Abstract
Significance: Broad-spectrum metalloproteinase (MMP) reduction along with inherent aspects of an extracellular matrix (ECM) dressing can bring about improved wound healing outcomes and shorter treatment duration. Initial reports of clinical effectiveness of a new ovine-based collagen extracellular matrix (CECM) dressing demonstrate benefits in chronic wound healing.
Recent Advances: CECM dressings are processed differently than oxidized regenerated cellulose/collagen dressings. CECM dressings consist primarily of collagens I and III arranged as native fibers that retain the three-dimensional architecture present in tissue ECM. As such, ovine-based ECM dressings represent a new generation of collagen dressings capable of impacting a broad spectrum of MMP excess known to be present in chronic wounds.
Critical Issues: While MMPs are essential in normal healing, elevated presence of MMPs has been linked to wound failure. Collagen has been shown to reduce levels of MMPs, acting as a sacrificial substrate for excessive proteases in a chronic wound. Preserving collagen dressings in a more native state enhances bioactivity in terms of the ability to affect the chronic wound environment. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response.
Future Directions: Collagen dressings that target broad-spectrum excessive MMP levels and can be applied early in the course of care may positively impact healing rates in difficult wounds. Next-generation collagen dressings offer broader MMP reduction capacity while providing a provisional dermal matrix or ECM.

Gregory Bohn, MD, FACS
Scope and Significance
Selecting treatment based on a scientific understanding of the pathological condition of a chronic wound is important in addressing cost and patient needs. Harmful effects of excessive metalloproteinase (MMP) activity in chronic wound healing should be a consideration of wound care decision making. Collagen dressings have been shown to reduce MMP levels, acting as a sacrificial substrate for excessive proteases in chronic wounds.1–3 New ovine-based collagen extracellular matrix (CECM) dressings (Endoform™ dermal template; Hollister Wound Care, Libertyville, IL) exhibit broad-spectrum MMP inhibition and can serve as a provisional matrix to affect cellular signaling and differentiation that may enhance wound healing.2
Translational Relevance
Degradation of collagen is important for the physiological remodeling of connective tissues during wound healing. Extracellular degradation of collagen fibers is mediated by MMPs and is a multistep process. A collagen dressing with a preserved structural component can serve as a provisional ECM dermal template and influence cellular interaction necessary to encourage keratinocyte migration.4 CECM dressings have been shown to retain the complex collagen architecture of native tissue ECM as well as the ECM-associated secondary molecules, including laminin, fibronectin, and glycosaminoglycans.5 Soluble components of the CECM biomaterial inhibited all MMPs tested, including collagen, stromelysins, and gelatinases.2
Clinical Relevance
While MMPs are essential in normal healing, elevated presence of MMPs has been linked to wound failure.6 Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response.7 A threshold spot test and quantifiable level of MMP activity have been proposed to help identify wounds that are failing and associated with elevated MMP/protease levels.7,8 Broad-spectrum MMP-modulating dressings, such as ovine-based CECM dressings, may affect multiple steps in the pathological process to correct abnormal physiology and promote healing.9
Background/overview
Wound treatment and wound care practice has undergone a transformation in the last 10 years as research in the field has improved understanding of the chronic wound condition. As chronic wounds have posed an increasing cost to the healthcare system, practitioners are focusing increased attention on cost-effective clinical treatments. It is estimated that U.S. wound care costs exceed $50 billion annually.10 Given the high cost of chronic wounds, clinicians may best benefit their patients by selecting treatments based on growing scientific understanding of the pathological condition. Restoring a more normal physiology to the chronic wound environment may trigger a positive healing progression, allowing wounds to complete the requisite steps to heal.
A patient's comorbid condition(s) can greatly influence healing.10 Diabetes, renal failure, peripheral vascular disease, and smoking are among factors associated with increased cost and poor healing outcomes.10 Any particular step in wound healing can be affected by a myriad of processes.6 When healing is interrupted or stalled, a chronic wound condition develops. Wounds that fail to heal have been evaluated in terms of the microenvironment and cellular biology. A growing understanding of the chronic wound microenvironment and resulting deficiencies in healing have led to the development and use of more effective treatments in managing the chronic wound condition. The harmful effects of excessive MMP activity in chronic wound healing are a growing interest of research and an increasingly central focus of decision making by wound care clinicians.
Discussion
Dynamic and reciprocal healing
Wound healing comes about as a result of a dynamic process that is reciprocal.9 Changes occurring between cells, and as cells relate to the matrix, signal new function and cellular activity so as to develop a more refined and mature ECM/cellular structure. The release of proteases in the right amounts, in the right places, and in a balanced and coordinated fashion prepares the wound for proliferation and cell migration.9 In the proliferation phase of wound healing, protease levels typically decline, but are still required and are important in allowing cellular migration, particularly of keratinocytes to migrate over the wound. MMPs are required for the breakdown of hemidesmosomes at the basement membrane so those cells can migrate. Maturation, contraction, and remodeling proceed in a similar manner.9 MMPs are involved as myofibroblasts work to improve and strengthen the wound matrix with type I collagen until equilibration with the surrounding tissue occurs.
