Abstract
The universal tRNA modification t6A is found at position 37 of nearly all tRNAs decoding ANN codons. The absence of t6A37 leads to severe growth defects in baker’s yeast, phenotypes similar to those caused by defects in mcm5s2U34 synthesis. Mutants in mcm5s2U34 can be suppressed by overexpression of tRNALysUUU, but we show t6A phenotypes could not be suppressed by expressing any individual ANN decoding tRNA, and t6A and mcm5s2U are not determinants for each other’s formation. Our results suggest that t6A deficiency, like mcm5s2U deficiency, leads to protein folding defects, and show that the absence of t6A led to stress sensitivities (heat, ethanol, salt) and sensitivity to TOR pathway inhibitors. Additionally, L-homoserine suppressed the slow growth phenotype seen in t6A-deficient strains, and proteins aggregates and Advanced Glycation End-products (AGEs) were increased in the mutants. The global consequences on translation caused by t6A absence were examined by ribosome profiling. Interestingly, the absence of t6A did not lead to global translation defects, but did increase translation initiation at upstream non-AUG codons and increased frame-shifting in specific genes. Analysis of codon occupancy rates suggests that one of the major roles of t6A is to homogenize the process of elongation by slowing the elongation rate at codons decoded by high abundance tRNAs and I34:C3 pairs while increasing the elongation rate of rare tRNAs and G34:U3 pairs. This work reveals that the consequences of t6A absence are complex and multilayered and has set the stage to elucidate the molecular basis of the observed phenotypes.
Keywords: t6A, tRNA, ribosome profiling, translation, modified nucleosides
INTRODUCTION
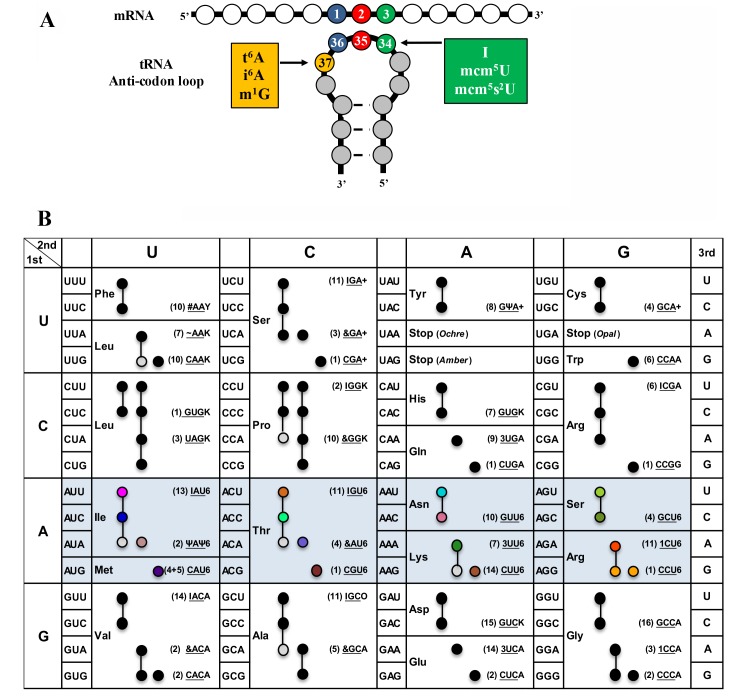
Modifications of the anticodon stem loop (ASL) of transfer RNA (tRNA) are critical for translational speed and accuracy. As the genetic code is degenerate, most tRNAs decode several codons 1. Nucleoside modifications ensure that the decoding process is stringent enough to discriminate between closely-related codons and yet relaxed enough to allow decoding of more than one codon 2,3. Different organisms use distinct but convergent strategies to optimize speed and accuracy of decoding by modifying specific tRNAs, predominantly at position 34 (the wobble base) and at position 37 (the dangling base) of the ASL (Figure 1A) 2,3. Modifications at position 34, such as ncm5U34 (5-carbamoylmethyluridine) or I34 (inosine) can expand decoding capacity, thereby allowing one tRNA to decode three synonymous codons 2,3,4. Likewise, most modifications at position 37 are critical for decoding, and their role can be complex. For example, i6A37 (N6-isopentenyladenosine) promotes decoding activity and increases fidelity of tRNACysGCA at its cognate codon, but also increases the misreading rate of tRNATyrGUA at the near-cognate UGC codon, which makes the effects of this modification on protein expression difficult to disentangle 5. In fact, the exact in vivo contributions of many ASL modifications to translational robustness are still poorly understood 6.
Figure 1. FIGURE 1: (A) Complex modifications found in the anticodon stem loop (ASL) of tRNA. (B) Codon table with decoding tRNAs, based on Johansson et al. 47.
Blue highlighted cells are decoded by t6A modified tRNAs in S. cerevisiae. In parenthesis is the number of genomic copies of that tRNA followed by the anticodon (underlined) and base at position 37. Black, grey, and colored circles indicate a codon decoded by that tRNA predicted by the wobble hypothesis, with grey indicating a tRNA less likely to decode that codon, and colors are matching those in Figure 6 and 7. For AUG, four genes code for tRNAiMet, and five code for tRNAeMet. Modification symbols are from Modomics 44. Ψ – pseudouridine, & - ncm5U, I – inosine, 3 – mcm5s2U, 1 – mcm5U, # – Gm, ~ – ncm5Um, Y – wybutosine, K – m1G, 6 – t6A, + – i6A, and O – 1-methylinosine.
Codon usage bias allows for fine-tuning of translation by ASL modifications. Codon choice (which codon in a synonymous set is used to encode a given amino acid) affects gene expression levels, protein production, accuracy, protein folding 7,8,9,10, and can even be used to predict gene function 11. Codon usage bias is not only driven by neutral processes such as mutation biases or GC%, but is also molded by selection 9,12. Although translation speed is a strong driving force of codon usage 9, the avoidance of codons with higher propensity for protein synthesis errors leading to misfolding is also an important factor in codon selection 13,14. ASL modifications play key roles in both these processes 6,7. In addition, systems level approaches integrating proteomics, codon usage, and modification profiling 15 have recently shown that tRNA modifications can modulate the expression of specific genes including stress-responsive genes 16,17. These tRNA modification tunable transcripts (MoTTs) respond to the proportion of modified tRNAs and regulate translation in response to cellular stress 18.
Threonyl-carbamoyl-adenosine (t6A) is a complex universal modification found at position 37 of nearly all ANN decoding tRNAs, as shown in Figure 1B (for an in depth review of t6A synthesis in all domains of life, see Thiaville et al. 19). t6A is formed in a two-step mechanism, where, in the cytoplasm of eukaryotes, the threonyl-carbamoyl-AMP (TC-AMP) intermediate is produced by Tcs1 or Tcs2 (previously named YrdC and Sua5, respectively) 20,21,22. TC-AMP is placed on tRNA by the threonyl-carbamoyl transferase complex (TCTC, previously referred to as KEOPS or EKC complex) made up of Tcs3 (Kae1), Tcs5 (Bud32), Tcs6 (Pcc1), and Tcs7 (Cgi121), whereas fungi have an additional member Tcs8 (Gon7)] 23,24,25. Yeast mitochondria use a minimum synthesis system to produce t6A-modified tRNAs, consisting of a mitochondrial-targeted Tcs2 and Tcs4 (Qri7) 26,27.
In yeast, the absence of t6A synthesis enzymes has been linked to many phenotypes including telomere shortening 28,29, transcription regulation defects 30, and respiration deficiency 31,32,33. The molecular basis for these pleiotropic phenotypes is far from understood, although it is expected that they should relate to translational defects in absence of t6A. In addition to the aberrant mis-initiation observed in the TCS2 mutant when the gene was discovered 34, the deletion of the TCS2 and TCS3 results in an increase in +1 and -1 frameshifts, as well as to mis-initiation at CUG codons of specific reporter genes 20,23. Further studies linked loss of TCS2 with increases in leaky scanning bypass of start codons, +1 frameshifts, read-through of UAG, UAA, and UGA stop codons, and an increase in internal ribosome entry site translation (IRES-dependent initiation of translation) 33. Polysome profiles of TCS2-depleted strains (PTET::TCS2, this strain requires doxycycline for expression of TCS2) revealed abnormal ribosome assembly, which could not be rescued by over-expressing the ternary complex (TC; eIF2α, -β, -γ, and Met-tRNAiMet), contrary to previously reported cases of ribosome assembly defects caused by inhibition of other tRNA modifications 33. Similarly, the over-expression of either TC or tRNAiMet (IMT4) did not rescue the slow-growth phenotype in absence of t6A 33. However, depletion of TCS2 leads to increased levels of the transcriptional activator GCN4, although in a non-canonical manner (Gcd– phenotype) 33. GCN4 is a positive regulator of genes expressed during amino-acid starvation, and is dependent on eIF2α phosphorylation by Gcn2, which monitors uncharged tRNAs 35,36. Over-expression of tRNAiMet or deletion of GCN2 did not reduce the high levels of GCN4 in a TCS2-depletion background 33. Paradoxically, GCN4 induction in the TCS2-depleted strain was independent of Gcn2 phosphorylation 33. In yeast, Gcn4 is also regulated at the translational level by four upstream open reading frames (uORFs), where the scanning ribosome initiates translation at the first AUG in the uORF leading to bypass of initiation at the AUG of the downstream ORFs 37. TCS2-depletion led to increased translation of the main ORF (GCN4) by bypassing the regulatory uORFs 33. Over-expression of TC or tRNAiMet did not reduce the leaky scanning seen in TCS2-depletion 33. Interestingly, mutations of Tcs3, Tcs5, and Tcs8 in yeast also increased GCN4 translation 38.
Evidence has emerged that some tRNA modifications can act as determinants of subsequent tRNA modification enzymes. Recently, the requirements of 2’-O-methylation of C32 and N34 has been linked to efficient wybutosine formation at m1G37 of tRNAPhe, a circuitry conserved from yeast to man 39,40,41. Additionally, in bacteria, presence of the t6A modification increases the efficiency of formation of the essential modification lysidine at U34 of tRNAIleCAU, and t6A is required for the charging of tRNAIle by IleRS 42,43. In yeast, parallels can also be made between t6A and 5-methoxycarbonylmethyliouridine (mcm5U) and its thiolated derivative (mcm5s2U) found at position 34 of several tRNAs. Both t6A and mcm5s2U modify tRNALysUUU, and mcm5U and t6A are found on tRNAArgUCU 44. Trm9 and Elp1-6 (Elongator complex) synthesise the mcm5 moiety, and the Ncs2/Ncs6 enzymes are responsible for thiolation 45,46. Deficiencies in mcm5s2U synthesis lead to slow growth, the inability to grow on non-fermentable carbon sources, and telomere shortening 45,47,48,49,50,51, which are similar to phenotypes seen in t6A biosynthesis mutants 20,23,29,33,34,38,52,53. Over-expression of a single tRNA, tRNALysUUU, suppresses all of the mcm5s2U phenotypes, and additional data suggest that mcm5s2U acts as a codon-dependent regulator of translation 52,53. Why elimination of mcm5s2U or t6A lead to similar phenotypes is unknown 2. One possibility is that the modification of A37 to t6A is required for the formation of the x5s2U derivatives, or vice versa, which has never been explored to date.
