anti-IL-2 complexes leads to a reduction in VLDL cholesterol and atherosclerosis. Global depletion of ILCs by anti-CD90.2 did not significantly affect lesion size indicating that different ILC subsets may have divergent effects on atherosclerosis.
Keywords: Innate lymphoid cells, atherosclerosis, IL-5, eosinophils
INTRODUCTION
The development of atherosclerotic lesions and the destabilization of established lesions are promoted by local arterial and systemic inflammation driven by innate and adaptive immune responses. Interferon-γ (IFN-γ)-secreting TH1 cells enhance lesion development, but the influence of TH2 or TH17 cells is uncertain 1. Innate lymphoid cells (ILCs) have emerged as important effector cells in both protective immunity against pathogens and immune/inflammatory diseases 2, 3. Each subset of ILCs, type 1, 2 or 3, secretes a distinct group of cytokines. The pattern of cytokine production corresponds approximately to that of its T cell counterpart: ILC1s secrete Th1-related cytokines, ILC2s secrete Th2-related cytokines and ILC3s secrete Th17-related cytokines. The influence of each of these ILC subsets to atherosclerosis is largely unknown. Natural killer (NK) cells, which are cytotoxic and IFN-γ-secreting innate cells that are phenotypically related to the ILC1 subset, appear to promote atherosclerotic lesion development in mice 4. The contribution of non-cytotoxic interferon-γ secreting ILC1s has not been addressed. Group 2 ILCs secrete IL-4, IL-5, IL-9 and IL-13 in response to IL-25, IL-33 and TSLP. These cells play a role in metabolic homeostasis by reducing adiposity, a function mediated in part by IL-5-dependent eosinophil activation 5-7. As of yet, there is no ILC-specific knockout mouse line that does not also have deficiencies in other lymphocyte populations 8. However, injections of anti-CD90 antibodies selectively deplete CD90-expressing ILCs in V-D-J recombinase-1 or −2 (rag1 or rag2) deficient mice that lack all lymphocytes, and this strategy has been used to assess the role of ILCs in health and disease 9, 10.
It was recently shown that administration of IL-2 complexed with a particular anti-IL-2 antibody (clone JES6-1) to rag1−/− mice selectively allows expansion of CD25+ ILC2s 11. In immunocompetent mice, treatment of mice with IL-2/anti-IL-2 complexes expands regulatory T cells (Treg) 12, 13, and has been proposed as a therapeutic approach for autoimmunity, graft vs. host disease and allograft rejection 14. IL-2/anti-IL-2 complex therapy reduces atherosclerotic lesion development in mice 15, 16, but the effect of this therapy on ILC expansion in atherosclerosis models in not known.
In this study, we investigated the influence of ILCs on atherosclerotic lesion development. We used antibody-mediated global ILC depletion and IL-2/anti-IL-2-driven expansion in ldlr−/−rag1−/− mice, which are atherosclerosis prone but lack adaptive immune cells. We show that IL-5-producing ILCs are present in atherosclerotic aortas. Global depletion of all CD90+ ILCs, which include the majority of all three groups of ILCs, leads to a reduction in type 1, 2 and 3 cytokine production in the spleen, with no net effect on atherosclerotic lesion development. However, IL-2/anti-IL-2 treatment results in a marked increase in ILC2s, eosinophilia, reduced VLDL cholesterol levels and protection against atherosclerotic lesion development. The results highlight the potential role of therapeutic expansion of type 2 ILCs for the treatment of atherosclerotic vascular disease.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement
