Abstract
Increased red blood cell (RBC) volume variation (RDW) has recently been shown to predict a wide range of mortality and morbidity: death due to cardiovascular disease, cancer, infection, renal disease, and more; complications in heart failure and coronary artery disease, advanced stage and worse prognosis in many cancers, poor outcomes in autoimmune disease, and many more. The mechanisms by which all of these diseases lead to increased RDW are unknown. Here we use a semi-mechanistic mathematical model of in vivo RBC population dynamics to dissect the factors controlling RDW and show that elevated RDW results largely from a slight reduction in the in vivo rate of RBC turnover. RBCs become smaller as they age, and a slight reduction in the rate of RBC turnover allows smaller cells to continue circulating, expanding the low-volume tail of the RBC population’s volume distribution, and thereby increasing RDW. Our results show that mildly extended RBC lifespan is a previously unrecognized homeostatic adaptation common to a very wide range of pathologic states, likely compensating for subtle reductions in erythropoietic output. A mathematical model-based estimate of the clearance rate may provide a novel early-warning biomarker for a wide range of morbidity and mortality.
Introduction
The complete blood count (CBC) is one of the most widely-used clinical tests, helping guide the diagnosis and treatment of almost all diseases. It summarizes basic features of circulating blood cell populations, including the variation in the volume of individual red blood cells (RBCs), quantified by its coefficient of variation (CV) and called the red cell distribution width or RDW. Recently, higher RDW has been found to be significantly associated with increased risk for a remarkably wide range of morbidity and mortality: all-cause mortality, mortality from heart disease, pulmonary disease, sepsis, and cancer; complications in heart failure and severity of coronary artery disease; advanced stage and grade for many cancers; development of diabetes, chronic obstructive pulmonary disease, stroke, anemia, and many more [1–8]. RDW thus represents a biomarker with tremendous potential to inform clinical practice as well as our understanding of the early stages of a wide range of fundamental disease processes, but we first must understand the pathophysiologic mechanisms linking these disparate disease processes to increased variation in RBC volume. Here we use a semi-mechanistic model of in vivo red blood cell population dynamics [9] to show that a delay in the in vivo clearance of RBCs is the major mechanism leading to elevated RDW. We find that RBC clearance rate is exquisitely controlled in healthy individuals and that it is reduced in a range of adverse clinical situations. Because RBCs become smaller as they age, this delay in clearance expands the low-volume tail of the volume distribution and increases RDW. Our study shows that RBC clearance is a previously unrecognized fundamental control point for human physiology, presumably adapting to subtle reductions in erythropoietic output to maintain circulating red cell mass in a wide range of the most important pathologic conditions.
New RBCs enter the circulation of a healthy human adult at a rate of more than 2 million per second, with equally massive flux through RBC maturation and clearance processes. Each RBC circulates for about 100–120 days, with its volume decreasing by about 30% during that time from an initial ~115 fl to a final ~80 fl [10–13]. See Fig. 1A for a schematic. The major intracellular RBC constituent is hemoglobin, and the typical RBC’s hemoglobin mass drops by about 20% from an initial ~35 pg on day 1 to a final ~28 pg 100–120 days later [10–13]. The RBC hemoglobin concentration increases slightly and is tightly regulated to ensure efficient oxygen delivery to the tissues. While the molecular and cellular mechanisms controlling these maturation and clearance processes are not fully understood [14], their net effects have been clearly established using RBC labeling and other studies [10,12–16,33–35], and it is therefore possible to estimate the rate of RBC clearance even in the absence of an accepted molecular clearance mechanism.
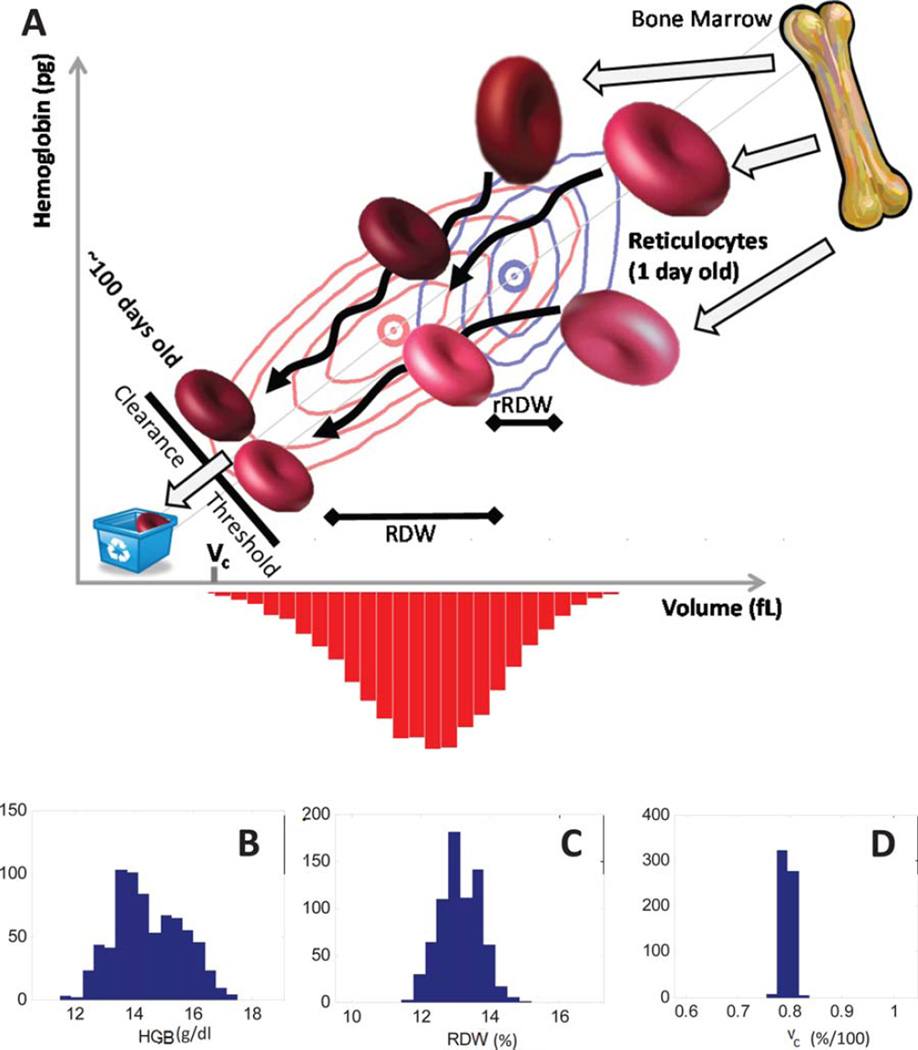
Figure 1.