Recently, both mcm5s2U and t6A have been associated to alterations of two central cell regulatory systems; the General Amino Acid Control system (GAAC) through activation of GCN4 38,54, which regulates > 1500 genes in response to nutritional cues 35, and Target of Rapamycin Complex (TORC), through alterations in Tor kinase activity 55,56,57,58 (reviewed in Thiaville and de Crécy-Lagard 59). Modulating the levels of t6A in Drosophila through expression of an unmodifiable tRNAiMet or overexpression of TCS3 led to alterations of Tor activity and changes in whole organism growth 56. Additionally, knock-down of Tcs3 (Kae1) or Tcs5 (Bud32) in Drosophila larvae activated the Unfolded Protein Response (UPR) 55.
Recent ribosome profiling studies of mutations in the mcm5s2U pathway (ncs6∆ and uba4∆) grown under nutrient-depleted conditions revealed pausing and accumulation of ribosomes at GAA, AAA, and CAA codons 54. Follow up studies also found codon-specific ribosome pausing in the absence of mcm5s2U (ncs2∆elp6∆), even in the absence of stress 60. Hypo-modified tRNAs cause slower decoding at GAA, AAA, and CAA codons that led to protein misfolding and aggregation of essential proteins, which prevent the cell from maintaining protein homeostasis during stressful events 60.
In this study, we sought to uncover the translational defects seen in a t6A deficient strain and to determine if there is a relationship between mcm5s2U and t6A. So far, the links between t6A and translation fidelity have been based on single gene reporters. Here, we sought to assess the functional impact of t6A at the genome scale, by combining genome-wide ribosome profiling and bioinformatics tools to catalogue all translational ambiguities in tcs2∆ and investigate the differential effects of t6A on distinct tRNAs.
RESULTS
mcm5s2U34 or t6A37 are not determinants for each other’s synthesis
The similarity of the phenotypes observed in strains deficient in mcm5s2U and t6A synthesis suggests that one of the modifications could be required for the synthesis of the other. To test this hypothesis, tRNAs from wild type (BY4741), mcm5s2U-deficient yeast strains (elp3∆, trm9∆, ncs2∆, and ncs6∆) and t6A synthesis mutants (tcs2∆-tcs8∆) were purified and analysed by HPLC.
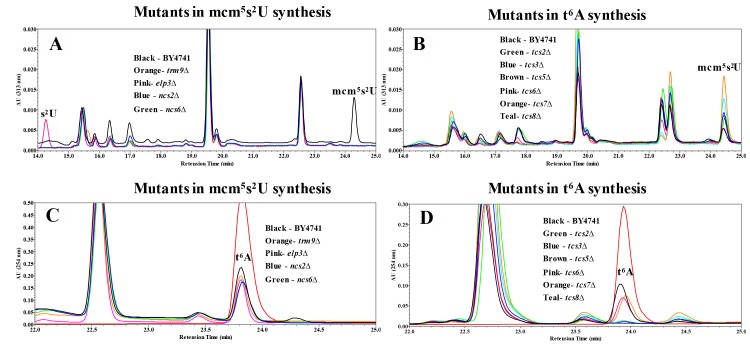
To determine how t6A synthesis deficiency affects mcm5s2U, HPLC analysis with detection at 313 nm (for detection of thio moieties) of nucleosides of tRNAs purified from elp3∆, trm9∆, ncs2∆, and ncs6∆ revealed the mcm5s2U peak at 24.35 minutes, which was unique to BY4741 but absent in all the mutants, and a peak at 14.20 minutes appeared only in elp3∆, indicating the presence of the s2U moiety in this strain (Figure 2A). The chromatographic patterns match previously published reports 50. Analysis of tRNAs purified from t6A biosynthesis mutants (tcs2∆-tcs8∆) revealed that all strains possessed the peak at 24.35 minutes corresponding to mcm5s2U, and none of the mutants showed the s2U peak at 14.20 minutes (Figure 2B). Interestingly, most mutants in t6A synthesis had higher levels of mcm5s2U (tcs6∆ is unchanged) as compared to BY4741, among which tcs7∆ was the highest (Figure 2B).
Figure 2. FIGURE 2: HPLC analysis examining the relationship between mcm5s2U34 and t6A37.
(A) Analysis of mutations in mcm5s2U synthesis with detection at 313 nm specific for thio-moieties. Black line = BY4741; Orange = trm9∆; Pink = elp3∆; Blue = ncs2∆; Green = ncs6∆. (B) Analysis of mcm5s2U in mutants for t6A synthesis for with detection at 313 nm. Black = BY4741; Green = tcs2∆; Blue = tcs3∆; Brown = tcs5∆; Pink = tcs6∆; Orange = tcs7∆; Teal = tcs8∆. (C) Analysis for t6A in mutants of mcm5s2U synthesis with detection at 254 nm. The color scheme is the same as part A, with the t6A standard in red. (D) Analysis for t6A in mutants of t6A synthesis with detection at 254 nm. Color scheme is the same as part B, with the t6A standard in red.
The HPLC profile at 254 nm revealed that all the mutants in mcm5s2U synthesis contained the same amount of t6A as the parental BY4741 strain, as indicated on Figure 2C by a peak at 23.57 minutes. Analysis of the t6A synthesis mutants (tcs2∆-tcs8∆) confirmed prior results of the absence of t6A in tcs2∆ and tcs3∆ 20,23, and revealed the absence of t6A in both tcs5∆ and tcs8∆ (Figure 2D). tcs6∆ and tcs7∆ were reduced for t6A relative to wild type by ~20% (Figure 2D), very similar to the reduction seen in a tcs6∆ mutant in the archaea Haloferax volcanii 61. These results indicate that mcm5s2U34 and t6A37 do not require one another for their synthesis, although eliminating t6A did increase levels of mcm5s2U.
Overexpression of tRNAs or Ternary Complex (TC) do not suppress the growth defects of tcs2∆
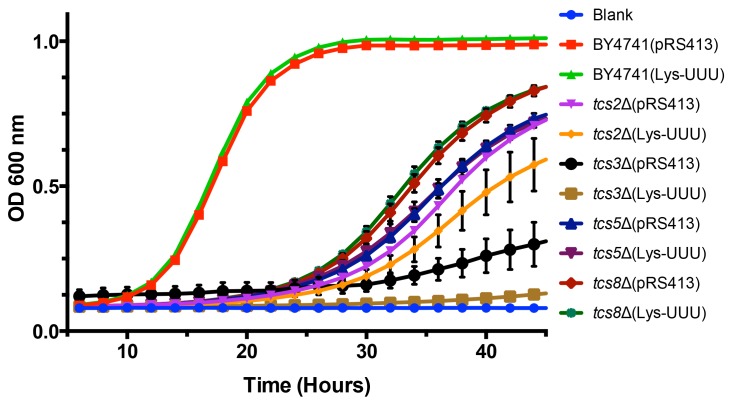
Overexpression of tRNALysUUU is sufficient to suppress all the phenotypes resulting from mutations of mcm5s2U synthesis enzymes 53. Therefore, we tested if this was also the case for mutations in the t6A synthesis pathway. To assess if tRNAs could suppress the slow growth rate seen in mutants of t6A synthesis, an expression plasmid containing tRNALysUUU was transformed into BY4741, tcs2∆, tcs3∆, tcs5∆, and tcs8∆ (tcs6∆ and tcs7∆ were not tested as they do not have a growth defect). Unlike in the case of mcm5s2U, the expression of tRNALysUUU did not suppress the growth defect observed in t6A synthesis mutants (Figure 3). Interestingly, it led instead to a small reduction in growth rate in the mutants (Figure 3). Further, we expressed the other tRNAs that decode ANN codons (tRNALysCUU, tRNAeMetCAU, tRNAIleAAU, tRNAIleUAU, tRNAThrUGU, tRNAArgACG, tRNAArgUCU, tRNAArgCCU, and tRNAGluUUC (does not contain t6A and decodes GAA, which is the most frequently used codon in S. cerevisiae)) in tcs2∆. None, of these individual tRNAs suppressed the growth defect of tcs2∆ (data not shown). To confirm the results of TCS2-depletion published by Lin et al. 33, plasmids over-expressing tRNAiMet, eIF2α, or TC were transformed into BY4741 and tcs2∆. In agreement with the previous results 33, neither tRNAiMet, eIF2α, nor TC suppressed the slow growth of tcs2∆ (Figure S2A), while the growth of tcs2∆ can be restored by expressing TCS2 in trans (Figure S2B).
Figure 3. FIGURE 3: Expression of tRNALysUUU does not suppress slow growth of mutants devoid of t6A.
BY4741, tsc2Δ, tsc3Δ, tsc5Δ, and tsc8Δ were transformed with plasmids expressing tRNALysUUU or empty vector. Data points are the average of 8 biological replicates. Error bars represent standard error of the mean (SEM).
Hence, unlike the suppression of mcm5s2U by tRNALysUUU, neither the overexpression of each ANN-tRNA, nor the overexpression of TC components could suppress the fitness defects observed in mutants of the t6A biosynthesis pathway. The effects of the loss of t6A thus appear to be more complex than those of the loss of mcm5s2U.
t6A-deficient strains are sensitive to heat and inhibitors of TOR, but growth can be partially rescued by L-homoserine
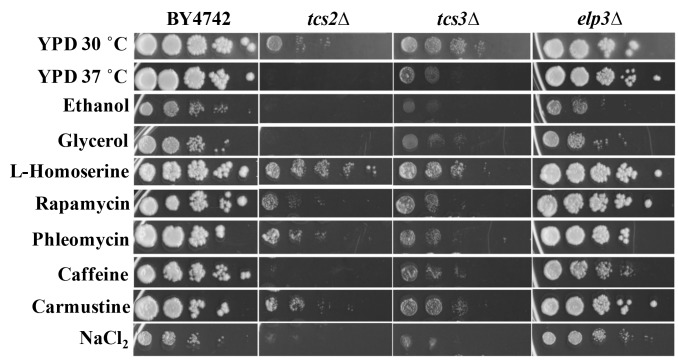
To better characterize how the absence of t6A was affecting cellular function, growth on several carbon sources and under different stress conditions was tested (Figure 4). t6A-deficient strains were found to be sensitive to heat stress, with tcs2∆ unable to grow at 37°C, and to salt stress, with both tcs2∆ and tcs3∆ affected by the presence of 1 M NaCl2. Also, tcs2∆ was unable to grow on 3% glycerol or 6% ethanol, but did grow slowly on 2% glucose (YPD), while tcs3∆ was able to grow slowly on all carbon sources (Figure 4). Addition of inhibitors of the TOR pathway such as caffeine (10 mM) 62 or rapamycin (10 nM) further reduced the growth of t6A-deficient strains (Figure 4). Interestingly, the addition of L-homoserine (1 mg/ml) partially suppressed the growth defects of the tcs2∆ strain, but not of the tcs3∆ strain. Several other chemical stresses did not affect growth of t6A- strains (Figure 4). These included the addition of DNA damaging agents such as phleomycin (8 μg/ml) or carmustine (1 mM). The elp3∆ strain was also tested in the same conditions as it is known that depending on the strain background, mutants in Elongator genes vary as to the degree of response to each of these stressors 63. The results presented in Figure 4 are consistent with the results recently published by the Schaffrath laboratory, reporting the lack of strong phenotypes of the elp3∆ strain when using the BY4741 background 64,
Figure 4. FIGURE 4: Stress phenotypes of t6A-deficient strains differ from a mcm5s2U-deficient strain.