RESULTS
Aortic CD90+CD127+CD25+ innate lymphoid cells produce type 2 cytokines
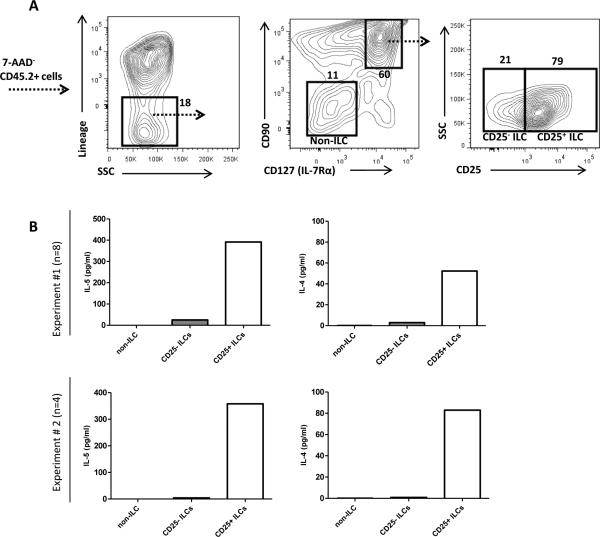
First we tested if hypercholesterolemia would influence levels of ILCs in the aorta. Mice were fed either high-fat diet (HFD) or chow diet for 10 weeks where after the aorta was digested and stained for the presence of ILCs, defined as CD45+lineage− (lin: CD11b, B220, Gr-1, CD3, CD5)CD90+CD127+ (Supplemental Fig. I A). As expected, the number of aortic CD45+ leukocytes was increased in atherosclerotic high-fat diet fed ldlr−/− mice (Supplemental Fig. I B). However, the number of CD90+CD127+ ILCs (Supplemental Fig. I C) or CD90+CD127+CD25+ ILCs(Supplemental Fig. I D) were not increased in the aorta of atherosclerotic high-fat diet fed ldlr−/− mice compared to chow fed ldlr−/− mice or C57BL/6 control mice. To further characterize these ILCs, we digested aortas from HFD-fed ldlr−/−rag1−/− mice. CD25+ and CD25− ILCs as well as CD90−CD127− non-ILCs were FACS sorted from aortic digests and stimulated with PMA and ionomycin (Supplemental Fig. II A). Supernatants were collected and analyzed for the presence of cytokines. Consistent with an ILC2 phenotype, lin−CD90+CD127+CD25+ cells produced the type 2 cytokines IL-4 and IL-5 (Fig. 1B) but not IFN-γ or IL-17 (Supplemental Fig. II B). We did not detect production of IL-13 from any sorted cell population (data not shown). Levels of splenic CD90+CD127+ or CD90+CD127+CD25+ ILCs in ldlr−/−rag1−/− were not affected by high-fat diet feeding (Supplemental Fig. II C-D).
Figure 1. Aortic CD25+ ILCs produce type 2 cytokines.
(A) Representative plot showing gating of aortic lin−CD90+CD127+ ILCs in rag1−/−ldlr−/− mice. Lin−CD90−CD127− (non-ILC), CD25− ILCs and CD25+ ILCs sorted by FACS and stimulated with PMA/ionomycin. (B) IL-5 and IL-4 in supernatants from stimulated cells were measured in two separate experiments (upper and lower panels, n=4-8 mice/experiment).
Depletion and expansion of ILCs in hypercholesterolemic mice
To evaluate the role of ILCs in atherosclerosis, we adopted two approaches previously used to study ILC depletion or expansion in the context of mouse models of immune defense and inflammatory diseases. For depletion, we treated ldlr−/−rag1−/− with anti-CD90.2 antibody (clone: 30H12), as described 10 (n=13). For expansion, we used anti-IL-2 antibody (clone: JES6-1)/IL-2 complexes that allow selective binding of IL-2 to CD25-expressing ILCs 11(n=12). A control group of mice was treated with a rat IgG2b antibody (n=13). Mice were fed a high fat, cholesterol containing diet (HFD) for seven weeks and injected twice a week with antibodies or IL-2 complexes for the last five weeks of HFD administration.
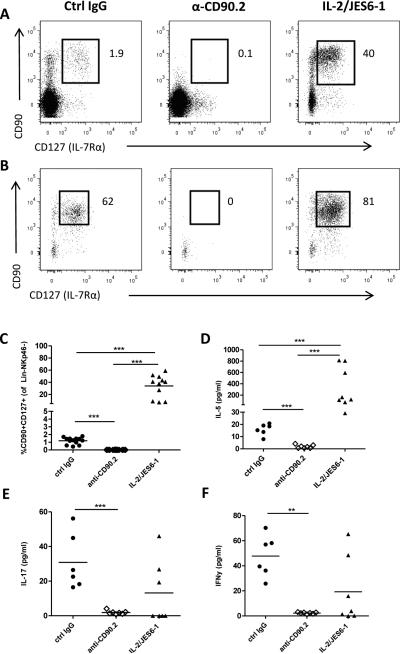
Treatment with anti-CD90.2 efficiently depleted lin−NKp46−CD90+CD127+ ILCs (see Supplemental Fig. III for gating strategy) in the spleen (Fig. 2A) and mesenteric lymph node (Figure 2B). Conversely, mice treated with IL-2/JES6-1 displayed markedly elevated levels of ILCs in secondary lymphoid organs (Fig. 2A-C). PMA/ionomycin-stimulated splenocytes from anti-CD90.2 treated mice did not produce ILC-related cytokines such as IL-5 (Fig. 2D), IL-17 (Fig. 2E) and IFN-γ (Fig. 2F). In contrast, PMA/ionomycin stimulated splenocytes from IL-2/JES6-1 treated mice produced elevated levels of IL-5 but equivalent levels of IFN-γ and IL-17 compared to controls (Fig. 2D-F), indicating a selective ILC2 expansion in the spleen.