RBC population dynamics and determinants of volume variation. Reticulocytes enter the circulation from the bone marrow (Panel A, top right). The volume and hemoglobin distribution for reticulocytes from a typical healthy individual is shown as blue contours enclosing 75, 50, and 25% of reticulocytes with a blue circle at the means. Reticulocytes mature into RBCs, circulating for ~100–120 days, becoming ~30% smaller and losing ~20% of their hemoglobin, with simulated trajectories shown as black arrows. The healthy individual’s total RBC volume and hemoglobin distribution is shown as red contours enclosing 90, 75, 50, and 25% of cells, with means shown as a red circle. Senescent RBCs are cleared and recycled before reaching a threshold that depends on the cell’s volume and hemoglobin, with vc (bottom left) designating that threshold for the typical RBC with hemoglobin concentration equal to the population mean. The variation in the RBC volume distribution (red histogram) is quantified by its coefficient of variation, called the red cell distribution width or RDW. The RDW is determined by (1) the mean volume, (2) the coefficient of variation in reticulocyte volume (rRDW), (3) the degree of heterogeneity in volume dynamics (black arrows) during maturation, and (4) the location of the clearance threshold (vc). See Supplementary Figs. S-1 and S-2 for additional schematics. Panels B–D show that vc is more tightly regulated (CV = 1.1%) in a healthy population (See “Healthy Patient Cohort” in Supplementary Methods n = 600) than HGB (CV = 7.9%), RDW (CV = 4.5%), or HCT (7.5% in Supporting Information Fig. S-3). x axis widths are the same fraction of the means.
The coefficient of variation in RBC volume (RDW) has historically been useful in distinguishing some causes of anemia such as iron deficiency but otherwise plays a marginal role in general medical diagnosis [8,17]. Reports in 2007 found a surprising significant association between elevated RDW and increased risk of poor prognosis in heart failure and coronary artery disease [2,4]. Hundreds of studies have followed finding remarkable associations between elevated RDW and a very wide range of morbidity and mortality [1–8]. The pathophysiologic processes common to all of these clinical states and leading to an increase in RBC volume variation are unknown.
Methods
Sample collection and clinical laboratory measurement
We measured CBCs and reticulocyte counts for more than 60,000 blood samples randomly selected from among those sent to the Massachusetts General Hospital (MGH) Clinical Laboratory. All reticulocyte and CBCs used in modeling were measured on an Abbott Cell-DYN Sapphire 4000 automated hematology analyzer (Abbott Hematology, Santa Clara, CA). All human studies were approved by the Partners Healthcare Institutional Review Board. See Supporting Information for more detail on patient cohorts and additional clinical laboratory testing.
Modeling of RBC population dynamics
We used a mathematical model of in vivo RBC population dynamics [9,34] to infer single-RBC rates of maturation and clearance for individual patients from single-RBC measurements of volume and hemoglobin content. See Fig. 1 and Supporting Information Figs. S-1 and S-2 for more detail. RBCs become smaller and lose hemoglobin as they age, with an initial fast phase of reduction followed by a slow phase [10,13,15,33]. The rates of the initial fast phase are quantified for each individual by model parameters βv and βh. The model quantifies the slower rate with the parameter α. Rates of volume and hemoglobin reduction vary for a single RBC over time and from one RBC to the next in the population. The model enables estimation of the magnitude of variation in rates of both hemoglobin (Dh) and volume reduction (Dv). The molecular trigger and mechanism for RBC clearance are not fully understood [14], but common proposed mechanisms and empirical measurements of RBC populations such as those in Fig. 1 (red contours) show that the probability of clearance is correlated with an RBC’s volume and hemoglobin, and the true clearance function can be approximated as a threshold function of RBC volume and hemoglobin. The probability of clearance for a particular RBC increases rapidly as the cell nears the clearance threshold, a line perpendicular to and intersecting the MCHC (mean corpuscular hemoglobin concentration) line at a volume equal to vc. An RBC with a hemoglobin concentration equal to the MCHC is most likely to be recycled when its volume reaches vc. RBCs with higher hemoglobin concentrations will circulate until reaching slightly lower volumes, and RBCs with lower hemoglobin concentrations will be cleared at slightly higher volumes. Details of model derivation, validation, and parameter estimation have previously been published [9]. All data analysis and modeling was done in MATLAB (MathWorks, Natick, Massachusetts). See “Modeling RBC Population Dynamics” in Supporting Information for more detail.