Disruption of normal healing
While MMPs are essential in normal healing, elevated presence of MMPs has been linked to wound failure.6 As an example, protease activity of acute wounds has been noted to be initially elevated, but to fall off as wound healing progresses during normal healing. Likewise, MMP/protease levels have been tracked through a normal healing process in surgical wounds and found to elevate after injury and then decline as the surgical wound heals.11,12 The effects of elevated protease activity in a wound break down the requisite matrix, but also interfere or change cell signaling.9,13 Without the matrix structure and signaling integrins of an intact ECM, wound fibroblasts do not respond and differentiate to provide needed next functions for subsequent steps in healing.9,14,15 Growth factors are degraded in the highly proteolytic environment,14 and cells become less or unresponsive to the growth factors.9,13 Additionally, when MMPs are elevated, the tissue inhibitors of metalloproteinases have been found to be low.6 Presence of damaged tissue, foreign material, bacteria, and biofilms are all known sources that may prolong high protease activity.16
Collagen degradation and role of a collagen dressing in restoring the microenvironment
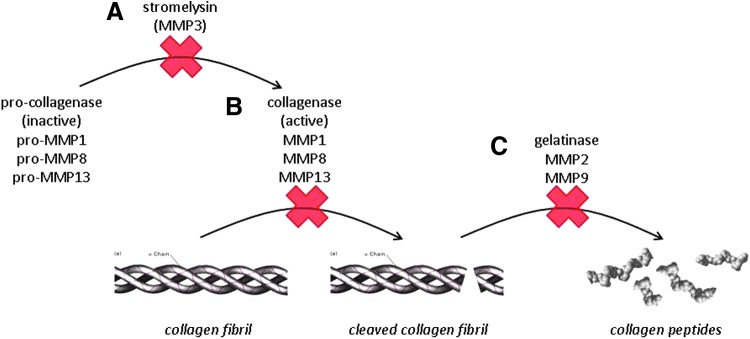
Collagen is a major structural protein present in all animals, used to support and connect bodily tissues; it has a high tensile strength and plays an important role in tissue repair. Degradation of collagen is important for the physiological remodeling of connective tissues during wound healing. Extracellular degradation of collagen fibers is mediated by MMPs and can be thought of as a multistep process. The conceptualized collagen degradation pathway in Fig. 1 demonstrates a number of factors and process steps that can be interrupted. Because there are numerous MMPs and disrupted processes that cause wound failure, normalizing multiple aberrant steps in healing is desirable.
Figure 1.
Collagen degradation pathway is a stepwise process. Activation of collagenase (A) initiates degradation of collagen by collagenase (B). Once the initial break is made in the collagen fibril by collagenase, gelatinase activity further cleaves collagen into smaller peptides (C).
MMP-modulating dressings, such as collagen-based dressings, may bring about healing in a chronic wound. Collagen dressings have been used effectively to modify the wound environment, control bacterial bioburden, protect growth factors from degradation, and stimulate cell growth and cell infiltration into the wound area.3,17,18 Collagen has been shown to reduce MMP levels, acting as a sacrificial substrate for excessive proteases in a chronic wound.1,2,19 As such, collagen can reduce the excessive protease activity in a wound to a level that may allow healing to proceed.
In addition, collagen binds with naturally occurring growth factors and prevents them from being broken down by damaging proteases. As the matrix slowly breaks down, the growth factors are released back into the wound in an active form while the damaging proteases remain inactive.20 Shi and Ronfard1 also noted that the application of collagen immediately reduced MMP inhibitory effects on keratinocyte migration. However, oxidized regenerated cellulose collagen (ORC/C) dressings may affect only one step in the collagen degradation process.3,19 A CECM dressing with a preserved structural component can serve as a provisional ECM dermal template and influence the cellular interaction necessary to encourage keratinocyte migration.9
Clinical monitoring of the chronic wound environment
Given that the clinical assessment may not be able to identify wounds with elevated protease activity, point-of-care MMP assessment methodology has been in development.8 A threshold spot test as well as a quantifiable level of MMP activity have been proposed to help identify wounds that are failing and associated with elevated MMP/protease levels.8
Wound duration is not a prerequisite for elevated protease activity; wounds of variable duration, both greater and less than 6 months, can have elevated protease activity.7 This elevated protease activity level may be an indication to use collagen buffering treatment of the wound. That is not to say, however, that lower levels of protease activity are not affecting a wound's ability to heal. Although the majority of uninfected nonhealing wounds do have elevated MMP levels, a percentage of nonhealing wounds will not have excess proteases.16 In addition, acceptable levels of MMPs vary according to wound type.