Figure 2. Depletion and expansion of ILCs by anti-CD90.2 or IL-2/JES6-1 treatment.
Representative flow cytometric staining of lineage-CD90+CD127+ ILCs in (A) spleen or (B) mesenteric lymph nodes in rag1−/−ldlr−/− treated with control IgG, anti-CD90.2 or IL-2/JES6-1. (C) Quantification of splenic ILCs comparing treatments. Supernatants from PMA/ionomycin treated spleens were analyzed for (D) IL-5, (E) IL-17 and (F) IFNγ content. ** p<0.01,*** p<0.001
Treatment with IL-2/anti-IL-2 complexes increases ILC2s in perivascular adipose tissue
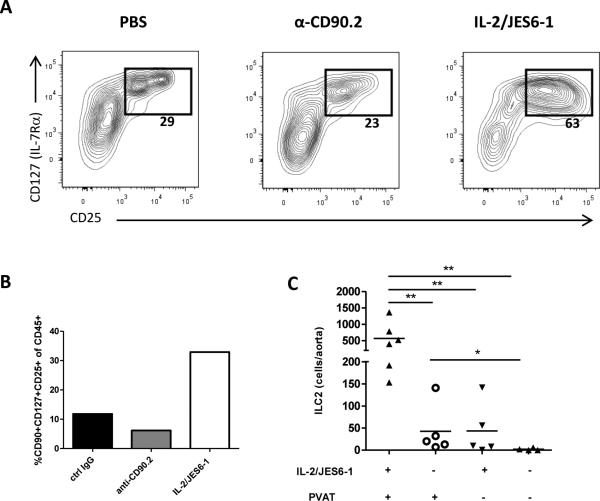
Previous studies have shown that dermal ILC2s are resistant to depletion by anti-CD90.2 treatment 11. To determine if we were able to deplete ILCs in the aorta, in a separate cohort, we injected HFD-fed ldlr−/−rag1−/− mice with PBS, anti-CD90.2 or IL-2/JES6-1 for two weeks, and analyzed cell in the aorta by flow cytometry. The percentage of aortic CD127+CD25+ ILC2s was only slightly reduced by anti-CD90.2 treatment (29% vs. 23% CD127+CD25+ of lin− cells; Fig. 3A). The CD127+CD25+ cells isolated from anti-CD90.2 treated mice displayed reduced levels of CD90 expression (Supplemental Fig. IV A). Aortic CD90dimCD127+CD25+ cells from anti-CD90.2 treated mice were sorted and stimulated with PMA/ionomycin. After 24h of stimulation, cytokines in supernatant were measured. These cells produced IL-5 and IL-4, but no detectable levels of IFN-γ or IL-17, indicating an ILC2 phenotype (Supplemental Fig. IV B-E). This suggests that anti-CD90.2 treatment reduces but does not completely deplete ILC2s in the aortic tissue.
Figure 3. IL-2/JES6-1 treatment expands ILC2s in the aorta of rag1−/−ldlr−/− mice.
(A) Percentages of aortic lin−CD25+CD127+ cells and (B) CD90+CD127+CD25+ cells compared between groups (3-4 mice/pool). (C) Comparison of ILC2 (lin−CD90+CD25+ST2+) cells from mice treated with or without IL-2/JES6-1 in aortas with or without perivascular adipose tissue (PVAT). * p<0.05, ** p<0.01,*** p<0.001.
Notably, IL-2/JES6-1 injections increased the proportion of CD127+CD25+ ILCs in the aorta (Fig. 3A, 3C). Next we investigated localization of expanded ILC2s in the tissue. Cell suspensions of aortas with or PVAT removed were stained. ILC2s (lin−CD90+CD25+ST2+) were primarily found in aortic PVAT, although we observed a minor increase of ILC2s in aorta stripped from PVAT after treatment with IL-2/JES6-1 (Fig. 3C).
