Abstract
Significance: The extracellular matrix (ECM) is known to be deficient in chronic wounds. Collagen is the major protein in the ECM. Many claims are made while extolling the virtues of collagen-based biomaterials in promoting cell growth and modulating matrix metalloproteinases. This review will explore the rationale for using topical collagen or ECM as an interface for healing.
Recent Advances: Rapid improvements in electrospinning and nanotechnology have resulted in the creation of third-generation biomaterials that mimic the native ECM, stimulate cellular and genetic responses in the target tissue, and provide a platform for controlled release of bioactive molecules and live cells. Although the major focus is currently on development of artificial tissues and organ regeneration, better understanding of the mechanisms that stimulate wound healing can be applied to specific deficits in the chronic wound.
Critical Issues: When choosing between the various advanced wound-care products and dressings, the clinician is challenged to select the most appropriate material at the right time. Understanding how the ECM components promote tissue regeneration and modulate the wound microenvironment will facilitate those choices. Laboratory discoveries of biomolecular and cellular strategies that promote skin regeneration rather than repair should be demonstrated to translate to deficits in the chronic wound.
Future Directions: Cost-effective production of materials that utilize non-mammalian sources of collagen or ECM components combined with synthetic scaffolding will provide an optimal structure for cellular ingrowth and modulation of the chronic wound microenvironment to facilitate healing. These bioengineered materials will be customizable to provide time-released delivery of bioactive molecules or drugs based on the degradation rate of the scaffold or specific signals from the wound.

Lisa J. Gould, MD, PhD
Scope and Significance
Chronic wounds are a major problem with costs of more than $50 billion annually to the United States. Overall, 40–60% of these wounds do not heal with standard moist wound care within 3 months and, therefore, are considered for more “advanced wound therapies” at a considerable additional cost.1
Translational Relevance
Matrices can be shown to provide cell attachment and growth factor binding sites in vitro, but it remains to be determined whether these vulnerary properties translate to improved healing in the chronic wound bed. Cells interact with the endogenous matrix through integrin binding, resulting in cell signaling that promotes wound healing. Interactions between tissue-engineered matrix substitutes and integrins have been demonstrated in vitro, suggesting that these interactions are one mechanism by which healing is facilitated when tissue-engineered matrices are applied topically.
Clinical Relevance
A better understanding of the intended purpose of tissue-engineered matrices when applied to chronic wounds will lead to optimization of the products. Specifically, if the purpose is only to bind and inactivate proteases, then these can be better targeted. If the intent is also to provide a scaffold for cellular ingrowth, the porosity and surface characteristics should be optimized. Ultimately, the matrices should be tailored to the goals of therapy, whether that is modulation of the wound microenvironment, providing the optimal cellular scaffold, reduction of scarring, or serving as a delivery device for cells or biologically active molecules.
Discussion of Findings and Relevant Literature
Extracellular matrix in acute and chronic wounds
The extracellular matrix (ECM) provides proteins and a structure that is critical for wound healing. Based on the premise that the ECM is dysfunctional in chronic wounds, a wide variety of topical therapies have been developed which supplement, replace, or modulate the ECM. Wound-care providers are constantly presented with new and ever more complex devices that are aimed at improving healing outcomes. Solid evidence is needed to support clinical decision making; however, most of the clinical data is derived from industry-supported trials and, therefore, is subject to inherent bias. The astute wound-care clinician's goal is to select topical therapies targeted at specific elements that are impeding healing. Therefore, it is important to link the pathophysiology of chronic wounds with the proposed mechanism of action for each element present in topical ECM products and to determine whether provision of those elements will facilitate healing. This review intends to combine the findings from basic and preclinical studies with clinical trials and case series to evaluate the efficacy of and rationale for using topical ECM devices and dressings. It is not intended to be an exhaustive dissertation on the molecular biology and cell signaling interactions related to the ECM. Rather, the goal is to tease out what is known about the ECM tissue-engineered devices and purified ECM components topically used in wound care as a guide to product selection.
Acute wounds heal via an orderly, highly orchestrated series of events. All the overlapping phases of wound healing rely on a dynamic interaction between cells and the ECM that promotes rapid resolution of inflammation and ingrowth of fibroblasts and keratinocytes, resulting in functional and durable healing. In contrast, chronic wounds exhibit a constellation of cellular and molecular abnormalities, many of which are the result of abnormal cellular-ECM interactions.2 Biochemical abnormalities of the ECM, as well as increased levels of proteases create an environment of persistent inflammation that is self-perpetuating. For example, in diabetes, collagen is glycosylated and although collagen gene expression is elevated, collagen deposition in wounds is decreased.3 Matrix metalloproteinase (MMP) and elastase activity are increased compared with nondiabetic controls, while tissue-derived inhibitors of metalloproteinases (TIMPs) are decreased, resulting in net degradation of the ECM.4 The degraded matrix components contribute to a prolonged inflammatory response.5 Diabetic fibroblasts are dysfunctional in many aspects with decreased migration and proliferation, decreased collagen production, elevated MMP-9 production, and reduced vascular endothelial growth factor production.6,7 Chronic wound fibroblasts from venous ulcers also exhibit phenotypic differences from normal, with altered MMP and TIMP activity resulting in destruction of the ECM.8 Keratinocyte activity with restoration of a complete and stable barrier function is critical for final wound closure. This activity depends on the ability of the keratinocytes at the wound edge to detach, migrate, and proliferate. Research models that utilize genetic knock-out mice, cultured cells from animals and humans, as well as an ex vivo human skin model have led to an understanding of the dependence on multiple cytokines, growth factors, intercellular communication, and genetic changes within the basilar keratinocytes that is required for successful re-epithelialization.9 To migrate over granulation tissue, keratinocytes express new integrins, which interact with exposed ECM components in the wound bed, specifically through connections to fibronectin, vitronectin, tenascin-C, and collagen I and III.10 In addition, laminins and type IV collagen in the basement membrane (BM) have been shown to modulate keratinocyte migration. Chronic wound keratinocytes have been shown to be hyperproliferative but deficient in migration, possibly due to absence of a key BM protein, LM-332.11,12 These examples, derived from human tissue, cell culture, and animal models, illustrate the complex, but critical relationship between the cells that populate the wound bed as well as wound margins and the matrix components. The fact that both the ECM and the cells are dysfunctional in chronic wounds may explain why addition of a single cytokine or matrix component has not translated to clinical efficacy in these biologically complex wounds.