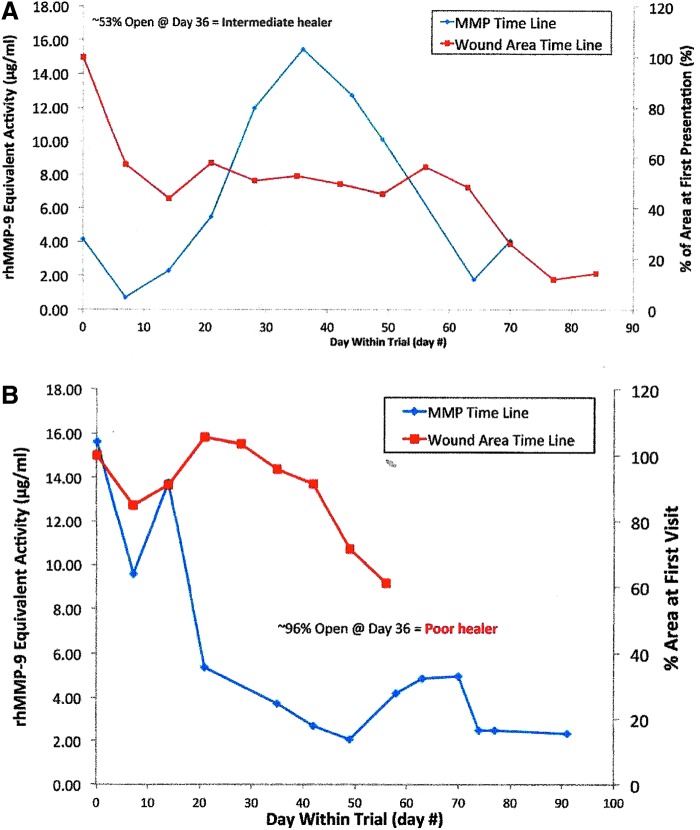
In a small pilot study performed at our facility, preliminary data showed that changing levels of MMP and protease levels did precede a change in venous leg ulcer (VLU) wound area. At the Institute for Wound Research (University of Florida, Gainesville, FL), current authors (G.S., Q.Y., and D.G.) performed a small pilot study of eight patients with VLUs. Wound fluid MMP levels were measured at weekly wound care visits for at least 36 days. At enrollment, initial MMP activity levels ranged from 1.54 to 15.62 μg/mL MMP-9 equivalents and wound sizes ranged from 6.10 to 17.28 cm2; each patient was classified as a “good,” “intermediate,” or “poor” healer.
In all participants, quantified changes in MMP levels could be associated with changes in wound area, although the sample size was too small to calculate the statistical significance. Change in protease activity, as measured at the weekly visit, appeared to predict a negative or positive change in the wound area. As MMP levels increased, the wound stalled and failed to progress; as MMP levels decreased, the wound area decreased. Difficult-to-heal or poor healing wounds with high protease levels responded once the elevated protease levels were reduced sufficiently to allow healing processes to be effective. Figure 2A and B display changes in wound area versus changes in MMP levels over time for one patient categorized as an intermediate healer and one patient categorized as a poor healer, respectively. Results of this pilot study have not yet been submitted for publication.
Figure 2.
(A) Sample time course of MMP levels and wound area changes in one VLU study patient from the “intermediate healer” group. (B) VLU of one patient with poor prognosis for healing was converted to healing wound after MMP levels were decreased. MMP, metalloproteinase; VLU, venous leg ulcer.
Differentiating collagen dressing biomaterials
ORC/C matrix dressings are processed through a digestive process and then reconstituted with oxidized reduced cellulose in a freeze drying process.20 This forms into a pad that is cut into the dressing shape.20 Inherent in the digestive processing is loss of MMP buffering capacity that intact collagen provides—resulting in a more narrow spectrum of MMP buffering activity.21 Chemically, cellulose and ORC are both classified as polysaccharides, sugar molecules linked together, forming a polymer. The main components of ORC are glucose and glucuronic acid.
To date, there is only one commercially available ECM collagen dressing and it is ovine based. Ovine-based CECM dressings are prepared from propria submucosa of ovine forestomach tissue using processes to delaminate and decellularize the tissue.2,22 Dressings are processed using a proprietary method that retains the complex collagen architecture of native tissue ECM.5 CECM dressings contain 90% natural, intact collagen, including types I, III, and IV; 10% is secondary ECM components.23 With this method, the collagen better retains its fibril length and density.23 Also retained during processing are the ECM-associated secondary molecules, including laminin, fibronectin, and glycosaminoglycans,23 which may also help protect from degradation the de novo matrix produced by the wound fibroblast. The ovine-based CECM dressing has been shown to support cell growth and promote cell differentiation, and its collagen structure is completely remodeled during the regenerative process.22
According to the manufacturer, protecting end users against pathogen transmission from ovine-based CECM dressings is addressed in several ways. With respect to the prion/transmissible spongiform encephalopathy (causative agent in Creutzfeldt–Jakob disease), the risk to end users is mitigated through control of the raw materials. The raw materials, ovine/sheep/forestomach tissue, are sourced exclusively from New Zealand sheep that are less than 1 year of age. New Zealand is recognized by international regulators as being prion free, with respect to infection of livestock. Additionally, while prion disease is known in sheep outside of New Zealand, there is a species barrier that protects humans from sheep prion transmission.24 This is evidenced by epidemiological studies that have failed to identify any instances of sheep prions causing disease in humans.25
In addition, during the manufacturing process, tissues are chemically disinfected before final processing and terminal sterilization. Chemical disinfection has been validated to inactivate both enveloped and nonenveloped DNA and RNA viruses. While New Zealand sheep are not known to carrying any pathogenic viruses, this validated chemical disinfection of the tissues mitigates risk of viral contamination of the final product. Bioburden risk is mitigated throughout the process through in-process monitoring controls. Terminal sterilization of the dressing through a validated ethylene oxide cycle ensures a sterility assurance level of six logs.