IL-2/anti-IL-2 complex treatment reduces atherosclerosis
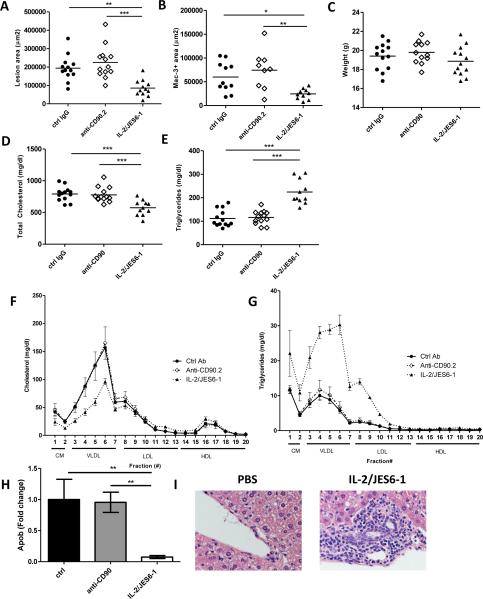
Aortic roots from mice fed HFD for seven weeks were sectioned and aortic sinus lesions were analyzed. Strikingly, mice treated with IL-2/JES6-1 had significantly reduced lesion size (85,608± 12 744 μm2) compared to either control IgG (194,121± 19,015 μm2) or anti-CD90.2 (225,220± 24 237 μm2) treated mice (Fig. 4A). Lesional area positive for macrophages (Mac-3+ area) was also reduced in IL-2 complex-treated mice (Fig. 4B, p<0.05). Although lesion size was reduced by IL-2/JES6-1, the percentage of macrophages, collagen or neutral lipids (Oil Red O) did not differ between groups (Supplemental Fig. V A-F). There was no significant effect on lesion size or composition after anti-CD90.2 treatment compared to control IgG treated mice.
Figure 4. IL-2/JES6-1 treatment reduces atherosclerosis and affects serum cholesterol and triglyceride levels.
Mice were fed high-fat diet (HFD) for seven weeks and treated with control IgG, anti-CD90.2 or IL-2/JES-1 for the last five weeks (n=12-13). (A)Lesion area and (B) macrophage area of lesions in aortic sinuses were quantified. (C) Weight, (D) total cholesterol and (E) triglycerides were measured in serum. High-performance liquid chromatography of serum for (F) cholesterol and (G) triglycerides (n=3/treatment). (H) Apolipoprotein B (Apob) mRNA expression in liver (n=7-11). In a follow-up experiment, mice were fed HFD for seven weeks and treated with PBS or IL-2/JES6-1 for the last four weeks (n=10-11). (I) H&E staining of liver sections (40×). * p<0.05, ** p<0.01,*** p<0.001.
IL-2/anti-IL-2 complex treatment reduces serum VLDL levels and is associated with reduced hepatic apolipoprotein B expression
We did not observe weight changes (Fig. 4C) as a consequence of either ILC depletion or expansion. However, injection of IL-2/JES6-1 caused a change in lipid profile. Levels of total cholesterol (Fig. 4D) were decreased while triglycerides (Fig. 4E) were increased in IL-2/JES6-1 treated mice. HPLC analysis revealed that the reduction in cholesterol was located in the chylomicron and VLDL fraction, while LDL and HDL levels were similar between groups (Fig. 4F). The increased levels of triglycerides were mainly located in the VLDL compartment (Fig. 4G). To test if expansion of CD25+ ILCs impacted lipoprotein production, we measured genes associated with lipid metabolism. Transcription of apolipoprotein B (apob) was markedly reduced in the livers from IL-2/JES6-1 treated mice (Fig. 4H), while hepatic lipase (lipc), apolipoprotein C-III (apoc3) and the triglyceride synthesizing enzyme diglyceride acetyltransferase (dgat) were expressed at similar levels between groups (Supplemental Fig. VI A-C).
Previous studies have demonstrated that type 2 ILCs may promote hepatic fibrosis 17. To address this we fed mice HFD for seven weeks and treated with PBS or IL-2/JES6-1 for the last four weeks. Liver was sectioned and stained with H&E as well as Masson's trichrome to detect collagen deposition. Strikingly, we found that mice treated with IL-2/JES6-1 displayed marked hepatic eosinophilic and lymphoid inflammation (Fig. 4I). Leukocyte accumulation was mainly found in portal areas (Supplemental Fig. VI D). Moreover, we observed moderate fibrosis in areas with intense leukocyte accumulation (Supplemental Fig. VI D) in IL-2/JES6-1 treated mice. The amount of ILC2s in liver digests was increased after IL-2 complex treatment (Supplemental Fig VI E).