Cells were grown at 30°C for 48 hours on YPD with 2% glucose except when 6% ethanol or 3% glycerol was used as a sole carbon source or heat stress at 37°C. Drugs were added to YPD at the following levels: L-homoserine, 1 mg/mL; Rapamycin, 10 nM; Phleomycin, 8 µg/mL; caffeine, 10 mM; carmustine, 1 mM; NaCl2, 1 M.
Homoserine acts as a toxic threonine analogue and incorporation of homoserine activates protein degradation pathways 65. To test whether activation of UPR could suppress the growth phenotype of t6A-depletion strain in yeast, t6A- strains were transformed with plasmids expressing UPR factors Xbp1, Kar2 (GRP78/BIP), Der1, and Hrd1. Expression of UPR-related factors were unable to rescue the slow-growth phenotype seen in t6A-deficient strains (data not shown).
t6A- strains accumulate aggregated proteins and advanced glycated end-products (AGEs)
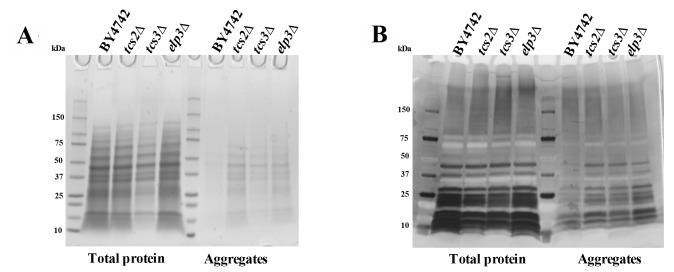
Double mutants elp6∆ncs2∆ (eliminating mcm5s2U) have been shown to contain increased amount of aggregated (insoluble) proteins, possibly due to alteration in translation speed 60. Equal amounts of total and insoluble proteins from BY4742, tcs2∆, tcs3∆ and elp3∆ were analysed by SDS-PAGE and Coomassie staining. Depletion of t6A in tcs2∆ and tcs3∆ increased the amount of aggregated or insoluble proteins similar to the single elp3∆ strain (Figure 5A), which is less than the amount of insoluble protein seen in the double elp6∆ncs2∆ strain 60. Prior experiments in E. coli and H. volcanii revealed that AGEs become more abundant when t6A levels are reduced 61,66. To assess levels of AGEs in our context, equal amounts of total and insoluble proteins from BY4742, tcs2∆, tcs3∆ and elp3∆ were separated by SDS-PAGE and visualized with a diol-specific silver stain for glycated proteins 67. Aggregated proteins extracted from tcs2∆, tcs3∆, and elp3∆ were all increased in AGEs relative to wild type (Figure 5B).
Figure 5. FIGURE 5: t6A deficient strains accumulate aggregated proteins and Advanced Glycated End-products (AGEs).
(A)Protein aggregation in BY4742 and mutant yeast cells. Yeast were grown in YPD to an OD600 = 0.8. Soluble and aggregated proteins were separated by SDS–PAGE and visualized by Coomassie blue staining. (B) AGEs were visualized by silver staining.
Ribosome assembly defects are observed in the t6A- strain
In light of the diverse phenotypes observed and because the previous analysis of translation defects had focussed on a handful of reporter proteins, we performed a global ribosome profiling analysis to assess the impact of t6A-deficiency at the genome scale. An essential step in ribosome profiling is ensuring high quality polysomes are prepared, which was assessed by sucrose gradient sedimentation and subsequent analysis with a fraction analyser. Polysomes prepared from tcs2∆ exhibited a “half-mer” phenotype, which is represented by a shoulder after the 80S peak on the chromatograph, blue arrow in Figure S3. Half-mers indicate excess 40S ribosome and incomplete assembly of the 80S particle, which may indicate problems with initiation 68. The half-mer phenotype of tcs2∆ was not seen in a prior publication examining a TCS2-depletion strain 33. These results may differ due to strain genotypes or technical differences in the preparation of the polysomes.
The absence of t6A leads to increased ribosome occupancy of arginine synthesis genes
A detailed description of purification of the ribosome-protected fragments (RPFs) and sequencing can be found in material and methods. Analysis of RPFs in tcs2∆ revealed 111 genes were decreased in RPFs and 196 genes were increased in RPFs relative to wild type. A complete list of these genes and their functional roles can be found in Table S3 and Table S4. To determine if any functional relationship existed with these genes, we performed gene ontology (GO) enrichment using YeastMine (http://yeastmine.yeastgenome.org) 69. The pathway for arginine biosynthesis was found to be enriched, P = 0.049, with five genes, ARG5,6, CPA2, ARG7, ARG1, and CPA1 identified. None of the arginine catabolism pathways were significantly increased (Table S4). Increased mRNA expression of arginine has been documented to act as an anti-oxidant to oxidative stress by an unknown mechanism 70. This antioxidative pathway acts through pyrroline-5-carboxylate (P5C) but PUT1, encoding a P5C synthesis enzyme, was not increased in RPFs in the mutant (Table S4). Of the 111 genes decreased in RPFs, five genes were identified matching the GO term polyphosphate metabolic process, P = 0.003, and no pathway enrichment was identified.
Depletion of t6A deregulates GCN4
The number of genes proposed to be regulated by Gcn4 varies greatly with the specific study, from less than 500 genes (microarrays measuring gene expression during histidine starvation) 35 to more than 2500 genes (predicted computationally by SGD). The most conservative estimate of Gcn4-induced genes was produced from a ChIP-Chip assay, which found 128 genes bound during immunoprecipitation of Gcn4 71. Comparison of the RPFs detected in tcs2∆ with the 128 well defined Gcn4-regulated genes reveals that 15 ORFs increased in RPFs are regulated by Gcn4 in tcs2∆, while no ORFs decreased in RPFs in tcs2∆ are regulated by Gcn4, Figure S4A. The 15 Gcn4-regulated genes with increased RPFs in tcs2∆ are involved in amino acid synthesis, with six in the arginine synthesis pathway (P = 1.3 x 10-5), Table S5.
Microarray analyses of conditional or point mutations in tcs3, tcs6, or tcs8 had been previously reported 38 and in all these studies, an up-regulation of Gcn4 regulated genes was observed, including genes in the arginine and histidine biosynthesis pathways, although the mRNA expression levels of GCN4 itself did not increase. The genes increased in each of the previous microarray studies were compared to the genes with increased RPFs in the ribosome profiling analysis of tcs2∆. Of the 196 genes with increased RPFs in tcs2∆, 29 were also increased in tcs3-18, 30 were also increased in tcs6-4, and 12 were also increased in tcs18-ts1, summarized in Figure S4B. 12 genes were increased in all four datasets (Table 1). 9 of these are under Gcn4 control, of which four are in the arginine synthesis pathway (Table 1).
Contrary to the previous microarray results that did not detect GCN4 induction, we found that RPFs mapping to GCN4 were increased 6-fold in tcs2∆ (Table S4). This difference may be due to an up-regulation of translation (detected by the increased levels of RPFs in tcs2∆), and not transcription (as measured by the microarrays). With the 6-fold increase in GCN4 expression, it is surprising so few Gcn4 inducible genes are increased in tcs2∆. Indeed, only 8% of RPFs increased in tcs2∆ are in common with the Gcn4p ChIP data.
Table 1.
Genes increased in expression in tcs2∆, tcs3-18, tcs6-4, and tcs8-ts1. *Under control of Gcn4 (see Table S5), &Arginine biosynthesis.
| Systematic name | Standard name | Description |
| YER069W | ARG5,6 | Acetylglutamate kinase and N-acetyl-gamma-glutamyl-phosphate reductase*& |
| YER175C | TMT1 | Trans-aconitate methyltransferase |
| YGL117W | Putative protein of unknown function | |
| YJL079C | PRY1 | Sterol binding protein involved in the export of acetylated sterols |
| YJR025C | BNA1 | 3-hydroxyanthranilic acid dioxygenase* |
| YJR109C | CPA2 | Large subunit of carbamoyl phosphate synthetase*& |
| YMR062C | ARG7 | Mitochondrial ornithine acetyltransferase*& |
| YMR095C | SNO1 | Protein of unconfirmed function* |
| YMR096W | SNZ1 | Protein involved in vitamin B6 biosynthesis* |
| YNL104C | LEU4 | Alpha-isopropylmalate synthase (2-isopropylmalate synthase)* |
| YOL058W | ARG1 | Arginosuccinate synthetase*& |
| YOR130C | ORT1 | Ornithine transporter of the mitochondrial inner membrane* |
A discrete but not a global increase in translational ambiguity is observed in the t6A- strain tcs2∆
In yeast, the RPF is 28 nucleotides long 72, hence to analyse the frame of each ribosome, only 28-mers that aligned uniquely to the genome and did not contain mismatches were used. 6.5 x 106 reads in wild type and 5.8 x 106 reads in tcs2∆ matched these strict criteria. For each read, the nucleotide at position +12, which corresponds to the ribosomal P-site, was determined and its identity defined the frame of the read, and hence the frame of the ribosome, Figure S5. Each ORF was divided into windows of approximately 300 nucleotides (minimum of 3 windows per ORF to a maximum of 9), and reads inside each window were mapped and enumerated 73. Since we cannot be sure if the ribosome associated with read is in frameshift or if that ribosome began translation of the ORFs out of frame, translational ambiguity is defined as the mapping of a read in a frame other than the frame of the annotated ORF.
There are four well-documented examples of +1 frameshifts occurring in S. cerevisiae and these were used to evaluate the frame analysis performed here. One known +1 frameshift, TRM140 (an AdoMet-dependent tRNA methyltransferase), was detected in both BY4742 and tcs2∆ and is illustrated in Figure S6A and B. For translation of full-length Trm140, the ribosome must undergo a +1 frameshift at nucleotide 832. As seen in Figure S6A and B, nearly 100% of the reads begin in Frame 0, then after base 832, nearly 100% of the reads are in the +1 frame.
87 and 213 ORFs were found to have potential translational ambiguities in BY4742 and tcs2∆, respectively (Table S6 and Table S7). GO term enrichment of these genes with translational ambiguities revealed a single biological process, cytoplasmic translation, was enriched in both strains, with 16 genes in BY4742 (P = 4 x 10-6) and 35 genes in tsc2∆ (P = 3 x10-11). In tcs2∆, 17 of the 79 ribosomal proteins had increased levels of translation ambiguities (Table S7).