Studies have shown that ORC/C reduces both MMP and serine protease activities, and is particularly effective against MMP-9 and elastase.3 Lobmann et al.6 found a reduction in the MMP-9: MMP-2 ratio in ORC/C-treated wounds. Smeets et al.18 reported a significant reduction of several key proteases, including gelatinases, elastase, and plasmin, in wound exudate of chronic venous ulcer patients treated with ORC/C compared to a control dressing.
However, in a scientific solid assay study, ORC/C extracts were found to be only modest inhibitors of MMP-1, MMP-3, MMP-8, MMP-12, MMP-13, MMP-14, and neutrophil elastase.2 In this same comparative study, the soluble components of the CECM biomaterial inhibited all MMPs tested, including collagen, stromelysins, and gelatinases. Similar inhibitory potency between ORC/C and CECM extracts were determined only for the gelatinases, MMP-2 and MMP-9. Other than MMP-2 and MMP-9, ORC/C was noted to have no significant impact on the panel of other tested MMPs, including MMP-1 and MMP-8, known to be involved in the collagen degradation process.2
A dressing material that can affect multiple steps in the pathological process would seem to be more effective in correcting abnormal physiology and promoting healing.9 Current ORC/C dressings do not contain other elements of a dermal template/ECM dressing.20 Ovine-based CECM dressings have buffering capacity for collagenases, MMP-1 and MMP-8, as well as gelatinases, MMP-2 and MMP-9.2 This broad spectrum of MMP inhibition may serve to protect and buffer other deleterious MMP activity in the chronic wound microenvironment. Other similarly classified collagen dressings such as Puracol® (Medline Industries, Inc., Mundelein, IL) and BioStep™ (Smith & Nephew, Hull, United Kingdom) have also incorporated more intact type I collagen in manufacturing to improve the functional buffering capacity of the collagen dressing. However, these dressings are not classified as CECM dressings.
Expert opinion seems to suggest that it might not be possible to restore a normal dynamic and reciprocal healing by affecting one single factor.9 Intervening with a dressing that has broad-spectrum MMP buffering capacity, that is, a more native collagen, and supplies other key factors such as elastin and fibronectin, may be a more complete treatment. For example, a CECM dressing has buffering capacity to lower MMP-10 (stromelysin-2),2 which degrades proteoglycans and fibronectin, constituents of the regenerated ECM that wound fibroblasts create. CECM dressings also have the capacity to lower MMP-12 (macrophage elastase), which degrades elastin in the ECM.26
Mechanisms of amniotic membrane dressings and collagen dressings
In advanced therapies such as amniotic membrane products, the collagen is more minimally processed and may offer a broad-range MMP inhibition. Processed amniotic tissues contain collagen types I, II, IV, V, and VI and typically differ from collagen dressings in that they also deliver growth factors.27 Epidermal growth factor, transforming growth factor beta, fibroblast growth factor, and platelet-derived growth factor have been assayed in amniotic tissues and thought to actively participate in tissue function.27,28 Amniotic tissues have also been studied as a source of pluripotent amniotic epithelial cells, which express surface markers associated with stem cells.27 Stem cells are not a component in ECM collagen dressings or ORC/C dressing derivatives.
How much collagen?
When the MMP buffering capacity of the collagen to impact MMP activity is exceeded, MMP levels in the wound increase.1 Applying sufficient collagen to affect protease levels for the duration of treatment application would seem to be necessary to buffer MMP activity effectively and change the chronic proteolytic wound environment. Shi et al.29 demonstrated that the attenuation effect of MMPs by porcine small intestine submucosa (SIS) diminished over time. The buffering effect was maximal at day 3, but subsided by day 7. Consumption of the collagen by MMPs occurred and the dressing was consumed.29 Thus, for a given chronic wound, a certain dosing of collagen would be necessary.