To assess liver function, we measured soluble markers of liver damage in serum. Levels of aspartate aminotransferase were similar between groups but alanine aminotransferase was increased by IL-2 complex treatment (Supplemental Fig. VI F-G). ILC2s have previously been associated with regulation of adipose tissue 18. Accordingly, mice treated with IL-2/anti-IL-2 complexes displayed reduced epididymal visceral adipose tissue (VAT) weight (Supplemental Fig. VI H) after high-fat diet feeding for seven weeks.
High serum levels of IL-5 and eosinophilia in mice treated with IL-2/JES6-1
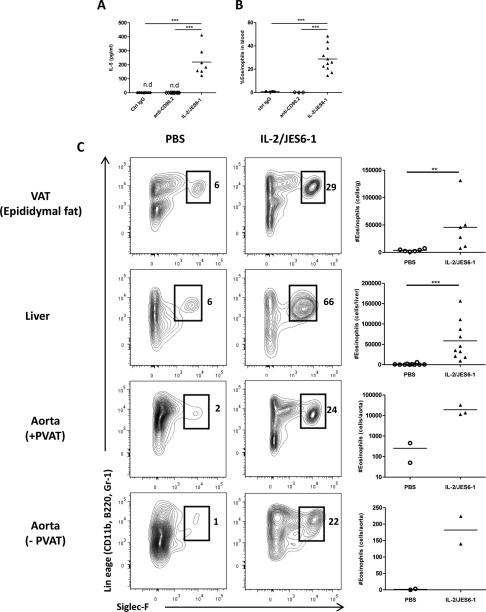
We measured the serum levels of cytokines in order to determine if either IL-2/JES6-1 or anti-CD90.2 treatment had systemic inflammatory effects. Serum levels of IFN-γ, IL-17, TNFα (not detected), MCP-1, IL-4 or IL-13 were not impacted by depletion or expansion of CD25+ ILCs (Supplemental Fig. VII A-E), although we observed a slight increase in IL-6 in mice treated with IL-2/JES6-1 (Supplemental Fig. VII F). Strikingly, treatment with IL-2/JES6-1 resulted in high levels of IL-5 in serum (Fig. 5A) and increased levels of eosinophils in blood (Fig. 5B).
Figure 5. IL-2/JES6-1 treatment causes high levels of IL-5 and recruitment of eosinophils to perivascular adipose tissue and liver.
(A) Levels of IL-5 (pg/ml) in serum and (B) eosinophils in blood of mice treated with control IgG, anti-CD90.2 or IL-2/JES6-1. (C) Eosinophils were quantified in visceral adipose tissue (VAT), liver and aorta ± perivascular adipose tissue (PVAT). Numbers indicate percentages of eosinophils (lin+Siglec-F+) of live CD45+ leukocytes. ** p<0.01,*** p<0.001.
We further investigated whether IL-2/anti-IL-2 complex treatment led to accumulation of eosinophils in tissues. Leukocyte fractions from tissues were isolated and analyzed by flow cytometry. Eosinophil accumulation to VAT and liver (Fig. 5C) was dramatically enhanced by IL-2/anti-IL-2treatment. Further, eosinophil accumulation in the aorta was increased 75-fold by IL-2/JES6-1 treatment (Fig. 5C) and 99% of the eosinophils were located in PVAT (182 ± 59 eosinophils in aorta without PVAT, 19064 ± 11400 eosinophils in aorta with PVAT).
IL-5 blockade inhibits IL-2 complex-induced eosinophilia but not affect atherosclerosis
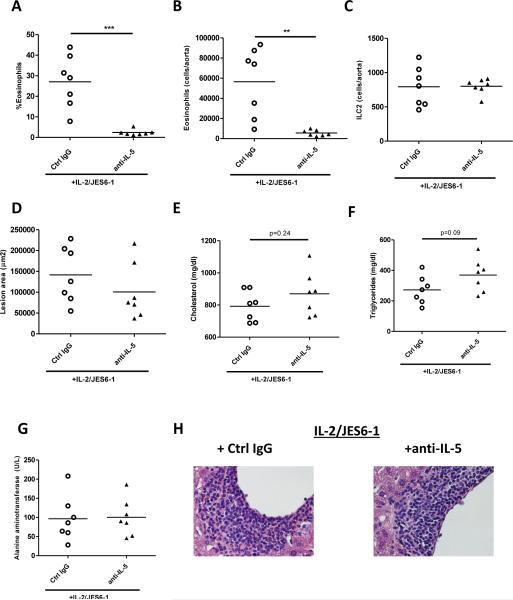
We hypothesized that the high levels of IL-5 induced after IL-2 complex injections affected atherosclerosis. To investigate this, mice (ldlr−/−rag1−/−) were fed high-fat diet for seven weeks and treated for the last four weeks with IL-2 complexes supplemented with anti-IL-5 (clone: TRFK5) or an isotype control (n=7/group). Anti-IL-5 efficiently blocked eosinophilia in IL-2/anti-IL-2 treated mice (>90% reduction, Fig. 6A) as well blocked eosinophil accumulation in the atherosclerotic aorta (>90% reduction, Fig. 6B). Moreover, accumulation of eosinophils was found to be reduced in the liver or the epididymal adipose tissue (Supplemental Fig. VIII A-B). Anti-IL-5 treatment did not affect levels of aortic ILC2s (Fig. 6C).