Interestingly, global analysis of translational ambiguities, by summing all reads used to determine frame, indicates that 80% of all reads from the ribosome profiling are in the correct, annotated frame (Frame 0), but there is a significant difference in translational ambiguities between wild type and mutant (P = 6.5 X 10-98, t-test), Figure S7. Interestingly, only 0.26% of all ORFs were identified as having potential translational ambiguities in tcs2∆. Thus, the data indicates that loss of t6A is causing ambiguities at discrete sequences, or codons, but is not causing a global, cataclysmic alteration of reading frame.
The number observed non-AUG starts doubles in the t6A-deficient strain
TCS2 (SUA5) was discovered in yeast as a suppressor of a translational initiation defect in the cyc1-362 allele 34. cyc1-362 contains an aberrant upstream and out of frame AUG resulting in ~2% of the normal Cyc1 protein levels. Suppressors would bypass the out of frame AUG and initiate at the correct downstream AUG, increasing the amount of Cyc1 34. To detect initiation of translation at non-conical codons, we parsed the profiling data with a strict set of parameters. To be considered a non-canonical start codon, a GUG, UUG, or GUC codons (the most frequently used non-AUG initiation codons in yeast) 74,75,76,77,78 had to be within 100 nucleotides upstream of the ORF of interest, and be in-frame with the downstream AUG with no stop codon between the candidate non-AUG and its downstream AUG. Finally, a minimum of 128 reads was required to cover the non-AUG site.
In yeast, there are two well-characterized occurrences of non-AUG initiation occurring upstream of the annotated AUG start site. ALA1 encodes both the cytoplasmic and mitochondrial alanyl-tRNA synthetase. The cytoplasmic form of Ala1p is translated from the annotated AUG, and the mitochondrial form is translated from a pair of ACG codons located at -25 and -24 relative to AUG 79. GRS1 encodes the cytoplasmic and mitochondrial glycyl-tRNA synthase. The cytoplasmic form of Grs1p is translated from the annotated AUG, and the mitochondrial form is translated from a UUG codon located at -26, relative to the AUG 78. In both BY4742 and tcs2∆, initiation at the upstream non-AUG codons can be detected for ALA1 and GRS1, Figure S8A and Figure S8B.
The analysis of non-AUG initiation was expanded to the entire profiling dataset. For the three codons analysed, tcs2∆ contain nearly twice as many non-AUG starts as BY4742. For initiation at UUG, BY4742 contained 140 genes, Table S8, and tcs2∆ contained 260, Table S9. For initiation at ACG, BY4742 contained 98 genes, Table S10, and tcs2∆ contained 169, Table S11. For initiation at GUG, BY4742 contained 62 genes, Table S12, and tcs2∆ contained 134, Table S13. None of these sets of genes contained any enrichment of GO terms.
t6A’s role in translation speed varies with the codon
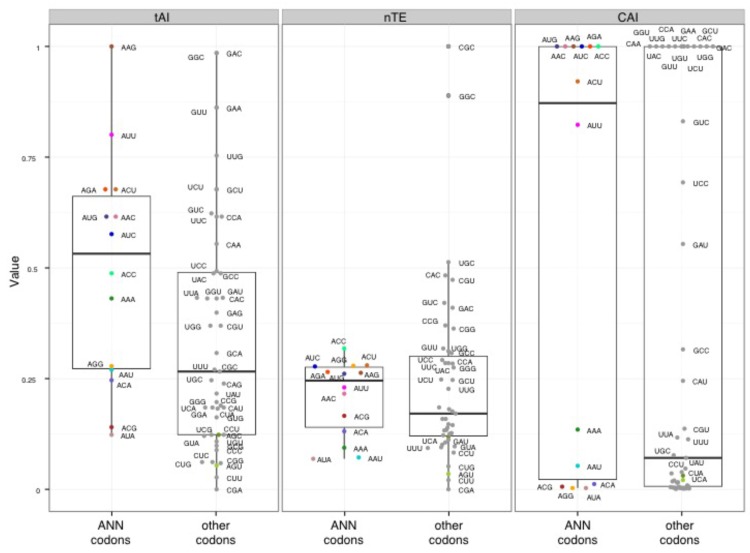
Different metrics have been developed to estimate translation efficiencies of individual codons based on the abundance of their cognate tRNAs, and the properties of the ASL they form. There are three major metrics commonly used to measure the translation efficiency of codons: the Codon adaptation index (CAI, 80), the tRNA adaptation index (tAI, 81), and the normalized Translational Efficiency (nTE, 82) (which is based on codon abundance in the transcriptome rather than codon frequency in the genome). Using the three metrics, we found that ANN codons in yeast have in average a higher estimated translational efficiency compared to other codons (Figure 6), suggesting that there is a statistical tendency for ANN-tRNAs to be in high supply in standard growth conditions. The predicted efficiencies vary greatly between ANN codons, with AAG always in high supply while AUA always in low supply (Figure 6).
Figure 6. FIGURE 6: Translation efficiency of t6A-dependent codons.
tAI – tRNA Adaptation Index; nTE – normalized Translational Efficiency; CAI – Codon Adaptation Index. Black and colored circles indicate a codon decoded by a tRNA predicted by the wobble hypothesis, with color matching Figure 1 and 7.
Ribosome profiling data allows for evaluation of translation speed by measuring codon occupancy at each site in the ribosome, with increased occupancy analogous to a decrease elongation rate, and vice versa. Using two different methods, the Codon Occupancy (CO) 83 and the Ribosome residence time (RRT) 84, a count of every codon occupying the ribosomal A (acceptor), P (peptidyl transfer), and E (exit) sites was compiled. Comparing the Log2 fold-change of CO and RRT in the A, P, and E sites of tcs2∆ and BY4742 produced a global summary of the consequences of t6A absence on decoding, Figure 7.
Figure 7. FIGURE 7: Measurements of ribosome pausing at the A-, P-, and E-sites of tcs2∆.
(A) Codon occupancy. (B) Ribosome residence time (RRT). Black and colored circles indicate a codon decoded by a tRNA predicted by the wobble hypothesis, with color matching Figure 1 and 6.
The ribosomal A site occupancy for t6A dependent codon site occupancy was increased for AUA, ACG, AGG, AUG, ACA, AAA, and AAU and decreased for AUU, AAC, AUC, AGA, ACU, ACC, and AAG. The codons with increased occupancy in the A-site fell into two categories: (i) codons that are decoded by rare tRNAs (only 1-4 copies of tRNAs genes are encoded in the chromosome) and (ii) codons that are decoded by a G34:U3 wobble, as for AAU (decoded by tRNAAsn(GUU)) (see Figure 1B for codon:anticodon pairs). The codons whose A-site occupancy decreased also fell into two categories: (i) codons decoded abundant by tRNAs (4-13 genes) and (ii) codons decoded by an I34:C3 wobble, as for AUC (decoded by tRNAIle(IAU)) and ACC (decoded by tRNAThr(IGU)) (Figure 1B). The pattern found for A-site occupancy, also held true for P-site and E-site occupancies. Interestingly, this pattern also held true for codons decoded by non t6A-containing tRNAs. AGU (G:U wobble) and CGG (decoded by the rare tRNAArgCCG) were increased in ribosome occupancy, while GUC and GCU (I:C or I:U) were decreased in ribosome occupancy (Figure 7). From this data, it appears that t6A is helping increase elongation rate of rare tRNAs and G34:U3 pairs and decrease the elongation rate of high abundance and I34:C3 pairs to homogenize the process of elongation.
DISCUSSION
The absence of t6A in yeast does not lead to catastrophic and global defects in translation, as would be expected from previous studies based on single reporter assays. Even with doubling of initiation at upstream non-AUG starts and a 2.5 fold increase in translational ambiguities, only a limited number of genes in the yeast genome were affected. This suggests that the severe and pleiotropic phenotypes caused by t6A deficiency may not be caused by global defects in translation, but instead of the subtler consequences of codon-specific translation defects caused by lack of t6A.
Role of t6A in decoding efficiency varies with the tRNA
The codon occupancy results presented in this study suggest that t6A helps rare cognate tRNAs and G:U mismatches (near-cognates) compete with Watson:Crick decoding tRNAs and slows decoding by high abundance tRNAs and tRNAs using the wobble U:C base pairings 85. This can be all the more critical for codons like AGG decoded by tRNAArgCCU that are known to be strongly inhibitory for translation efficiency 86,87,88. Another important role for t6A is stabilizing the interaction between the first base of the mRNA codon and position 36 (the third nucleoside) of the tRNA anticodon, preventing decoding of near-cognates by tRNAiMetCAU 89. This is exemplified by tRNAfMet of E. coli that contains an unmodified A37 and can efficiently decode GUG and UUG while the eukaryotic tRNAiMet contains t6A37 and rarely decodes non-AUG codons 90,91,92,93. The examination of alternative start-sites presented here supports the role of t6A preventing tRNAiMet from recognizing near-cognates and restricting translation initiation to AUG codons.
The codon occupancy for non-t6A containing tRNAs is also altered (Figure 7), and this is more dramatic than what is seen in ncs2∆elp6∆ 60. This could be due to an alteration in competition between cognate and near-cognate tRNAs. This was previously demonstrated for tRNAArgUCU (tRNAArgIII), which naturally exists in t6A modified and unmodified forms 94. The modified version of tRNAArgUCU can outcompete the unmodified form for the cognate codon and binds more tightly to tRNASerGGA involving a U36:G34 mismatch 94. CGG codons (decoded by tRNAArgCCG) and UGG codons (tRNATrpCCA) are increased in both the codon occupancy and RRT assays (Figure 7). One can speculate that the slower decoding at CGG and UGG is due to competition between tRNAArgCCG or tRNATrpCCA with an unmodified near-cognate tRNAArgCCU.
A recent global analysis of yeast ribosome profiling data has shown that frequent codons are decoded more quickly than rare codons, and AT-rich codons are decoded more quickly than GC-rich codons 84. It seems that the difference could be even larger if tRNA modifications are altered, as shown here with the absence of t6A, and as is already know for several other ASL modifications. The absence of Queuosine (Q34) is known to have opposite effects on decoding depending on identity of the 3rd base of the codon 88,95, and the depletion of mcm5s2U synthesis can alter the decoding rates of tRNAs that do not possess this modification 60. An emerging general trend for the ASL modifications is to homogenize the kinetics of individual tRNA binding (competition) during translation and to alter the speed of translation to ensure proper protein folding, a concept that was predicted by Toshimichi Ikemura in 1981 96.
Could defects in translation speed cause the pleiotropic phenotypes of t6A-?
Analysis of codon stretches in yeast 20 revealed that the genes with the longest stretches of t6A-dependent codons encode poly-Asn (poly-N) proteins that contain up to 31 consecutive AAU/AAC codons. These include GPR1, required for glucose activation of the cAMP pathway 97, and SWI1, a subunit of the SWI/SNF chromatin remodelling complex required for transcription of many genes involved in sugar catabolism, as well as meiosis cell mating type (see summary in SGD 98). If the protein expression of these two genes is reduced in the absence of t6A (that is a decrease in elongation speed of these transcripts possibly due to stalling), many of the phenotypes seen in t6A- strains (e.g., no growth on galactose, chromatin remodelling defects and telomere shortening 33,99,100) can be explained. Unfortunately, the presence of these repeats (90 nts) are longer than the RPFs (28 nts) sequenced, so these genes could not be analysed in the ribosome profiling and further studies are needed to test this hypothesis.