Results
Because , an increase in RDW must mathematically be caused by an increase in RBC volume variance or a decrease in mean volume (MCV), or both. The causes of increased volume variance can be categorized based on when they act during the RBC lifecycle: an increased variance in the volume of young RBCs called reticulocytes [18] exiting the bone marrow, an increase in the accumulation of volume variation during RBC maturation in the circulation, or a delay in the clearance of senescent RBCs. Thus, the cause of increased RDW must be one of the following four possibilities:
Decreased mean volume.
Increased reticulocyte volume variance.
Increased heterogeneity in the rate of RBC volume reduction occurring in the peripheral circulation.
Delayed RBC clearance.
A combination of these sources may also be involved, but we first investigate each separately.
If one of these four factors is dominant in elevating RDW, it must be narrowly distributed in healthy people, i.e., sufficiently specific to show robust independent association with a wide range of morbidity and mortality. A possible role for MCV in mortality and morbidity risk has been assessed in some of the studies identifying RDW associations [1–8], and it is unlikely to be the dominant factor in RDW elevation. The second possible cause of elevated RDW is increased reticulocyte volume variation (rRDW). rRDW is not narrowly distributed in healthy people, fluctuating significantly with a CV of more than 8% (see Supporting Information Fig. S-4). The third and fourth potential causes, heterogeneity of the volume reduction processes occurring while RBCs circulate, and the rate of RBC clearance, have not been possible to quantify routinely until now. We use a recently developed mathematical model of in vivo RBC population dynamics [9] to estimate the heterogeneity in volume reduction (Dv), and to estimate the rate of RBC clearance as a threshold function of an RBC’s volume and hemoglobin (vc). See Supporting Information “Reticulocyte and Complete Blood Counts” and “Modeling RBC Population Dynamics” for more detail. Dv varies significantly in a healthy population with a CV > 20% (Supporting Information Fig. S-4) and is unlikely to be the dominant cause. In contrast, the rate of RBC clearance as estimated by a threshold function (vc) is exquisitely controlled in healthy individuals with a CV of about 1%, as compared to CVs of 4% for RDW and 7% for HCT and HGB (see Fig. 1B–D and Supporting Information Fig. S-3). RBC clearance delay is therefore a good candidate for the mechanism underlying elevations of RDW in many diseases.
We now provide the following additional evidence that delayed RBC clearance, as estimated by vc, causes elevated RDW in general and in many pathologic conditions: (1) decreased vc correlates with RDW much more strongly than the other potential causes; (2) individuals with increased RDW have a mild increase in average RBC age that only a causal relationship between clearance delay and elevated RDW can explain; (3) decreased vc correlates with all-cause mortality risk just as strongly as RDW; (4) among individuals with normal CBCs a low vc is associated with a higher odds ratio for future RDW-associated pathology like anemia; and (5) individuals with low vc have a higher relative risk for current latent pathology like decreased iron stores, supporting the conclusion that in response to this sort of pathologic state, RBC clearance is delayed, temporarily compensating for decreased erythropoietic output, and over time causing an increase in RDW.