Figure 6. Co-administration of anti-IL-5 with IL-2 complexes abrogates ILC-mediated eosinophil accumulation to the atherosclerotic aorta but does not affect lesion size or hepatic inflammation.
Mice were fed high-fat diet for seven weeks and treated for the last four weeks with IL-2/JES6-1 combined with either anti-IL-5 or an isotype control. Levels of eosinophils in (A) blood and (B) number of eosinophils in the atherosclerotic aorta comparing groups. (C) Frequency of aortic ILC2 (lin−CD90+CD127+CD25+ST2+). (D) Lesion area in the aortic root and levels of (E) triglycerides and (F) cholesterol. (G) Serum levels of alanine aminotransferase. (H) H&E staining of liver sections (40× magnification). n=7/group. ** p<0.01,*** p<0.001.
Aortic sinus lesions were comparable between mice treated with IL-2/anti-IL-2 complexes plus either control IgG (141 594± 25 343 μm2) or anti-IL-5 (100 582± 25 470 μm2; Fig. 6D, p=0.2) and cholesterol levels were similar (Fig. 6E). The serum levels of triglycerides was higher in the anti-L5 treated group, but the difference did not reach statistical significance (Fig. 6F). No difference in body weight or epididymal VAT was observed (Supplemental Fig. VIII C-D). Notably, while eosinophils were dramatically reduced in the liver, levels of alanine aminotransferase (Fig. 6G) and aspartate aminotransferase (Supplemental Fig. VIII E) and hepatic inflammation assessed by histology (Fig. 6H, Supplemental Fig. VIII F) were similar between the two treatment groups.
DISCUSSION
ILCs have been shown to play important roles in inflammatory diseases and in maintaining barrier homeostasis. Previous work has demonstrated that several cytokines influence atherosclerotic disease in different ways 1. Many of these cytokines, previously considered to be TH-cell derived have recently been shown to be produced by ILCs. Our study demonstrates that ILCs, in particular CD25+ ILC2s, can influence atherosclerosis. A few recent studies have indirectly suggested a role for ILCs in atherosclerosis. The ILC2 activating cytokines IL-25 19 and IL-33 20 have been shown to reduce atherosclerosis. Also, the transcription factor Id3 was shown to regulate IL-5 production of lin−Sca1+CD117+CD90+ ILCs present in the aorta of atherosclerotic mice 21. However, this is the first study to directly examine the effects of ILC expansion or depletion on atherosclerosis.
We demonstrate that IL-5-producing CD25+ ILCs are present in atherosclerotic mouse aortas. These cells are expanded by treatment of mice with IL-2/anti-IL-2 complexes, inhibiting atherosclerotic lesion formation. IL-5 has been shown to be atheroprotective by promoting the secretion of natural antibodies by B-1 cells 22. As ldlr−/−rag1−/− mice lack B-1 cells, our model allows us to study the B-1 cell independent effects of IL-5. One such effect is the role for IL-5 in inducing mobilization and proliferation of eosinophils. We found that IL-2/JES6-1 treatment generated a marked increase in circulating and tissue eosinophils. However, blockade of IL-5 in mice given IL-2 complexes did not significantly affect atherosclerosis. This intriguing finding suggests that CD25+ ILCs may act in an IL-5 independent manner to limit atherosclerotic burden.