Several stress-induced transcription factors are also increased in tcs2∆ context, including NSF1 (YPL230W), a transcriptional regulator of genes involved in growth on non-fermentable carbon sources (see summary in SGD 98), Sol4 (YGR248W), which functions in the pentose phosphate pathway (see summary in SGD 98), and Smc6 (YLR383W), a component of the SMC5-SMC6 complex that plays a key role in the removal of X-shaped DNA structures (see summary in SGD 98) (Table S4). Up-regulation of any of these transcription factors would have wide-ranging effects and could explain some of the pleiotropic phenotypes seen under t6A deficiency.
Ribosome profiling of ncs2∆ revealed genes with increased translation activity tend to play a role in amino acid metabolism, and GCN4 is significantly increased 54. Comparison of genes increased in tcs2∆ and ncs6∆ revealed 19 genes found in each of these mutants, Table S14. Increased genes included GCN4 and several members of the arginine biosynthesis pathway, Table S14. Twelve genes are decreased in both tcs2∆ and ncs6∆, including two ribosomal protein subunits and two phosphatases, Table S15. GCN4 is also increased in the ribosome profiling of ncs2∆elp6∆ 60. A common theme seen in disrupting ASL modifications is the de-repression of Gcn4, in a non-canonical Gcn2-independent manner, activating a small subset of Gcn4 regulated genes. The mechanism of activation and the importance of Gcn4 activation are not understood at this point in time.
Restoring protein homeostasis suppresses the slow growth of tcs2∆
An explanation for the similarity of phenotypes seen in both Elongator and t6A mutants could be due to the disruption of protein homeostasis. Unlike the mutations in the Elongator modification, the slow growth observed during disruption of t6A biosynthesis cannot be suppressed by overexpression of tRNAs (Figure 3). However, L-homoserine rescued the growth of tcs2∆, but not tcs3∆ (Figure 4). Further studies are required to explain this suppression, but homoserine is a toxic intermediate, which acts as a threonine analogue, and it was recently shown that the ubiquitin pathway and the proteasome are crucial in alleviating homoserine toxicity 65.
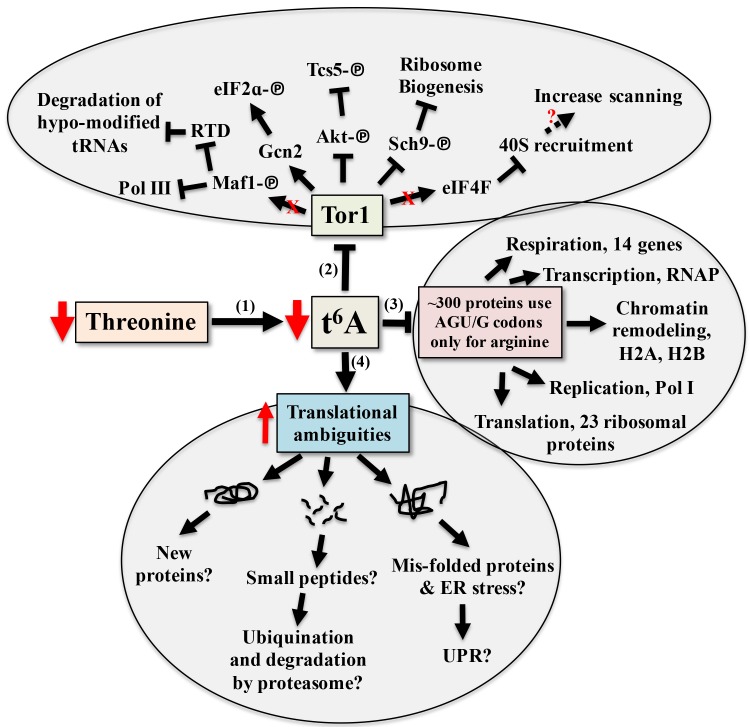
Model for the cellular response to reduction of t6A
To date, tcs2 and members of the TCTC complex have been implicated in transcriptional regulation. While there is no empirical evidence eliminating this possibly, the evidence presented here and in other works suggests transcriptional changes seen when perturbing t6A biosynthetic genes are part of an adaptive response by the cell to cope with translational errors. The response to alterations in t6A levels involves a combination of both independent and interrelated events, summarized in Figure 8. The model proposes that t6A acts as a sensor of nutritional levels as t6A varies in response to the availability of threonine, Figure 8-1 101. As t6A levels decline, Tor1 activity decreases, Figure 8-2 56. As a master controller, declines in Tor1 activity reduces the growth potential of the cell 55,56,102 and has wide ranging affects, from blocking Pol I, Pol III 103, and the RTD (Rapid tRNA Degradation pathway to prevent degradation of hypo-modified tRNAs) 104 to lessening translation initiation and ribosome biogenesis 105.
Figure 8. FIGURE 8: Model for the cellular response to reduction of t6A.
(1) Reduction of threonine lowers the level of t6A and (2) decreases the activity of the master controller Tor, which reduces anabolism and the growth potential of the cell through multiple pathways. (3) ~300 proteins only use t6A encoding tRNAs for arginine. (4) Potential outcomes of increased translation ambiguities seen in the absence of t6A.
A reduction in t6A may also lower the translation rate of specific proteins due to codon usage, Figure 8-3. Arginine is one of two amino acids in yeast that are incorporated both by t6A-containing tRNAs (AGA/G codons) and by tRNAs lacking t6A (CGN codons), Figure 1B 44. The AGA/G codons are known to be frequent sites of frameshifting, reviewed in 106. Using the codon usage database 107, yeast genes were ranked according to their use of t6A-dependent or t6A-independent Arg codons. Around 300 genes used only t6A-dependent Arg codons and GO analysis showed a strong enrichment for genes of the aerobic respiration and electron transport pathways (P - value 10-9, Holm-Bonferroni test), which could explain the respiratory deficiency phenotype displayed by t6A- yeast strains (Table S16 and S17). 23 ribosomal proteins, RNA Polymerase subunits (RNAP), and proteins of the chromatin remodelling complexes H2A and H2B use t6A-dependent Arg codons. Only 12 of these t6A-dependent Arg genes were decreased in RPFs in tcs2∆. Proteomic studies are now underway to confirm if this specific set of genes are translated less efficiently in the t6A- strains.
As t6A levels decrease, translation fidelity decreases (Figures S7, S8 and Tables S6-13). Increase in translation ambiguity could lead to new protein products, which may be non-functional or toxic 108. Out-of-frame decoding could increase synthesis of small peptides 108 and misfolded proteins could lead to the activation of the unfolded protein response (UPR) 55, and to the activation of catabolic pathways 109,110, Figure 8-4. Additional proteomic studies are underway to measure misfolding and amino acid misincorporation rates in t6A- strains to further characterize these multi-layered and complex phenotypes.
MATERIALS AND METHODS
Strains and growth conditions
A list of all organisms used in this study can be found in Table S1. Yeast strains were grown on YPD (DIFCO Laboratories) at 30°C. Synthetic minimal media (SD), with or without agar, with or without dropout supplements (-uracil, -ura; -leucine, -leu; -histidine, -his) were purchased from Clontech (Palo Alto, CA) and prepared as recommended by the manufacturer. Glucose (Glu, 2% w/v), Glycerol (Gly, 4% w/v), Ethanol (EtOH, 6% v/v) 5-fluoro-orotic acid (5-FOA, 0.1% w/v) and G418 (300 μg/mL) were used when appropriate. Yeast transformations were carried out using frozen competent cells as described by 111 with plating onto the appropriate media. The S. cerevisiae tcs2∆::KanMX4 strain, VDC9100, was created as previously described 27. All strains were genotyped using oligonucleotides targeting inside and outside the gene of interest, in addition to the location of the replacement cassette. Oligonucleotides are listed in Table S2. VDC9100 (tcs2∆) harboring tRNA over-expression plasmids were created using the plasmid shuffle technique by first transforming with pBN204 (TCS2 complementation plasmid), then transforming with tRNA plasmids, and finally curing pBN204 from VDC9100 using SD-leu+5-FOA media.
Yeast growth assays
Growth curves were performed using a Bioscreen C MBR (Oy Growth Curves AB Ltd, Finland) at 30°C and at maximum shaking. A 250 µl culture was used in each well, and 5 biological replicates were used for each condition. Yeast cultures were grown in the listed media to saturation, normalized to an OD600 of 1, and diluted 200 times in the listed media before loading on the Bioscreen. The growth curves presented are averages of 5 biological replicates. Significance was determined using a 2-way ANOVA and Fisher’s LSD using Prism 6 (GraphPad).
For phenotype screens and tRNA over-expression assays, yeast cultures were grown in the media listed in the figure to saturation, normalized to an OD600 of 1.0 and 5 µL of 1:10 serial dilutions were spotted on the listed media with the supplements listed in the figure and text. Galactose (2% w/v) was added when needed.
Extraction and digestion of bulk tRNAs
Bulk tRNAs were prepared as previously described using acid buffered-phenol (phenol saturated with 50 mM sodium acetate, pH 5.8) and alcohol precipitation 20. Nucleosides were prepared as described in 61 by hydrolyzing bulk tRNA with 10 units of Nuclease P1 (Sigma) overnight at 37°C, with the addition of 0.01 units of phosphodiesterase I (Sigma) and 3 µL E. coli alkaline phosphatase (Sigma). The hydrolyzed nucleosides were further purified by filtering through a 5 kD MWCO filter (Millipore) (to remove enzymes), dried in a CentriVap Concentrator, and suspended in 20 µL of water prior to analysis by HPLC or LC-MS/MS.
HPLC and LC-MS/MS Analysis
t6A was detected by HPLC as described by 112 using a Waters 1525 HPLC with Empower 2 software and detected with a Waters 2487 UV-vis spectrophotometer with simultaneous detection at 254 nm and 313 nm (for thio-derivatives). Separation was performed on an Ace C-18 column heated to 30°C, using 250 mM ammonium acetate (Buffer A) and 40% acetonitrile (Buffer B) run at 1 mL/min. 100 µg of nucleosides were injected and separated using a complex step gradient 112. Levels of t6A were measured by integrating the peak area from the extraction ion chromatograms. The ratio of Ψ-modified base/m22G was used to normalize for tRNA concentration across samples. Levels for mutant strains were expressed relative to wild type levels. Results were confirmed by LC–MS/MS at the Donald Danforth Plant Science Center, St. Louis MO, as described in 20. The MS/MS fragmentation data, as well as a t6A standard provided by D. Davis (University of Utah) were also used to confirm the presence of t6A.