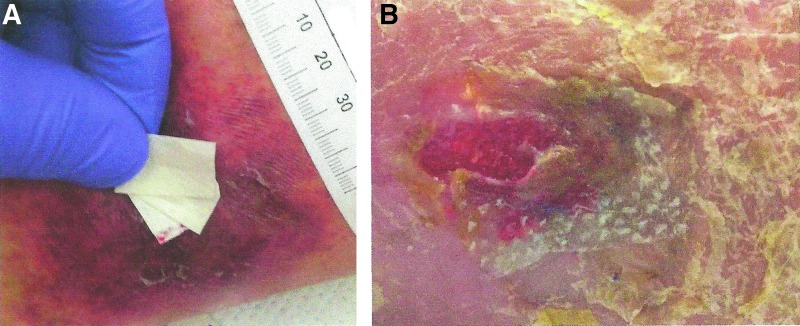
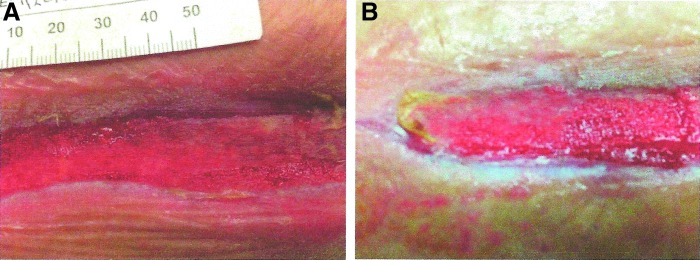
Clinical observation of the effects of CECM dressings in a wound with excessive protease activity may provide insight into what may be adequate MMP buffering capacity for a wound. One may hypothesize that when the collagen dressing is consumed, the buffering capacity of wound MMPs is lost. Applying sufficient layers of dressings (Fig. 3A) so that on the return clinic visit, there is remaining collagen dressing covering the wound (Fig. 3B) may be a visual validation that sufficient collagen has been applied to the wound, but more research is needed to quantify the amount of collagen required for effective wound treatment.
Figure 3.
(A) Collagen dosing by layering ovine CECM dressings to ensure that at least one layer of dressing remains to buffer MMP activity during treatment period. (B) CECM dressing acting as the sacrificial substrate is consumed by protease activity in a VLU. CECM, collagen extracellular matrix.
Effectiveness of collagen dressings in treating VLUs
Protease activity in chronic wounds has been noted to result from a combined effect rather than one particular elevated protease.7 Multiple studies note elevated protease levels in VLUs, and methods of VLU treatment, including compression that target lowering elevated protease, have been shown to be comparatively more effective.30–33 For example, at 12 weeks of treatment, 41% of venous ulcers were reported healed using ORC/C dressings compared to a nonadherent dressing (31%).31 A study of porcine SIS matrix collagen dressing plus compression in chronic leg ulcers demonstrated 55% healing at 12 weeks compared to 34% receiving standard of care and compression.32 Comparing SIS collagen to standard of care in 84 VLUs, an interim analysis at 12 weeks showed a healing rate of 71% with SIS and 46% with standard of care.33
Treatments that lower MMP levels in pressure and diabetic ulcers have been shown to improve healing as well.3,34 In a study, including venous ulcers, diabetic foot wounds, and incisional wounds, Liden and May35 reported 50% closure at 12 weeks with ovine-based CECM dressings with an average surface area reduction of 73.4%. Bohn and Gass17 reported a healing rate of 97% of VLUs at 12 weeks with weekly application of CECM dressing and compression; 50% of wounds were closed by 7–8 weeks using CECM dressings. Authors suggested that compared with other collagen dressings requiring at least twice weekly application, the once weekly application of CECM dressings saved healthcare system dollars in terms of reduced facility fees, material costs, and home nursing visits.17
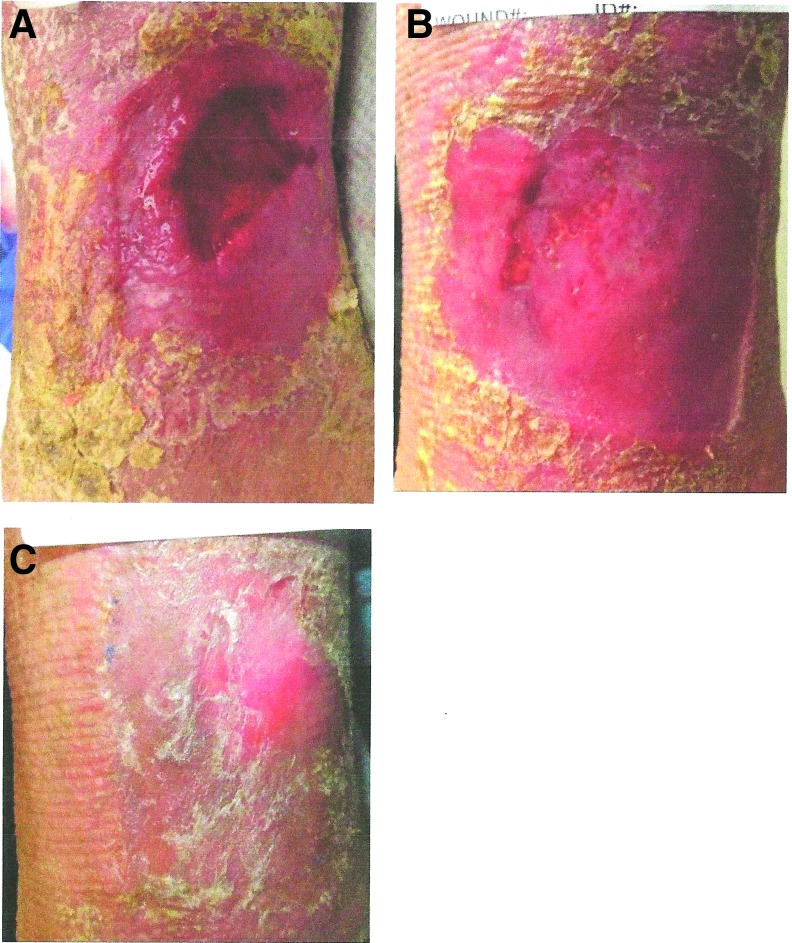
In addition to compression, application of a CECM dressing with broad-spectrum activity to modulate MMP activity may translate into improved healing rates for patients with VLUs. Once degradation is normalized, other cellular processes can proceed. Case Study 1 of a VLU demonstrates how a broad-spectrum activity collagen dressing may improve outcomes and meet quality measures in wound care (Fig. 4). While the patient's wound exhibits characteristics that would predict poor healing, the wound responded quickly to broad spectrum MMP buffering and CECM matrix in the dressing.
Figure 4.