The atheroprotective potential of ILC2s in our study likely relates in part to alterations in lipid metabolism. Expansion of CD25-expressing ILCs resulted in reduced VLDL cholesterol levels. This atheroprotective phenotype was associated with reduced expression of the Apob gene in the liver. However, mRNA levels of Lipc, Dgat2 or Apoc3 were not impacted by IL-2/JES6-1 treatment, showing that other aspects of liver function were intact. As IL-5 blockade did not affect cholesterol levels, the cholesterol-lowering effect of IL-2 complexes may possibly be mediated by IL-13, other ILC2 related soluble mediators or by cell-cell dependent mechanisms. Arguing against a potential role of IL-13 in the current study is the fact that IL-2 complex treatment did not increase levels of IL-13 in serum, although it is difficult to estimate how well local production of IL-13 in the tissue is reflected in the circulation. Also, we cannot completely exclude the role of other CD25-expressing cells in affecting cholesterol. Surprisingly, the IL-2/JES6-1 treated mice exhibited elevated triglyceride levels. Others have shown that deficiency in IL-5 production results in increased adiposity 23. Conversely, we observed a decrease in epididymal VAT in mice treated with IL-2/anti-IL-2 complexes, which have high levels of circulating IL-5. Since anti-IL-5 appeared no to fully block the rise in triglycerides in high fat diet-fed ldlr−/−rag1−/− treated with IL-2/anti-IL-2 complexes, thus the elevated numbers of eosinophils may not be responsible for the IL-2/anti-IL-2 effect on serum triglycerides. Possibly, ILC2 activation compromises storage of triglyceride in adipose tissue, leading to high levels of triglycerides in the serum, through an IL-5 independent mechanism. Altogether our data suggests that ILC2s may affect atherosclerosis primarily by influencing metabolism rather than acting directly in the lesion. However, further investigation is required to determine if there are direct atheroprotective effects of ILC2s within lesions, such as by influencing macrophage phenotype. We found that HFD did not influence numbers of ILCs in the aorta or percent ILCs in the spleen. However, the proportion of CD25+ ILCs to total leukocytes was reduced in the atherosclerotic artery, indicating that relative levels of anti-atherogenic CD25+ ILCs drop as atherosclerosis progresses.
The role of eosinophils in atherosclerosis has not yet been addressed in experimental models. Human carotid plaques were found to have expression of eotaxin and CCR3 but no significant levels of eosinophils were detected18. We found that most eosinophils were localized to the PVAT and not to actual lesion, indicating that eosinophils do not influence lesion development through actions within the plaque. Further studies utilizing eosinophil-deficient mice models are needed to further understand the role of eosinophils in atherosclerosis development.
Treatment with IL-2/JES6-1 immune complexes has been shown to reduce atherosclerosis in ldlr−/− mice, which has been attributed to the expansion of regulatory T cells 15, 16. The ldlr−/−rag1−/− mouse lacks Tregs, and the only known CD25+ cells that will respond to IL-2/JES6-1 are ILCs 11. Thus, our findings suggest that part of the effect of IL-2/JES6-1 treatment observed in these studies may be due to expansion of CD25-expressing ILCs. It should be noted that expansion of ILCs is likely more pronounced in ldlr−/−rag1−/− than in immunocompetent mice because of reduced competition for available IL-2 complexes in the absence of regulatory T cells.
Given the atheroprotective effect of IL-2/JES6-1 treatment, it may be considered surprising that depletion of ILCs by anti-CD90.2 did not affect lesion size or composition. There are several possible explanations for this finding. First, depletion of ILCs by anti-CD90.2 injections removes several different classes of ILCs with potentially opposite effects on atherosclerosis. It is conceivable, given knowledge from cytokine knockout mice 24, 25, that ILC1s, ILC3s and CD90-expressing NK cells may promote atherosclerosis while ILC2s may be atheroprotective. The role of these subsets needs to be addressed in future studies. Second, treatment of mice with CD90 effectively depleted all splenic cells capable of secreting these cytokines, but failed to remove all ILC2s from the aortic wall. This is consistent with previous reports of failure of anti-CD90.2 treatment to deplete dermal ILC2s 11. It is possible that aortic ILCs, rather than lymphoid organ resident ILCs, determine atherosclerotic progression in ldlr−/−rag1−/− mice. Third, to avoid infections, our ldlr−/−rag1−/−mice were given antibiotics from birth and for the duration of the experiment. Finally, our study does not address the potentially important interaction of ILCs with T-cells and B-cells.
In summary, we demonstrate that expansion of CD25-expressing ILCs, including ILC2s, results in atheroprotection in ldlr−/−rag1−/− mice. Our study highlights the potential of ILC modulation as a pharmacological target for the treatment of cardiovascular disease and demonstrates the interaction between innate type 2 immunity and metabolism.
Supplementary Material
SIGNIFICANCE.