Ribosome profiling
Purification of RPFs and Library Preparation
Ribosome Profiling was performed as described previously by Baudin-Baillieu et al. 113,114. Briefly, polysomes were prepared from two biological replicates of the parental BY4742 strain and tcs2∆ (VDC9100) grown from a preculture diluted into 500 mL YPD in an Erlenmeyer flask. Cells were harvested at OD600 0.6, chilled on ice, and cycloheximide was added to a final concentration of 50 µg/mL. Polysomes were harvested in cold lysis buffer (0.1 mM Tris-HCl, pH 7.4, 10 mM NaCl2, 3 mM MgCl2, and 50 µg/mL cycloheximide), and were aliquoted at approximately 40-50 OD260 units per tube and rapidly frozen in liquid nitrogen and stored at -80°C. Monosomes were prepared by digesting polysome extracts for 1 hour at room temperature with 15 units RNaseI (Ambion) per OD unit. The digested polysomes were purified on sucrose gradients prepared by casting the sucrose gradients (31% sucrose, 50 mM Tris-acetate pH 7.6, 50 mM NH4Cl, 12 mM MgCl2, and 1 mM DTT) with three freeze-thaw cycles. The samples were loaded on the gradients and centrifuged in a Beckman SW41 rotor at 39,000 rpm at 4°C for 3 hours. Fractions were collected using an ISCO (Teledyne, Lincoln, NE) instrument at a 0.5 mL/min flow rate. Ribosome protected fragments (RPFs) of mRNA were purified using acid phenol (unbuffered), chloroform, and ethanol precipitation, then stored at -20°C. The 28-nucleotide RNA fragments were selected on 15% acrylamide gels containing 7 M urea. A 28 nt RNA oligonucleotide (oNTI 199 5'-AUGUACACGGAGUCGACCCGCAACGCGA-3') was used as a size marker. After migration, gels were incubated for 30 minutes in a 10 % solution of SYBR-Gold (Life Technologies) and visualized with a UV lamp at 300 nm, and the 28 nt fragments were excised. The excised gel pieces were loaded into the pierced 1.5 mL tubes inside a 2 mL tube, and centrifuged for 1 minute at 16,000 x g. RNA was precipitated with glycogen ethanol overnight at -20°C. RPFs were depleted of major rRNA contamination by subtractive hybridization using biotinylated oligonucleotides (Table S2) and were recovered by reacting with MageneShere Paramagnetic streptavidin particles (Promega). The supernatants containing the RPFs were recovered and the RNA was precipitated, as described above. RNA size and quality was checked with a Small RNA Chip on a Bioanalyzer 2100 (Agilent). A directional RNA-Seq library was prepared by IMAGIF (Centre de Recherche de Gif - www.imagif.cnrs.fr) Gif-sur-Yvette, France using the TruSeq Small RNA Sample Prep Kit (Illumina) and the v1.5 sRNA 3' Adaptor (Illumina) according to the manufacturer’s protocol and verified using Bioanalyzer Small RNA Analysis kit (Agilent). Sequencing was performed at the Microarray and Genomic Analysis Core Facility at the University of Utah Huntsman Cancer Institute on an Illumina HiSeq 1500 and subjected to a 50 cycle run.
Sequencing, quality control, and read-mapping
Sequencing and bioinformatics analysis were performed as described in 114. In brief, four sequencing libraries were prepared from the 28-mer RPFs purified from two biological replicates of BY4742 and tcs2∆. The libraries were sequenced on an Illumina HiSeq 1500 with 50-cycle single-end reads to maximize the numbers of reads for each small RNA library. Approximately 2 x 109 reads were obtained for each sample. Quality was assessed using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and adaptors were removed using CutAdapt 115. To remove contaminating reads corresponding to rRNA, reads were mapped against an rRNA index from the Saccharomyces Genome Database (SGD) using Bowtie2 with the default settings 116. Reads not mapping to rRNA were mapped against the SacCer3 index (SGD) 117 using Bowtie2. Approximately 1.3 x 108 reads from each sample were mapped to the S. cerevisiae genome. Differential expression between samples was determined using a DESeq, with multiple testing correction using Benjamini and Hochberg 118,119,120. Significant differences between wild type and mutant were declared based on an adjusted alpha of 0.05. Precision of the biological replicates was very high with R = 0.9881 and R = 0.9959 for BY4742 and tcs2∆, respectively (Figure S1A and B). Post-sequencing analysis to identify differential expression, frameshifts, read-through, and non-AUG starts was performed as described in 73. Sequences were deposited at NCBI GEO database under accession number GSE72030.
Functional Classification of Genes
Lists of genes produced from the above analysis were analysed using YeastMine 69, an interactive database for querying the Saccharomyces Genome Database (SGD,www.yeastgenome.org) 69 to produce Gene Ontology enrichments and pathway enrichments.
Detection of protein aggregates and AGEs
Proteins were extracted from cells grown to mid-log and total proteins were extracted as described in 121. Aggregates were isolated as described by 122. Total proteins and aggregates were separated on 4-20% denaturing polyacrylamide gels with Coomasie blue staining. AGEs were identified by diol-specific silver staining 67.
SUPPLEMENTAL MATERIAL
All supplemental data for this article are also available online at http://microbialcell.com/researcharticles/global-translational-impacts-of-the-loss-of-the-trna-modification-t6a-in-yeast/.
Funding Statement
This work was supported by the National Institutes of Health (grant number R01 GM70641 to V.dC-L.), by FONDECYT 1140522 and FONDAP 15090007 grants to A.G., and CONICYT grant AT24121519 to D.R.B. P.C.T. was funded in part by a Chateaubriand Fellowship from the French Embassy in the United States. RL was supported by a grant from ANR N° 11-BSV6-011-01 to ON. G.C. was funded by the Medical Research Council and the Gates Cambridge Scholarship. We thank Alan Hinnebush and Sebastian Leidel for plasmids and advice. The authors acknowledge Alex Moskalenko from the University of Florida Research Computing (http://researchcomputing.ufl.edu) for providing computational support that have contributed to the research results reported in this publication. The authors wish to acknowledge Henri Grosjean for his storied career in tRNA biology and modification. He has provided all of us with immeasurable inspiration and advice, and we wish him a happy retirement.
References
- 1.Crick FHC. Codon—anticodon pairing: The wobble hypothesis. J Mol Biol. 1966;19(2):548–555. doi: 10.1016/S0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 2.El Yacoubi B, Bailly M, de Crécy-Lagard V. Biosynthesis and Function of Posttranscriptional Modifications of Transfer RNAs. Annu Rev Genet. 2011;46(August):120820103026000. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 3.Grosjean H, de Crécy-Lagard V, Marck C. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS Lett. 2010;584(2):252–264. doi: 10.1016/j.febslet.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 4.Novoa EM, Pavon-Eternod M, Pan T, Ribas de Pouplana L. A role for tRNA modifications in genome structure and codon usage. Cell. 2012;149(1):202–213. doi: 10.1016/j.cell.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Lamichhane TN, Blewett NH, Crawford AK, Cherkasova V a, Iben JR, Begley TJ, Farabaugh PJ, Maraia RJ. Lack of tRNA modification isopentenyl-A37 alters mRNA decoding and causes metabolic deficiencies in fission yeast. Mol Cell Biol. 2013;33(15):2918–2929. doi: 10.1128/MCB.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novoa EM, Ribas de Pouplana L. Speeding with control: codon usage, tRNAs, and ribosomes. 2012;(34):1–8. doi: 10.1016/j.tig.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Ran W, Higgs PG. The influence of anticodon-codon interactions and modified bases on codon usage bias in bacteria. Mol Biol Evol. 2010;27(9):2129–2140. doi: 10.1093/molbev/msq102. [DOI] [PubMed] [Google Scholar]
- 8.Ran W, Kristensen DM, Koonin E V. Coupling between protein level selection and codon usage optimization in the evolution of bacteria and archaea. MBio. 2014;5(2):1–11. doi: 10.1128/mBio.00956-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ran W, Higgs PG. Contributions of Speed and Accuracy to Translational Selection in Bacteria. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet. 2011;12(1):32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KrisKo A, Copic T, Gabaldón T, Lehner B, Supek F. Inferring gene function from evolutionary change in signatures of translation efficiency. Genome Biol. 2014;15(3):R44. doi: 10.1186/gb-2014-15-3-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace EWJ, Airoldi EM, Drummond DA. Estimating selection on synonymous codon usage from noisy experimental data. Mol Biol Evol. 2013;30(6):1438–1453. doi: 10.1093/molbev/mst051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond DA, Wilke CO. The evolutionary consequences of erroneous protein synthesis. Nat Rev Genet. 2009;10(10):715–724. doi: 10.1038/nrg2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond DA, Bloom JD, Adami C, Wilke CO, Arnold FH. Why highly expressed proteins evolve slowly. Proc Natl Acad Sci U S A. 2005;102(40):14338–14343. doi: 10.1073/pnas.0504070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dedon PC, Begley TJ. A system of RNA modifications and biased codon use controls cellular stress response at the level of translation. Chem Res Toxicol. 2014;27(3):330–337. doi: 10.1021/tx400438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston M a, D’Silva S, Kon Y, Phizicky EM. tRNAHis 5-methylcytidine levels increase in response to several growth arrest conditions in Saccharomyces cerevisiae. RNA. 2013;19:243–256. doi: 10.1261/rna.035808.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CTY, Dyavaiah M, DeMott MS, Taghizadeh K, Dedon PC, Begley TJ. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010;6(12):1–9. doi: 10.1371/journal.pgen.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu C, Begley TJ, Dedon PC. tRNA modifications regulate translation during cellular stress. FEBS Lett. 2014;588(23):4287–4296. doi: 10.1016/j.febslet.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiaville PC, Iwata-Reuyl D, de Crécy-Lagard V. Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t(6)A), a universal modification of tRNA. RNA Biol. 2014;11(12):1529–1539. doi: 10.4161/15476286.2014.992277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Yacoubi B, Lyons B, Cruz Y, Reddy R, Nordin B, Agnelli F, Williamson JR, Schimmel P, Swairjo MA, de Crécy-Lagard V. The universal YrdC/Sua5 family is required for the formation of threonylcarbamoyladenosine in tRNA. Nucleic Acids Res. 2009;37(9):2894–2909. doi: 10.1093/nar/gkp152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deutsch C, El Yacoubi B, de Crécy-Lagard V, Iwata-Reuyl D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J Biol Chem. 2012;287(17):13666–13673. doi: 10.1074/jbc.M112.344028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauhon CT. Mechanism of N6-Threonylcarbamoyladenonsine (t6A) Biosynthesis: Isolation and Characterization of the Intermediate Threonylcarbamoyl-AMP. Biochemistry. 2012;51(44):8950–8963. doi: 10.1021/bi301233d. [DOI] [PubMed] [Google Scholar]