(A–C) Case study: impacting healing of a chronic VLU with ovine-based CECM dressing. A 69-year-old female patient presented with a nonhealing VLU present for 9 months. Wound measured 3.5 × 4.1 cm (14.35 cm2) (A). Patient's ABI was 0.85. Wound area and relative abnormal arterial perfusion would be negative predictors of healing by 24 weeks, according to risk factors established by Kantor and Margolis.36 Given these negative prognostic indicators, this VLU may have as high as a 78% chance of not healing at 24 weeks.36,37 This patient's wound was treated with CECM dressings from the first day of treatment in the clinic, following debridement. Compression wrap therapy was used. Significant healing was noted in the first 21 days (B). Patient had healed by 35 days, despite negative risk factors at presentation (C). ABI, Ankle to Brachial Index.
Structural components of CECM dressing
As the MMP protease levels approach a modulated activity level during which cellular processes of healing progress, the CECM dressing may lend itself to a cellular supportive role. As collagen is no longer consumed by elevated proteases, the structural dermal template of the dressing may become incorporated into the wound (Fig. 5A, B). The collagen material remains in the wound and it appears that the granulation tissue is growing into and incorporating the collagen as it would a provisional matrix. Ovine-based CECM dressing material tested in vitro has been shown to enhance cellular adhesion and infiltration.23
Figure 5.
(A, B) Collagen is no longer consumed and remains in the wound. Granulation tissue formation is noted. The dressing appears to be incorporating into the wound. Photographs reprinted with permission from Hollister Wound Care.
Study of ECMs has more clearly defined the importance of cellular signaling and participation in healing by the structural components of an ECM. As a provisional matrix, cellular signaling and differentiation could be affected to enhance wound healing.9 After processing, ovine-based CECM has been shown to retain structural elements of an ECM2,23 such that cell signaling9 may be a causative factor in the dressing's effectiveness.
Products to use with collagen dressings
Collagen dressings are typically used with a cover dressing, or they tend to dry out. There are varied choices to consider in selecting a cover dressing. Hydrocolloid dressings seem to work well. The hydrocolloid helps maintain moisture balance while keeping the collagen in place, and may prevent friction and shear. Foam dressings also help manage moisture and drainage. When combined with an antimicrobial, a foam dressing can work well over collagen as a secondary dressing. Managing bioburden may assist in correcting protease production. Maintaining a protease management strategy as well as a bioburden management strategy are key concepts in chronic wound healing. Using combination dressings allows the practitioner to select an appropriate strategy based on the wound state, and may improve healing.
Early application of collagen
Collagen dressings should be used early in the course of care to expedite a return to a more normal reciprocal healing process that leads to closure. Practitioners should not only consider collagen dressings in a chronic wound, but also in an acute wound to help limit the development of protease excess. The ECM in the collagen dressing may incorporate into the acute wound and assist in healing.