Delayed RBC clearance is strongly correlated with elevated RDW
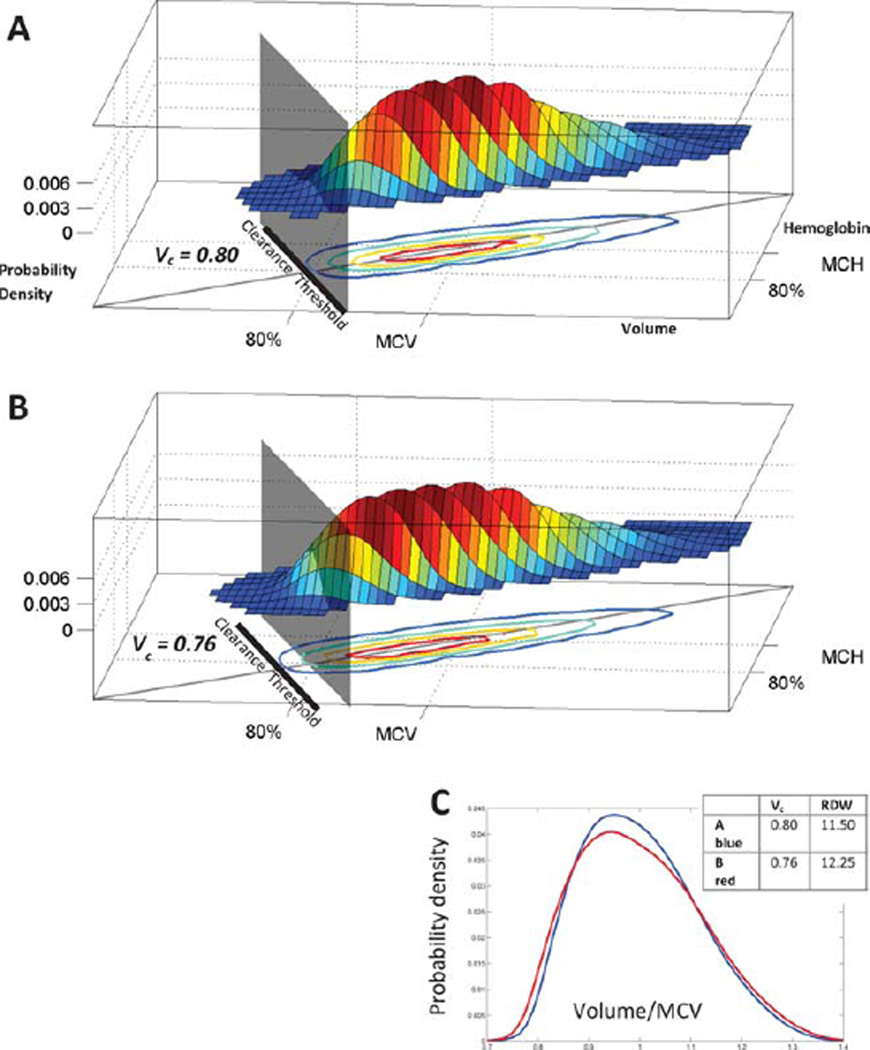
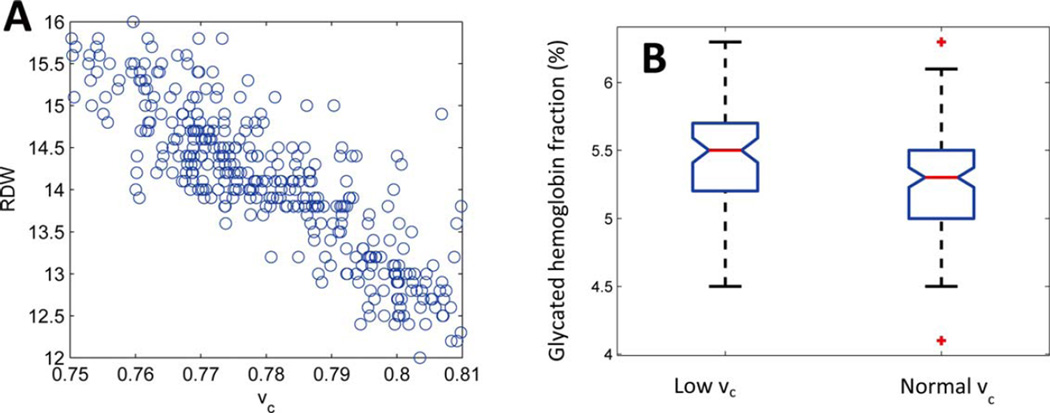
Delayed clearance enables RBCs to continue circulating with smaller volumes than would be possible otherwise, expanding and extending the low-volume tail of the RBC volume distribution. The MCV will decrease slightly, but the major effect is an elevation in the variance, increasing the RDW, as we will demonstrate. Figure 2A shows the volume and hemoglobin distribution for a healthy individual with a vc of 0.80. Using the model of RBC population dynamics, we simulated the effect of delaying RBC clearance by lowering vc to 0.76 (Fig. 2B). The low-volume tail expands beyond the original vc marked with a gray plane at 0.80. This decrease in vc raises the RDW from 11.75% to 12.5% (Fig. 2C). If delayed clearance is the major mechanism for increases in RDW, we would expect to find a strong correlation between vc and RDW. Figure 3A shows that the correlation coefficient between vc and RDW is −0.91 in a randomly selected population of 450 patients receiving blood counts at Massachusetts General Hospital. See “Random Selection of Complete Blood Counts (Correlation Cohort)” in Supplementary Methods for more detail. The correlation between RDW and vc is much stronger than the correlation between RDW and any of the other three possible causes of an increased RDW: −0.23 for MCV, 0.64 for rRDW, and 0.41 for Dv, as shown in Supporting Information Fig. S-6.
Figure 2.
Delayed RBC clearance increases RDW. Panel A shows the RBC volume and hemoglobin distribution for a healthy individual with an estimated RBC clearance threshold vc = 0.80. Volume and hemoglobin are scaled by their means. Contours enclose 95, 75, 50, and 25% of RBCs. A gray plane and black line show the clearance threshold. A gray diagonal line through the origin shows the mean RBC hemoglobin concentration (MCHC). Panel B models the effect of lowering that individual’s clearance threshold by 5% to 0.76 (thick black line). The gray plane remains at the original vc and shows that the low-volume tail expands through that plane, increasing the RDW from 11.5 to 12.25 (Panel C).
Figure 3.
RDW and glycated hemoglobin depend on vc. Panel A shows RDW and vc for 450 randomly selected patients with CBCs at Massachusetts General Hospital. See “Correlation Cohort” in Methods. The correlation coefficient (ρ) is −0.91. Panel B shows boxplots of glycated hemoglobin for 74 non-diabetic individuals with vc < 0.775 (median vc = 0.77) and 115 individuals with vc > 0.79 (median vc = 0.80). See “A1c Cohort” in Methods for more detail. The red horizontal lines show the medians of each group (5.5% and 5.3%). These medians are different according to a Kruskall–Wallis test with P < 0.002. The low vc group had a median RDW of 14.6%, and the high vc group had a median RDW of 12.9%. The notches show the 95% confidence intervals for the medians. The boxes extend from the 25th to the 75th percentile for each group.