- 23.El Yacoubi B, Hatin I, Deutsch C, Kahveci T, Rousset J-P, Iwata-Reuyl D, Murzin AG, de Crécy-Lagard V. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 2011;30(5):882–893. doi: 10.1038/emboj.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrochia L, Guetta D, Hecker A, Forterre P, Basta T. Functional assignment of KEOPS/EKC complex subunits in the biosynthesis of the universal t6A tRNA modification. Nucleic Acids Res. 2013;41(20):9484–9499. doi: 10.1093/nar/gkt720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrochia L, Crozat E, Hecker A, Zhang W, Bareille J, Collinet B, van Tilbeurgh H, Forterre P, Basta T. In vitro biosynthesis of a universal t6A tRNA modification in Archaea and Eukarya. Nucleic Acids Res. 2013;41(3):1953–1964. doi: 10.1093/nar/gks1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan LCK, Mao DYL, Neculai D, Strecker J, Chiovitti D, Kurinov I, Poda G, Thevakumaran N, Yuan F, Szilard RK, Lissina E, Nislow C, Caudy A a, Durocher D, Sicheri F. Reconstitution and characterization of eukaryotic N6-threonylcarbamoylation of tRNA using a minimal enzyme system. Nucleic Acids Res. 2013;41(12):6332–6346. doi: 10.1093/nar/gkt322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiaville PC, El Yacoubi B, Perrochia L, Hecker A, Prigent M, Thiaville JJ, Forterre P, Namy O, Basta T, de Crécy-Lagard V. Cross Kingdom Functional Conservation of the Core Universally Conserved Threonylcarbamoyladenosine tRNA Synthesis Enzymes. Eukaryot Cell. 2014;13(9):1222–1231. doi: 10.1128/EC.00147-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng F-L, Hu Y, Shen N, Tong X-J, Wang J, Ding J, Zhou J-Q. Sua5p a single-stranded telomeric DNA-binding protein facilitates telomere replication. EMBO J. 2009;28(10):1466–1478. doi: 10.1038/emboj.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downey M, Houlsworth R, Maringele L, Rollie A, Brehme M, Galicia S, Guillard S, Partington M, Zubko MK, Krogan NJ, Emili A, Greenblatt JF, Harrington L, Lydall D, Durocher D. A genome-wide screen identifies the evolutionarily conserved KEOPS complex as a telomere regulator. Cell. 2006;124(6):1155–1168. doi: 10.1016/j.cell.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Kisseleva-Romanova E, Lopreiato R, Baudin-Baillieu A, Rousselle J-C, Ilan L, Hofmann K, Namane A, Mann C, Libri D. Yeast homolog of a cancer-testis antigen defines a new transcription complex. EMBO J. 2006;25(15):3576–3585. doi: 10.1038/sj.emboj.7601235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecker A, Graille M, Madec E, Gadelle D, Le Cam E, van Tilbergh H, Forterre P. The universal Kae1 protein and the associated Bud32 kinase (PRPK), a mysterious protein couple probably essential for genome maintenance in Archaea and Eukarya. Biochem Soc Trans. 2009;37(1):29–35. doi: 10.1042/BST0370029. [DOI] [PubMed] [Google Scholar]
- 32.Oberto J, Breuil N, Hecker A, Farina F, Brochier-Armanet C, Culetto E, Forterre P. Qri7/OSGEPL, the mitochondrial version of the universal Kae1/YgjD protein, is essential for mitochondrial genome maintenance. Nucleic Acids Res. 2009;37(16):5343–5352. doi: 10.1093/nar/gkp557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin C a, Ellis SR, True HL. The Sua5 protein is essential for normal translational regulation in yeast. Mol Cell Biol. 2010;30(1):354–363. doi: 10.1128/MCB.00754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampsey M, Na JG, Pinto I, Ware DE, Berroteran RW. Extragenic suppressors of a translation initiation defect in the cyc1 gene of Saccharomyces cerevisiae. Biochimie. 1991;73(12):1445–1455. doi: 10.1016/0300-9084(91)90177-3. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol. 2001;21(13):4347–4368. doi: 10.1128/MCB.21.13.4347-4368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foiani M, Cigan AM, Paddon CJ, Harashima S, Hinnebusch AG. GCD2, a translational repressor of the GCN4 gene, has a general function in the initiation of protein synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11(6):3203–3216. doi: 10.1128/MCB.11.6.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 38.Daugeron M-C, Lenstra TL, Frizzarin M, El Yacoubi B, Liu X, Baudin-Baillieu A, Lijnzaad P, Decourty L, Saveanu C, Jacquier A, Holstege FCP, de Crécy-Lagard V, van Tilbeurgh H, Libri D. Gcn4 misregulation reveals a direct role for the evolutionary conserved EKC/KEOPS in the t6A modification of tRNAs. Nucleic Acids Res. 2011;39(14):6148–6160. doi: 10.1093/nar/gkr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guy MP, Phizicky EM. Conservation of an intricate circuit for crucial modifications of the tRNAPhe anticodon loop in eukaryotes. RNA. 2015;21(1):61–74. doi: 10.1261/rna.047639.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guy MP, Podyma BM, Preston MA, Shaheen HH, Krivos KL, Limbach PA, Hopper AK, Phizicky EM. Yeast Trm7 interacts with distinct proteins for critical modifications of the tRNAPhe anticodon loop. RNA. 2012;18(10):1921–1933. doi: 10.1261/rna.035287.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noma A, Kirino Y, Ikeuchi Y, Suzuki T. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J. 2006;25(10):2142–2154. doi: 10.1038/sj.emboj.7601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiaville PC, El Yacoubi B, Köhrer C, Thiaville JJ, Deutsch C, Iwata-Reuyl D, Bacusmo JM, Armengaud J, Bessho Y, Wetzel C, Cao X, Limbach PA, RajBhandary UL, de Crécy-Lagard V. Essentiality of threonylcarbamoyladenosine (t(6)A), a universal tRNA modification, in bacteria. Mol Microbiol. 2015:1–23. doi: 10.1111/mmi.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taniguchi T, Miyauchi K, Nakane D, Miyata M, Muto A, Nishimura S, Suzuki T. Decoding system for the AUA codon by tRNAIle with the UAU anticodon in Mycoplasma mobile. Nucleic Acids Res. 2013;41(4):2621–2631. doi: 10.1093/nar/gks1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machnicka M a, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, Helm M, Bujnicki JM, Grosjean H. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res. 2013;41((Database issue)):D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Björk GR, Huang B, Persson OP, Byström AS. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA. 2007;13(8):1245–1255. doi: 10.1261/rna.558707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewez M, Bauer F, Dieu M, Raes M, Vandenhaute J, Hermand D. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc Natl Acad Sci U S A. 2008;105(14):5459–5464. doi: 10.1073/pnas.0709404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson MJO, Esberg A, Huang B, Björk GR, Byström AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol. 2008;28(10):3301–3312. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jäger G, Nilsson K, Björk GR. The phenotype of many independently isolated +1 frameshift suppressor mutants supports a pivotal role of the P-site in reading frame maintenance. PLoS One. 2013;8(4):e60246. doi: 10.1371/journal.pone.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urbonavicius J, Stahl G, Durand JMB, Ben Salem SN, Qian Q, Farabaugh PJ, Björk GR. Transfer RNA modifications that alter +1 frameshifting in general fail to affect -1 frameshifting. RNA. 2003;9(6):760–768. doi: 10.1261/rna.5210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang B, Johansson MJO, Byström AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11(4):424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C, Huang B, Eliasson M, Rydén P, Byström AS. Elongator Complex Influences Telomeric Gene Silencing and DNA Damage Response by Its Role in Wobble Uridine tRNA Modification. PLoS Genet. 2011;7(9):e1002258. doi: 10.1371/journal.pgen.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauer F, Hermand D. A coordinated codon-dependent regulation of translation by Elongator. Cell Cycle. 2012;11(24):4524–4529. doi: 10.4161/cc.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esberg A, Huang B, Johansson MJO, Byström AS. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell. 2006;24(1):139–148. doi: 10.1016/j.molcel.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 54.Zinshteyn B, Gilbert W V. Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling. PLoS Genet. 2013;9(8):e1003675. doi: 10.1371/journal.pgen.1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rojas-Benítez D, Ibar C, Glavic Á. The Drosophila EKC/KEOPS complex: roles in protein synthesis homeostasis and animal growth. Fly (Austin) 2013;7(3):168–172. doi: 10.4161/fly.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rojas-Benitez D, Thiaville PC, de Crécy-Lagard V, Glavic A. The Levels of a Universally Conserved tRNA Modification Regulate Cell Growth. J Biol Chem. 2015;290(30):18699–18707. doi: 10.1074/jbc.M115.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibar C, Cataldo VF, Vásquez-Doorman C, Olguín P, Glavic A. Drosophila p53-related protein kinase is required for PI3K/TOR pathway-dependent growth. Development. 2013;140(6):1282–1291. doi: 10.1242/dev.086918. [DOI] [PubMed] [Google Scholar]
- 58.Scheidt V, Juedes A, Baer C, Klassen R, Schaffrath R. Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling. Microb Cell. 2014;1(12):416–424. doi: 10.15698/mic2014.12.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiaville PC, de Crecy-Lagard V. The emerging role of complex modifications of tRNALysUUU in signaling pathways. Microb Cell. 2015;2(1):1–4. doi: 10.15698/mic2015.01.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nedialkova DD, Leidel SA. Optimization of Codon Translation Rates via tRNA Modifications Maintains Proteome Integrity. Cell. 2015;161(7):1606–1618. doi: 10.1016/j.cell.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naor A, Thiaville PC, Altman-Price N, Cohen-Or I, Allers T, de Crécy-Lagard V, Gophna U. A Genetic Investigation of the KEOPS Complex in Halophilic Archaea. PLoS One. 2012;7(8):e43013. doi: 10.1371/journal.pone.0043013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuranda K, Leberre V, Sokol S, Palamarczyk G, François J. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol Microbiol. 2006;61(5):1147–1166. doi: 10.1111/j.1365-2958.2006.05300.x. [DOI] [PubMed] [Google Scholar]
- 63.Alings F, Sarin LP, Fufezan C, Drexler HC a, Leidel S a. An evolutionary approach uncovers a diverse response of tRNA 2-thiolation to elevated temperatures in yeast. 2014;RNA21(2):202–212. doi: 10.1261/rna.048199.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klassen R, Grunewald P, Thüring KL, Eichler C, Helm M, Schaffrath R. Loss of Anticodon Wobble Uridine Modifications Affects tRNALys Function and Protein Levels in Saccharomyces cerevisiae. PLoS One. 2015;10(3):e0119261. doi: 10.1371/journal.pone.0119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kingsbury JM, McCusker JH. Homoserine toxicity in Saccharomyces cerevisiae and Candida albicans homoserine kinase (thr1Delta) mutants. Eukaryot Cell. 2010;9(5):717–728. doi: 10.1128/EC.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katz C, Cohen-Or I, Gophna U, Ron EZ. The ubiquitous conserved glycopeptidase Gcp prevents accumulation of toxic glycated proteins. MBio. 2010;1(3):1–10. doi: 10.1128/mBio.00195-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119(1):115–119. doi: 10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- 68.Anderson J, Phan L, Cuesta R, Carlson BA, Pak M, Asano K, Björk GR, Tamame M, Hinnebusch AG. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12(23):3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balakrishnan R, Park J, Karra K, Hitz BC, Binkley G, Hong EL, Sullivan J, Micklem G, Cherry JM. YeastMine--an integrated data warehouse for Saccharomyces cerevisiae data as a multipurpose tool-kit. Database J Biol Databases Curation. 2012;2012:bar062. doi: 10.1093/database/bar062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura A, Kotani T, Sasano Y, Takagi H. An antioxidative mechanism mediated by the yeast N-acetyltransferase Mpr1: Oxidative stress-induced arginine synthesis and its physiological role. FEMS Yeast Res. 2010;10(6):687–698. doi: 10.1111/j.1567-1364.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 71.Harbison CT, Gordon DB, Lee TI, Rinaldi NJ, Macisaac KD, Danford TW, Hannett NM, Tagne J, Reynolds DB, Yoo J, Jennings EG, Zeitlinger J, Pokholok DK, Kellis M, Rolfe PA, Takusagawa KT, Lander ES, Gifford DK, Fraenkel E, Young RA. Transcriptional regulatory code of a eukaryotic genome. Nature. 2004;431(7004):99–104. doi: 10.1038/nature02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolin SL, Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988;7(11):3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Legendre R, Baudin-Baillieu A, Hatin I, Namy O. RiboTools: A Galaxy toolbox for qualitative ribosome profiling analysis. Bioinformatics. 2015;31(15):2586–2588. doi: 10.1093/bioinformatics/btv174. [DOI] [PubMed] [Google Scholar]