A major advantage of ovine CECM dressings is that they are classified for reimbursement as a “collagen dressing” as opposed to an “advanced wound care matrix dressing.” As such, these dressings are relatively inexpensive ($10–12 each) and can be applied from the initial visit, rather than waiting the requisite 3–8 weeks of moist wound healing application that is typically required by payors before initiating an advanced wound care matrix dressing.38,39 In addition, the dressing can be applied by patients, nurses, or any other caregiver in any care setting. Earlier, MMP-targeted treatment may reduce the need for advanced wound therapies later in the course of care, and lead to reduced costs and healing times.17
Summary
With growing scientific understanding of essential steps in healing and tissue repair, improved methods of treatment will logically result. Clinicians can now treat the wound to bring about changes in the pathophysiological state to repair the process of healing. Proteases and cellular dysfunction are key concepts in wound repair, and need to be addressed by the wound care clinician. Treating the wound to affect multiple levels of dysfunction and pathological process seems to offer improvements in the healing of chronic wounds. Serial monitoring of MMP levels throughout care, in response to treatment or intervention, gives the clinician added insight as to the therapy effect.
Take-Home Messages.
• While MMPs are essential in normal healing, elevated presence of MMPs has been linked to wound failure.
• Degradation of collagen is important for the physiological remodeling of connective tissues during wound healing; extracellular degradation of collagen fibers is mediated by MMPs and involves several steps.
• Collagen dressings have been shown to reduce MMP levels, acting as a sacrificial substrate for excessive proteases in a chronic wound.
• Processed ovine-based CECM dressings retain the complex collagen architecture of native tissue ECM as well as the ECM-associated secondary molecules, including laminin, fibronectin, and glycosaminoglycans.
• Ovine-based CECM dressings have broad-spectrum buffering capacity for collagenases, MMP-1 and MMP-8, as well as gelatinases, MMP-2 and MMP-9, which may serve to protect and buffer other deleterious MMP activity in the chronic wound microenvironment.
Similar to engineering modifications that have taken place with medications, the processing of collagen dressings that preserves essential elements of the intact collagen and dermal template has led to next-generation dressing biomaterial CECM. Data from preliminary case series suggest that clinicians may achieve better outcomes in chronic wound healing in a shorter timeframe using a broad-spectrum ovine-based CECM dressing compared to previous available treatments.17,34 Controlled clinical trials are needed to further analyze the comparative and cost-effectiveness of ovine-based CECM dressings in various wound types.
Abbreviations and Acronyms
- ABI
Ankle to Brachial Index
- CECM
collagen extracellular matrix
- DFU
diabetic foot ulcer
- ECM
extracellular matrix
- MMP
metalloproteinase
- ORC/C
oxidized regenerated cellulose/collagen
- SIS
small intestine submucosa
- TSE
transmissible spongiform encephalopathy
- VLU
venous leg ulcer
Acknowledgment and Funding Source
Karen Beach, medical writer, was financed by Hollister Wound Care to assist in editing this article.
Author Disclosure and Ghostwriting
Gregory Bohn, Brock Liden, and Greg Schultz have served as consultants to Hollister. No competing financial interests exist for Qingping Yang or Daniel J. Gibson. The content of this article was expressly written by all authors listed. No ghostwriters were used to write this article.
About the Authors
Gregory Bohn, MD, FACS is a practicing surgeon and serves as medical director of the Westshore Medical Center for Wound Care and Hyperbaric Medicine. He is board certified in General Surgery and Hyperbaric Medicine. He received certifications in wound care by the American Board of Wound Healing and the American Board of Wound Management. Dr. Bohn is currently president of the American Board of Wound Healing, and a Fellow of the American College of Hyperbaric Medicine and of the American Professional Wound Care Association. Brock Liden, DPM, is a practicing podiatric physician near Columbus, OH. He is actively engaged in clinical research and product research & development, with specific focus on collagen products and limb salvage. Gregory Schultz, PhD, is a Professor of Obstetrics and Gynecology at the University of Florida, and director of the Institute for Wound Research. Qingping Yang, MS, is a research scientist at the Institute of Wound Research at the University of Florida. She has conducted extensive research on bacterial biofilms in chronic wounds. Daniel J. Gibson, PhD, is an Assistant Professor at the University of Florida and studies the molecular and cellular basis of wound healing and fibrosis. Dr. Gibson has developed point-of-care molecular and imaging assays to improve clinical insight into pathologies, including chronic skin wounds, corneal fibrosis, and diabetic retinopathy. With a background in mechanical engineering, Dr. Gibson has also created systemic models for biological processes to help improve medical treatment decision making and research focus.