Delayed RBC clearance and elevated RDW are associated with increased mean RBC age
A delay in RBC clearance will not only increase RDW, it will also enable cells to circulate beyond the time when they would ordinarily have been cleared, thus increasing the average RBC age and lifespan. None of the other three possible causes of increased RDW will have a consistent effect on RBC age. We can use the model to estimate the magnitude of the expected clearance delay required to generate the ~0.5% increase in RDW shown to be broadly associated with morbidity and mortality [3,19]. Model simulations show that a delay in clearance of <3%, for instance a drop in vc from 0.79 to 0.77, can increase the RDW by ~0.5% (e.g., from 13.2 to 13.7%) and the mean RBC age by 3–7% (e.g., from 50 to 51.5–53.5 days). We therefore sought to test this model-generated prediction of increased mean RBC age by estimating mean RBC age and vc. There are a number of ways to estimate mean RBC age, and the best method depends on a number of factors, including whether an absolute or relative estimate is needed. To test the hypothesis of clearance delay, we need to estimate only the change in mean RBC age relative to an individual’s baseline. We do not need an absolute estimate of mean RBC age, which likely varies by 20% in healthy individuals and more in those with chronic illness [14]. Labeling studies are required for estimates of absolute mean RBC age and life span, but the glycated hemoglobin fraction can be used to estimate mean RBC age relative to baseline in non-diabetic individuals. The glycated hemoglobin fraction (hemoglobin A1c or HbA1c) is routinely measured to assess glucose control in diabetic patients. HbA1c is proportional to the mean plasma glucose concentration and to the mean RBC age [20–22]. In non-diabetic individuals, HbA1c is linearly dependent on mean RBC age [14,20,23–27]. We can therefore test the prediction that elevated RDW is associated with elevated mean RBC age by measuring vc and HbA1c in non-diabetics.
Based on published estimates of in vivo glycation rates in nondiabetics, a ~3–7% increase in RBC lifespan would be expected to increase HbA1c by about 0.2–0.3% (e.g., from 5.3% to about 5.5% or 5.6%) [20]. Figure 3B compares HbA1c for a nondiabetic group with normal vc (median 0.80) and a nondiabetic group with low vc (median 0.77). See “Prospective Study of Dependence of Glycated Hemoglobin Fraction on vc (‘A1c Cohort’)” in Supplementary Methods for more detail. The normal vc group had a median HbA1c of 5.3% and a median RDW of 12.9%. The low vc group with an estimated 3–7% increase in average RBC age had a median HbA1c of 5.5% and a median RDW of 14.6%. We find that delayed RBC clearance and increased RDW in non-diabetics is associated with elevated HbA1c and thus increased mean RBC age, supporting the conclusion that delayed RBC clearance is the predominant cause of elevated RDW. Because vc and RDW are so strongly correlated (Fig. 3A), we are able to find additional support for our prediction that delayed clearance and elevated RDW are associated with increased mean RBC age in studies that measured HbA1c and RDW in non-diabetics. At least two recent independent studies of 15,343 nondiabetics in the United States and 4,845 nondiabetics in Sweden find this same association between increased RDW and increased HbA1c with high statistical significance [28,29]. We thus find evidence in three independent studies of more than 20,000 non-diabetics that RBC clearance delay and increased RDW are significantly associated with increased HbA1c and thus increased mean RBC life span. See Supplementary Methods “Prospective Study of Dependence of Glycated Hemoglobin Fraction on vc (A1c Cohort)” for more detail.
Delayed RBC clearance is associated with increased odds of all-cause mortality
If delayed RBC clearance elevates RDW in disease, then we would expect low vc to be as predictive of all-cause mortality as elevated RDW. In a case-control study of 900 patients, we fit a logistic regression model for odds of death as a function of the patient’s vc. A decrease in vc of 1 standard deviation (SD), for instance from 0.79 to 0.78, increases the odds of death by about e0.23 = 1.26 or 26% (P value < 10−10). An increase of 1 SD in RDW, for instance from 12.5 to 13.1%, increases the odds of death by about the same 26% (P value < 10−10). The logistic regression coefficients for vc and RDW have a correlation coefficient of −0.99, strongly supporting the hypothesis that reductions in vc explain the association between increased RDW and increased risk of all-cause mortality. See Table I and Supplementary Methods “Case Control Study of RDW and vc Association with All-Cause Mortality (‘Mortality Rate Cohort’)” for more detail.
TABLE I.
Odds of All-cause Mortality as a Function of vc and RDW
| Odds ratio for 1 SD change | Coefficient | Standard error | P value | |
|---|---|---|---|---|
| log (odds of death) = Constant+β1 · vc | ||||
| Constant | 17.6 | 1.4 | <10−10 | |
| vc | 1.26 | −22.8 | 1.8 | <10−10 |
| log (odds of death) = Constant+β2 · RDW | ||||
| Constant | −6.50 | 0.43 | <10−10 | |
| RDW | 1.26 | 0.44 | 0.03 | <10−10 |
Logistic regression modeling shows vc contains the same predictive information as RDW for all-cause mortality. It is not possible to fit a model containing both RDW and vc as predictors, because they are strongly collinear, as would be expected from Fig. 3A.