Atherosclerosis is an inflammatory disease of the major arteries and a major cause of myocardial infarction and stroke. Leukocytes are important in regulating the disease and immune pathways are considered potential targets of next-generation pharmaceuticals for the treatment of cardiovascular disease. In this study we investigated the role of a newly discovered leukocyte subset termed innate lymphoid cells (ILCs) in a mouse model of atherosclerosis.
We either depleted or expanded ILCs in order to study the effect on atherosclerosis. Although ILC-depletion did not significantly affect atherosclerosis, expansion of CD25-expressing ILCs led to a dramatic reduction of atherosclerosis and was associated with reduced VLDL cholesterol levels and increased levels of the type 2 cytokine IL-5. However, the role of ILCs in affecting atherosclerosis was, in our experimental setting, not contingent on IL-5. We propose that expansion of type 2 ILCs may have beneficial effects on atherosclerosis through various mechanisms, including lipid metabolism.
Acknowledgments
SOURCES OF FUNDING
This work was supported by National Institutes of Health grant HL087282 (AHL), and fellowships from the Swedish Heart-Lung Foundation and the Tegger Foundation (DE).
Abbreviations
- Dgat
Diglyceride acetyltransferase
- FACS
Fluorescence activated cell sorting
- HFD
High-fat diet
- IL
Interleukin
- IL-2/JES6-1
IL-2-anti-IL-2 complex
- ILC
Innate lymphoid cell
- IFN
Interferon
- PMA
Phorbol 12-myristate 13-acetate
- PVAT
Perivascular adipose tissue
- rag1
Recombination activating gene 1
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Ann Rev Path. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: New insights into function and development. Curr Opin Immunol. 2015;32C:71–77. doi: 10.1016/j.coi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, To K, Kanellakis P, Hosseini H, Deswaerte V, Tipping P, Smyth MJ, Toh BH, Bobik A, Kyaw T. Cd4+ natural killer t cells potently augment aortic root atherosclerosis by perforin- and granzyme b-dependent cytotoxicity. Circ Res. 2015;116:245–254. doi: 10.1161/CIRCRESAHA.116.304734. [DOI] [PubMed] [Google Scholar]
- 5.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–6. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashiguchi M, Kashiwakura Y, Kojima H, Kobayashi A, Kanno Y, Kobata T. Il-33 activates eosinophils of visceral adipose tissue both directly and via innate lymphoid cells. Eur J Immunol. 2015;45:876–8. doi: 10.1002/eji.201444969. [DOI] [PubMed] [Google Scholar]
- 8.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate cd4+ t-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roediger B, Kyle R, Yip KH, Sumaria N, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of t reg cells with il-2–mab complexes: Induction of resistance to eae and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of t cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 14.Bayer AL, Pugliese A, Malek TR. The il-2/il-2r system: From basic science to therapeutic applications to enhance immune regulation. Immunol Res. 2013;57:197–209. doi: 10.1007/s12026-013-8452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foks AC, Frodermann V, ter Borg M, Habets KL, Bot I, Zhao Y, van Eck M, van Berkel TJ, Kuiper J, van Puijvelde GH. Differential effects of regulatory t cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, Bobik A, Agrotis A. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands cd4+cd25+foxp3+ regulatory t cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–1266. doi: 10.1161/CIRCULATIONAHA.112.099044. [DOI] [PubMed] [Google Scholar]
- 17.McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF, Pflanz S, Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, Thompson JF, Sukhova GH, Libby P, Lee RT. Overexpression of eotaxin and the ccr3 receptor in human atherosclerosis: Using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. doi: 10.1161/01.cir.102.18.2185. [DOI] [PubMed] [Google Scholar]
- 19.Mantani PT, Duner P, Bengtsson E, Alm R, Ljungcrantz I, Soderberg I, Sundius L, To F, Nilsson J, Bjorkbacka H, Fredrikson GN. Il-25 inhibits atherosclerosis development in apolipoprotein e deficient mice. PloS one. 2015;10:e0117255. doi: 10.1371/journal.pone.0117255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. Il-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry HM, Oldham SN, Fahl SP, Que X, Gonen A, Harmon DB, Tsimikas S, Witztum JL, Bender TP, McNamara CA. Helix-loop-helix factor inhibitor of differentiation 3 regulates interleukin-5 expression and b-1a b cell proliferation. Arterioscler Thromb Vasc Biol. 2013;33:2771–2779. doi: 10.1161/ATVBAHA.113.302571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. Il-5 links adaptive and natural immunity specific for epitopes of oxidized ldl and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The il-17a/il-17ra axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the ldlr-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.