References
- 1.Shi L, Ronfard V. Biochemical and biomechanical characterization of porcine small intestine submucosa (SIS): a mini review. Int J Burns Trauma 2013;3:173–179 [PMC free article] [PubMed] [Google Scholar]
- 2.Negron L, Lun S, May BC. Ovine forestomach matrix biomaterial is a broad spectrum inhibitor of matrix metalloproteinases and neutrophil elastase. Int Wound J 2014;11:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix for treatment of diabetic foot ulcers. Wound Repair Regen 2002;10:16–25 [DOI] [PubMed] [Google Scholar]
- 4.Brett D. A review of collagen and collagen-based wound dressings. Wounds 2008;20:347–356 [PubMed] [Google Scholar]
- 5.Floden EW, Malak SF, Basil-Jones MM, et al. . Biophysical characterization of ovine forestomach extracellular matrix biomaterials. J Biomed Mater Res B Appl Biomater 2011;96:67–75 [DOI] [PubMed] [Google Scholar]
- 6.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002;45:1011–1016 [DOI] [PubMed] [Google Scholar]
- 7.Serena TE. Development of a novel technique to collect proteases from chronic wounds. Adv Wound Care (New Rochelle) 2014;3:729–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson DJ, Schultz GS. Molecular wound assessments: matrix metalloproteinases. Adv Wound Care (New Rochelle) 2013;2:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fife CE, Carter MJ, Walker D, Thomson B. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds 2012;24:10–17 [PubMed] [Google Scholar]
- 11.Tarlton JF, Vickery CJ, Leaper DJ, Bailey AJ. Postsurgical wound progression monitored by temporal changes in the expression of matrix metalloproteinase-9. Br J Dermatol 1997;137:506–516 [DOI] [PubMed] [Google Scholar]
- 12.Baker EA, Leaper DJ. Profiles of matrix metalloproteinases and their tissue inhibitors in intraperitoneal drainage fluid: relationship to wound healing. Wound Repair Regen 2003;11:268–274 [DOI] [PubMed] [Google Scholar]
- 13.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997;5:23–32 [DOI] [PubMed] [Google Scholar]
- 15.Hakkinen L, Koivisto L, Gardner H, et al. . Increased expression of beta 6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol 2004;164:229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Consensus. The Role of Proteases in Wound Diagnostics. An Expert Working Group Review. London: Wounds International, 2011 [Google Scholar]
- 17.Bohn GA, Gass K. Leg ulcer treatment outcomes with new ovine collagen extracellular matrix dressing: a retrospective case series. Adv Skin Wound Care 2014;27:448–454 [DOI] [PubMed] [Google Scholar]
- 18.Smeets R, Ulrich D, Unglaub F, Wöltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J 2008;5:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen B, Kemp L, Essler A, Wallenfang-Sohle K, Stadler R. Rebalancing wound biochemistry improves healing: a clinical study examining effect of Promogran. Wound Repair Regen 2004;12:A4 [Google Scholar]
- 20.Cullen B, Watt PW, Lundqvist C, et al. . The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol 2002;34:1544–1556 [DOI] [PubMed] [Google Scholar]
- 21.Fleck CA, Simman R. Modern collagen wound dressings: function and purpose. J Am Col Certif Wound Spec 2011;2:50–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irvine SM, Cayzer J, Todd EM, et al. . Quantification of in vitro and in vivo angiogenesis stimulated by ovine forestomach matrix biomaterial. Biomaterials 2011;32:6351–6361 [DOI] [PubMed] [Google Scholar]
- 23.Lun S, Irvine SM, Johnson KD. A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials 2010;31:4517–4529 [DOI] [PubMed] [Google Scholar]
- 24.Prusiner SB, Scott M, Foster D, et al. . Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 1990;63:673–686 [DOI] [PubMed] [Google Scholar]
- 25.Fediaevsky A, Tongue SC, Nöremark M, Calavas D, Ru G, Hopp P. A descriptive study of the prevalence of atypical and classical scrapie in sheep in 20 European countries. BMC Vet Res 2008;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J Biol Chem 1996;271:13055–13060 [DOI] [PubMed] [Google Scholar]
- 27.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99 [DOI] [PubMed] [Google Scholar]
- 28.Kay H, Nelson D, Wang Y, eds. The Placenta: From Development to Disease, 1st ed. Hoboken, NJ: Wiley-Blackwell, 2011 [Google Scholar]
- 29.Shi L, Ramsay S, Ermis R, Carson D. In vitro and in vivo studies on matrix metalloproteinases interacting with small intestine submucosa wound matrix. Int Wound J 2012;9:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen 2008;16:642–648 [DOI] [PubMed] [Google Scholar]
- 31.Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care 2002;11:335–341 [DOI] [PubMed] [Google Scholar]
- 32.Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. OASIS Venus Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 2005;41:837–843 [DOI] [PubMed] [Google Scholar]
- 33.Demling RH, Niezgoda JA, Haraway GD, Mostow EN. Small intestinal submucosa wound matrix and full-thickness venous ulcers: preliminary results. Wounds 2004;16:18–22 [Google Scholar]
- 34.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37 [DOI] [PubMed] [Google Scholar]
- 35.Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care 2013;26:164–167 [DOI] [PubMed] [Google Scholar]
- 36.Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–964 [DOI] [PubMed] [Google Scholar]
- 37.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163–168 [DOI] [PubMed] [Google Scholar]
- 38.Aetna. Clinical policy bulletin: wound care. Number: 0244. Last reviewed February 13, 2015. www.aetna.com/cpb/medical/data/200_299/0244.html (last accessed September28, 2015)
- 39.Health Net. National Medical Policy. Wound Care. Last reviewed June 2015. www.healthnet.com (last accessed September28, 2015)