Delayed RBC clearance has increased odds of future RDW-associated pathology
If delayed RBC clearance causes elevated RDW in response to latent or incipient pathology, then a decrease in vc should precede both the elevation in RDW and the future clinical appearance of RDW-associated pathologic conditions. Risk of all-cause anemia is one condition associated with elevated RDW [5,6]. We first assessed the predictive value of vc for anemia relative to the reference range for vc in Fig. 1D, defining “low” as beneath the 5th percentile (0.7798). Among people with normal CBCs, we expect that those with low vc have delayed RBC clearance as compensation for some latent pathophysiologic process. Because some of these underlying disease processes will lead to anemia, we expect the odds of future anemia in the low vc group to be higher than that in a control group with normal vc. We identified 299 patients with normal CBCs who went on to develop anemia in the subsequent 3 months due to any cause. At the time of their normal CBC as many as 3 months prior to anemia, 15% (44/299) had a low vc. In contrast, in a separate group of 98 patients with normal CBCs and no decrease in HCT in the subsequent year, only 3% (3/98) had a low vc. The odds ratio for the association between low vc and future anemia is 4.8, with 95% confidence interval (1.5, 15.8), supporting the conclusion that delayed RBC clearance precedes pathologic conditions associated with elevated RDW like all-cause anemia. See Supplementary Methods “Case Control Study of vc-based Prediction of All-Cause Anemia (‘Anemia Prediction Cohort’)” for more detail.
If delayed RBC clearance causes elevations in RDW, then changes in vc should precede changes in existing CBC indices independent of any reference range. We tested this hypothesis in the same patient population by comparing the predictive accuracy of varying thresholds of vc to the predictive accuracy of varying thresholds of existing CBC indices: HCT, HGB, MCV, RDW, MCH, and MCHC. We find vc is highly predictive of future anemia almost regardless of the specific threshold chosen, reaching a maximum odds ratio of more than 8.0. The existing CBC indices have no thresholds yielding an odds ratio statistically different from 1.0, with the exception of RDW, for which there were a minority of thresholds with significant odds ratios, none of which surpassed 2.0. See Supplementary Methods “Odds Ratios of Future Anemia for Thresholds of CBC parameters and vc” and Supporting Information Table S-3 for additional detail. vc thus adds substantial quantitative predictive power to existing CBC indices even for entirely normal CBCs, supporting the hypothesis that delayed RBC clearance is an early response to a wide range of pathologic conditions and the major cause of elevated RDW.
A delay in RBC clearance may represent either adaptation or maladaptation. We believe RBC clearance delay adaptively compensates for the mild anemia associated with many of the diseases linked to elevated RDW, maintaining circulating red cell mass and oxygen carrying capacity despite reduced erythropoietic output. Prior studies have shown that increases on the order of 0.5% in RDW (e.g., from 13.2% to 13.7%) are associated with increased morbidity and mortality risk [3,19]. Our modeling suggests that this 0.5% increase in RDW can be caused by a delay in RBC clearance that increases mean RBC age by as little as 3%, e.g., from a mean age of 50 days to a mean age of 51.5 days. Modeling also predicts that this magnitude of clearance delay will additionally cause a slight decrease in MCV, a finding also commonly seen with elevated RDW [3]. We hypothesize that this ~3% delay in RBC clearance relative to the individual’s baseline is adaptive compensation for a corresponding ~3% decrease in erythropoietic output. A ~3% reduction in erythropoietic output implies a ~3% reduction in reticulocyte production relative to baseline, e.g., a reduction in reticulocyte count from 1.00% to 0.97%. In a typical complete blood count of ~50,000 RBCs, the absolute number of reticulocytes would drop from 500 to 485. This ~3% relative drop in reticulocyte count (~15 fewer absolute reticulocytes counted per 50,000 RBCs in a CBC) is far too small to be detected by current reticulocyte count assays which show >3× more imprecision (~10%) even on repeat measurements of the same patient blood sample and have coefficients of variation in healthy populations of at least 30% based on our own results and those previously published [30].
Delayed RBC clearance is associated with a higher relative risk for current latent pathology
Some patients become anemic due to iron deficiency. Iron deficiency anemia is associated with elevated RDW at the time of diagnosis [6]. If RBC clearance delay is an adaptive response to subtle decreases in erythropoietic output, then low vc should signal decreasing iron availability—even in some individuals whose HCT has not decreased at all, precisely because their delayed RBC clearance is compensating for any diminished erythropoietic output. We tested this hypothesis in a distinct group of healthy patients, none of whom was anemic. We measured ferritin levels in these individuals with normal CBCs and low vc, and compared them to controls with vc above the median. See Supplementary Methods “Prospective Study of Ferritin Levels (‘Ferritin Cohort’)” for more detail. Because we believe that RBC clearance delay is a common response to a wide range of pathologic conditions in addition to iron deficiency, we would expect many randomly selected individuals with normal CBCs and low vc to be compensating for pathologic conditions other than decreasing iron stores. We therefore expect the absolute prevalence of decreased iron stores to be modest, but we also expect higher relative prevalence of decreased iron stores in individuals with low vc as compared to that for individuals with higher vc. We found decreased iron stores (ferritin in the bottom 5% of the normal range or below) in 10/68 (16%) patients with low vc and in 4/132 (3%) patients with vc above the median. Thus, among patients with entirely normal CBCs, the relative risk of decreased iron stores for those with low vc is 4.9 with a 95% confidence interval of (1.6, 14.7), supporting the conclusion that low vc and the delayed RBC clearance it estimates represent a compensatory response to this pathologic condition. It is possible that the mild decrease in ferritin found in the low vc individuals leads to an iron-restricted erythropoiesis which directly alters RBC morphology in a way that mimics a low vc without actually extending the RBC lifespan. HbA1c provides an estimate of mean RBC age in non-diabetics [14,20,23–27]. We measured hemoglobin A1c levels in the nondiabetics among these individuals with decreased ferritin and low vc and found a statistically significant increase relative to the nondiabetics in the group with normal ferritin and vc, supporting the conclusion that the decreased ferritin is in fact associated with delayed RBC clearance. See Supporting Information Fig. S-7. Calculating vc may thus help identify patients with delayed RBC clearance who would benefit from further diagnostic workup for diseases often underlying iron deficiency such as occult GI bleeding due to undiagnosed colon cancer, or insufficient dietary intake during important developmental periods [31,32]. The predictive accuracy of vc in these particular study populations with these specific current and future pathologic states needs to be validated in other populations with other latent disease or future pathology. Model parameter estimation is robust to the mild erythropoietic perturbations expected for many of the diverse conditions shown to be associated with elevated RDW. Estimated parameters are less accurate in rapidly changing or severe conditions such as acute blood loss or significant hemolysis.