- 74.Pearson CE. Repeat associated non-ATG translation initiation: one DNA, two transcripts, seven reading frames, potentially nine toxic entities! PLoS Genet. 2011;7(3):e1002018. doi: 10.1371/journal.pgen.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen S-J, Lin G, Chang K-J, Yeh L-S, Wang C-C. Translational efficiency of a non-AUG initiation codon is significantly affected by its sequence context in yeast. J Biol Chem. 2008;283(6):3173–3180. doi: 10.1074/jbc.M706968200. [DOI] [PubMed] [Google Scholar]
- 76.Clements JM, Laz TM, Sherman F. Efficiency of translation initiation by non-AUG codons in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8(10):4533–4536. doi: 10.1128/MCB.8.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang C-P, Chen S-J, Lin C-H, Wang T-L, Wang C-C. A single sequence context cannot satisfy all non-AUG initiator codons in yeast. BMC Microbiol. 2010;10(1):188. doi: 10.1186/1471-2180-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang K-J, Wang C-C. Translation initiation from a naturally occurring non-AUG codon in Saccharomyces cerevisiae. J Biol Chem. 2004;279(14):13778–13785. doi: 10.1074/jbc.M311269200. [DOI] [PubMed] [Google Scholar]
- 79.Tang H-L, Yeh L-S, Chen N-K, Ripmaster T, Schimmel P, Wang C-C. Translation of a yeast mitochondrial tRNA synthetase initiated at redundant non-AUG codons. J Biol Chem. 2004;279(48):49656–49663. doi: 10.1074/jbc.M408081200. [DOI] [PubMed] [Google Scholar]
- 80.Sharp PM, Li WH. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15(3):1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.dos Reis M, Savva R, Wernisch L. Solving the riddle of codon usage preferences: a test for translational selection. Nucleic Acids Res. 2004;32(17):5036–5044. doi: 10.1093/nar/gkh834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pechmann S, Frydman J. Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nat Struct Mol Biol. 2013;20(2):237–243. doi: 10.1038/nsmb.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stadler M, Fire A. Wobble base-pairing slows in vivo translation elongation in metazoans. RNA. 2011;17(12):2063–2073. doi: 10.1261/rna.02890211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gardin J, Yeasmin R, Yurovsky A, Cai Y, Skiena S, Futcher B. Measurement of average decoding rates of the 61 sense codons in vivo. Elife. 2014;3:1–20. doi: 10.7554/eLife.03735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kramer EB, Farabaugh PJ. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. . RNA. 2007;13(1):87–96. doi: 10.1261/rna.294907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farabaugh PJ, Björk GR. How translational accuracy influences reading frame maintenance. EMBO J. 1999;18(6):1427–1434. doi: 10.1093/emboj/18.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawakami K, Pande S, Faiola B, Moore DP, Boeke JD, Farabaugh PJ, Strathern JN, Nakamura Y, Garfinkel DJ. A rare tRNA-Arg(CCU) that regulates Ty1 element ribosomal frameshifting is essential for Ty1 retrotransposition in Saccharomyces cerevisiae. Genetics. 1993;135(2):309–320. doi: 10.1093/genetics/135.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manickam N, Nag N, Abbasi A, Patel K, Farabaugh PJ. Studies of translational misreading in vivo show that the ribosome very efficiently discriminates against most potential errors. RNA. 2014;20(1):9–15. doi: 10.1261/rna.039792.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dube SK, Marcker K a, Clark BF, Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- 90.Miller JH. GUG and UUG are initiation codons in vivo. Cell. 1974;1(2):73–76. doi: 10.1016/0092-8674(74)90063-4. [DOI] [Google Scholar]
- 91.Files JG, Weber K, Coulondre C, Miller JH. Identification of the UUG codon as a translational initiation codon in vivo. J Mol Biol. 1975;95(2):327–330. doi: 10.1016/0022-2836(75)90398-8. [DOI] [PubMed] [Google Scholar]
- 92.Stewart JW, Sherman F, Shipman N a, Jackson M. Identification and mutational relocation of the AUG codon initiating translation of iso-1-cytochrome c in yeast. J Biol Chem. 1971;246(24):7429–7445. [PubMed] [Google Scholar]
- 93.Baralle FE, Brownlee GG. AUG is the only recognisable signal sequence in the 5′ non-coding regions of eukaryotic mRNA. Nature. 1978;274(5666):84–87. doi: 10.1038/274084a0. [DOI] [PubMed] [Google Scholar]
- 94.Weissenbach J, Grosjean H. Effect of threonylcarbamoyl modification (t6A) in yeast tRNA Arg III on codon-anticodon and anticodon-anticodon interactions. A thermodynamic and kinetic evaluation. . Eur J Biochem. 1981;116(1):207–213. doi: 10.1111/j.1432-1033.1981.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 95.Zaborske JM, Bauer DuMont VL, Wallace EWJ, Pan T, Aquadro CF, Drummond DA. A Nutrient-Driven tRNA Modification Alters Translational Fidelity and Genome-wide Protein Coding across an Animal Genus. PLoS Biol. 2014;12(12):e1002015. doi: 10.1371/journal.pbio.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes: Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. . J Mol Biol. 1982;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- 97.Kraakman L, Lemaire K, Ma P, Teunlssen AWRH, Donaton MC V, Van Dijck P, Winderickx J, De Winde JH, Thevelein JM. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol Microbiol. 1999;32(5):1002–1012. doi: 10.1046/j.1365-2958.1999.01413.x. [DOI] [PubMed] [Google Scholar]
- 98.Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, Fisk DG, Hirschman JE, Hitz BC, Karra K, Krieger CJ, Miyasato SR, Nash RS, Park J, Skrzypek MS, Simison M, Weng S, Wong ED. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2012;40(D1):700–705. doi: 10.1093/nar/gkr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kato M, Slack FJ. Ageing and the small, non-coding RNA world. 2012:1–7. doi: 10.1016/j.arr.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Na JG, Pinto I, Hampsey M. Isolation and Characterization of SUA5, a Novel Gene Required for Normal Growth in Saccharomyces cerevisiae. Genetics. 1992;151(August):791–801. doi: 10.1093/genetics/131.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller JP, Hussain Z, Schweizer MP. The involvement of the anticodon adjacent modified nucleoside N-(9-(BETA-D-ribofuranosyl) purine-6-ylcarbamoyl)-threonine in the biological function of E. coli tRNAile. Nucleic Acids Res. 1976;3(5):1185–1201. doi: 10.1093/nar/3.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rohde JR, Bastidas R, Puria R, Cardenas ME. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr Opin Microbiol. 2008;11(2):153–160. doi: 10.1016/j.mib.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turowski TW, Karkusiewicz I, Kowal J, Boguta M. Maf1-mediated repression of RNA polymerase III transcription inhibits tRNA degradation via RTD pathway. RNA. 2012;18(10):1823–1832. doi: 10.1261/rna.033597.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 1999;13(24):3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farabaugh PJ. Programmed translational frameshifting. Annu Rev Genet. 1996;30:507–528. doi: 10.1146/annurev.genet.30.1.507. [DOI] [PubMed] [Google Scholar]
- 107.Tumu S, Patil A, Towns W, Dyavaiah M, Begley TJ. The gene-specific codon counting database: a genome-based catalog of one-, two-, three-, four- and five-codon combinations present in Saccharomyces cerevisiae genes. Database (Oxford) 2012;2012:bas002. doi: 10.1093/database/bas002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Powers ET, Balch WE. Costly mistakes: translational infidelity and protein homeostasis. Cell. 2008;134(2):204–206. doi: 10.1016/j.cell.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 109.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 110.Herzog B, Popova B, Jakobshagen A, Shahpasandzadeh H, Braus GH. Mutual cross talk between the regulators Hac1 of the unfolded protein response and Gcn4 of the general amino acid control of Saccharomyces cerevisiae. Eukaryot Cell. 2013;12(8):1142–1154. doi: 10.1128/EC.00123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gietz RD, Schiestl RH. Frozen competent yeast cells that can be transformed with high efficiency using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2(1):1–4. doi: 10.1038/nprot.2007.17. [DOI] [PubMed] [Google Scholar]
- 112.Pomerantz SC, McCloskey JA. Analysis of RNA hydrolyzates by liquid chromatography-mass spectrometry. Methods Enzymol. 1990;193(1983):796–824. doi: 10.1016/0076-6879(90)93452-q. [DOI] [PubMed] [Google Scholar]
- 113.Baudin-Baillieu A, Legendre R, Kuchly C, Hatin I, Demais S, Mestdagh C, Gautheret D, Namy O. Genome-wide Translational Changes Induced by the Prion [PSI+]. Cell Rep. 2014;8(2):439–448. doi: 10.1016/j.celrep.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 114.Baudin-Baillieu A, Hatin I, Legendre R, Namy O. Translation Analysis at the Genome Scale by Ribosome Profiling. Methods in Molecular Biology. 2016;1361:105–124. doi: 10.1007/978-1-4939-3079-1_7. [DOI] [PubMed] [Google Scholar]
- 115.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1) doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 116.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Engel SR, Dietrich FS, Fisk DG, Binkley G, Balakrishnan R, Costanzo MC, Dwight SS, Hitz BC, Karra K, Nash RS, Weng S, Wong ED, Lloyd P, Skrzypek MS, Miyasato SR, Simison M, Cherry JM. The reference genome sequence of Saccharomyces cerevisiae: then and now. G3 (Bethesda) 2014;4(3):389–398. doi: 10.1534/g3.113.008995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anders S, EMBL. Analysing RNA-Seq data with the DESeq package. 2012 Available at: https://www.bioconductor.org/help/course-materials/2011/BioC2011/LabStuff/DESeq.pdf. [Google Scholar]
- 120.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 121.Kushnirov V V. Rapid and reliable protein extraction from yeast. Yeast. 2000;16(9):857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 122.Koplin A, Preissler S, Llina Y, Koch M, Scior A, Erhardt M, Deuerling E. A dual function for chaperones SSB-RAC and the NAC nascent polypeptide-associated complex on ribosomes. J Cell Biol. 2010;189(1):57–68. doi: 10.1083/jcb.200910074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.