Discussion
We propose that modulation of the RBC clearance threshold is a fundamental adaptation central to the homeostatic response to a very wide range of pathologic conditions presumably involving decreased erythropoietic output. The mathematical model makes assumptions about incompletely understood pathophysiologic processes, and while the model has been extensively tested, it will be less accurate in conditions where the assumptions are less valid. Future studies motivated by this work uncovering new molecular and dynamic details of the processes controlling RBC maturation and clearance will enable model improvements which will likely enhance predictive accuracy. We have focused on vc because it is the dominant overall cause of elevated RDW, but it is likely that other potential contributors to RDW, including MCV, rRDW, and Dv, will also be relevant and even more important in some particular conditions and for some particular patients, and characterizing their contributions in addition to that of vc will provide additional insight.
Calculating vc enables the estimation of the RBC clearance rate and therefore the identification of cohorts of patients in the latent or incipient stage of many important diseases, while the disease or its complications are still invisible to current clinical methods. Translational studies of these newly-accessible patient cohorts are likely to reveal new mechanisms of disease as well as even more powerful biomarkers for specific diseases and their treatments, both those in routine practice and those in development. The broad predictive value of increased RDW suggests that there are many different molecular signals funneled through the common cellular-scale homeostatic adaptation of delayed RBC clearance. Our study shows how mathematical modeling of large amounts of clinical laboratory data can lead to new understanding of human pathophysiology, revealing the mechanistic basis for statistical association between existing biomarkers and important clinical outcomes, and providing a conceptual foundation for more detailed mechanistic investigations and for the rational development of novel predictors with even more robust clinical diagnostic power and utility.
Supplementary Material
Acknowledgments
The authors thank Rosy Jaromin for informatics support; Maureen Hames-English, Francine Molay, Nancy Stanganelli, Debera Grzybek, Kent Lewandrowski, and other members of the MGH Clinical Laboratories and Clinical Research Program for assistance with logistics. They thank the Partners Healthcare Research Patient Data Repository for help retrieving medical record information. All RBC population modeling was performed on the Harvard Medical School Orchestra Computing Cluster. Computational support and resources were also provided by Partners Healthcare Research Computing. The authors thank Don Wright, Martin Krockenberger, Nigel Llewellyn-Smith, Agim Beshiri, and others at Abbott Hematology for technical support. They thank Frank Bunn, Carlo Brugnara, David Louis, Ralph Weissleder, Tim Mitchison, Rebecca Ward, Marc Kirschner, Judy Glaven, Roy Malka, and Lorette Noiret for helpful discussions and feedback on the manuscript.
Conflict of interest: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. JMH is an inventor on a patent application submitted by Massachusetts General Hospital.
Contract grant sponsor: NIDDK; Contract grant number: K08DK083242.
Contract grant sponsor: NIH Director’s New Innovator Award; Contract grant number: DP2DK098087.
Contract grant sponsor: Abbott Hematology.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Author Contributions
HHP performed blood count measurements, maintained instruments, and was responsible for all additional clinical laboratory testing. HRP maintained clinical laboratory instruments and assisted with additional sample testing. JMH designed and managed study, analyzed data, and wrote the article.
References
- 1.Horne BD. A changing focus on the red cell distribution width: Why does it predict mortality and other adverse medical outcomes? Cardiology. 2012;122:213–215. doi: 10.1159/000341244. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure—Data from the CHARM program and the duke databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 3.Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JL, Ronnow BS, Horne BD, et al. Usefulness of a complete blood count-derived risk score to predict incident mortality in patients with suspected cardiovascular disease. Am J Cardiol. 2007;99:169–174. doi: 10.1016/j.amjcard.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Spell DW, Jones DV, Harper WF, et al. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev. 2004;28:37–42. doi: 10.1016/j.cdp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Pascual-Figal DA, Bonaque JC, Manzano-Fernandez S, et al. Red blood cell distribution width predicts new-onset anemia in heart failure patients. Int J Cardiol. 2012;160:196–200. doi: 10.1016/j.ijcard.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horne BD. The red cell distribution width: What is its value for risk prognostication and for understanding disease pathophysiology? Cardiology. 2011;119:140–141. doi: 10.1159/000331434. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JM, Mahadevan L. Physiological and pathological population dynamics of circulating human red blood cells. Proc Natl Acad Sci USA. 2010;107:20587–20592. doi: 10.1073/pnas.1012747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willekens FLA, Werre JM, Groenen-Dop YAM, et al. Erythrocyte vesiculation: A self-protective mechanism? Br J Haematol. 2008;141:549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 11.Willekens FL, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–751. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 12.Waugh RE, Narla M, Jackson CW, et al. Rheologic properties of senescent erythrocytes—Loss of surface-area and volume with red-blood-cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 13.Franco RS, Puchulu-Campanella ME, Barber LA, et al. Changes in the properties of normal human red blood cells during in vivo aging. Am J Hematol. 2013;88:44–51. doi: 10.1002/ajh.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109–114. doi: 10.1002/ajh.21298. [DOI] [PubMed] [Google Scholar]
- 15.Gifford SC, Derganc J, Shevkoplyas SS, et al. A detailed study of time-dependent changes in human red blood cells: From reticulocyte maturation to erythrocyte senescence. Br J Haematol. 2006;135:395–404. doi: 10.1111/j.1365-2141.2006.06279.x. [DOI] [PubMed] [Google Scholar]
- 16.Willekens FLA, Roerdinkholder-Stoelwinder B, Groenen-Dopp YAM, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–751. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 17.Beutler E, Waalen J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donofrio G, Chirillo R, Zini G, et al. Simultaneous measurement of reticulocyte and red-blood-cell indexes in healthy-subjects and patients with microcytic and macrocytic anemia. Blood. 1995;85:818–823. [PubMed] [Google Scholar]
- 19.Patel KV, Ferrucci L, Ershler WB, et al. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–523. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–4291. doi: 10.1182/blood-2008-04-154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladyzynski P, Wojcicki JM, Bak M, et al. Validation of hemoglobin glycation models using glycemia monitoring in vivo and culturing of erythrocytes in vitro. Ann Biomed Eng. 2008;36:1188–1202. doi: 10.1007/s10439-008-9508-x. [DOI] [PubMed] [Google Scholar]
- 22.Bunn HF, Gabbay KH, Gallop PM. Glycosylation of hemoglobin—Relevance to diabetes-mellitus. Science. 1978;200:21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- 23.Gram-Hansen P, Mourits-Andersen HT, Eriksen JE, et al. Glycosylated hemoglobin (HbA1c) as an index of the age of the erythrocyte population in non-diabetic patients. Eur J Haematol. 1990;44:201–203. doi: 10.1111/j.1600-0609.1990.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 24.Jansen H, Stolk RP, Nolte IM, et al. Determinants of HbA1c in nondiabetic dutch adults: Genetic loci and clinical and lifestyle parameters, and their interactions in the lifelines cohort study. J Inter Med. 2013;273:283–293. doi: 10.1111/joim.12010. [DOI] [PubMed] [Google Scholar]
- 25.Ladyzynski P, Wojcicki JM, Bak MI, et al. Hemoglobin glycation rate constant in nondiabetic individuals. Ann Biomed Eng. 2011;39:2721–2734. doi: 10.1007/s10439-011-0366-6. [DOI] [PubMed] [Google Scholar]
- 26.Leslie RDG, Cohen RM. Biologic variability in plasma glucose, hemoglobin A1c, and advanced glycation end products associated with diabetes complications. J Diabet Sci Technol. 2009;3:635–643. doi: 10.1177/193229680900300403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mock DM, Widness JA, Veng-Pedersen P, et al. Measurement of posttransfusion red cell survival with the biotin label. Transf Med Rev. 2014;28:114–125. doi: 10.1016/j.tmrv.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engstrom G, Smith JG, Persson M, et al. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. Journal of Internal Medicine. 2014;276:174–183. doi: 10.1111/joim.12188. [DOI] [PubMed] [Google Scholar]
- 29.Veeranna V, Zalawadiya SK, Panaich SS, et al. The association of red cell distribution width with glycated hemoglobin among healthy adults without diabetes mellitus. Cardiology. 2012;122:129–132. doi: 10.1159/000339225. [DOI] [PubMed] [Google Scholar]
- 30.Piva E, Brugnara C, Chiandetti L, et al. Automated reticulocyte counting: State of the art and clinical applications in the evaluation of erythropoiesis. Clin Chem Lab Med. 2010;48:1369–1380. doi: 10.1515/CCLM.2010.292. [DOI] [PubMed] [Google Scholar]
- 31.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron-deficiency. N Engl J Med. 1991;325:687–694. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 32.Rockey DC, Cello JP. Evaluation of the gastrointestinal-tract in patient with iron-deficiency anemia. N Engl J Med. 1993;329:1691–1695. doi: 10.1056/NEJM199312023292303. [DOI] [PubMed] [Google Scholar]
- 33.Malka R, Delgado FF, Manalis SR, et al. In Vivo Volume and Hemoglobin Dynamics of Human Red Blood Cells. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JM. Red Blood Cell Population Dynamics. Clin Lab Med. 2015;35:43–57. doi: 10.1016/j.cll.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golub MS, Hogrefe CE, Malka R, et al. Developmental plasticity of red blood cell homeostasis. Am J Hematol. 2014;89:459–466. doi: 10.1002/ajh.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.