Individual matrix components
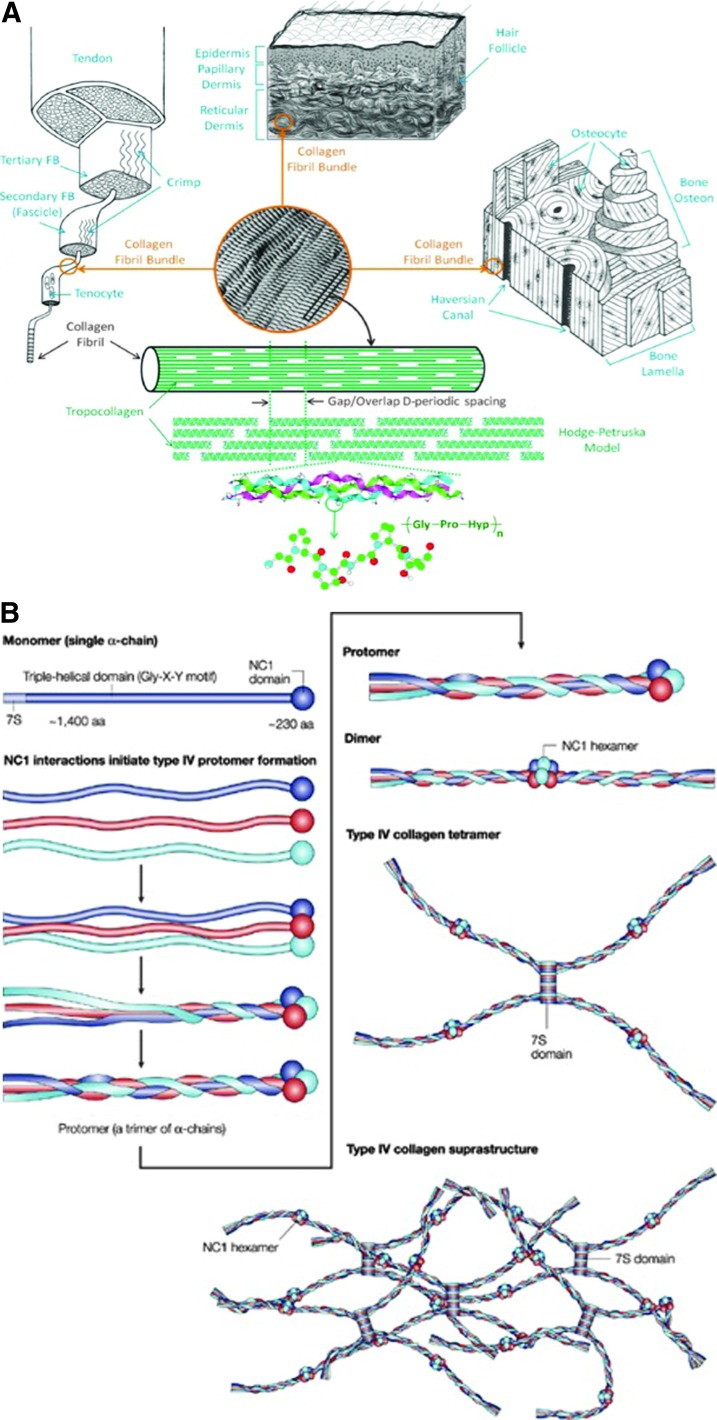
Collagen is the most abundant protein in the body and is critical for structural support of the skin. In addition to its scaffold function, collagen is a critical signaling molecule in the ECM. Collagen has a unique triple helical structure that arises from the unusual abundance of three amino acids: glycine (Gly), proline, and hydroxyproline. Each chain is an α helix composed of the repetitive triplet Gly-Xxx-Yyy, where the Xxx and Yyy positions are typically occupied by proline and hydroxyproline residues. Three left-handed chains make up the right-handed superhelical collagen molecule. Intra- and inter-molecular cross-linking leads to the formation of fibrils and fibers, leading to remarkable tensile strength13 (Fig. 1). Although collagen I accounts for 80–85% of the collagen in the skin, the collagen family comprises more than 29 members. A critical review of the differences between the collagens known to be present in the ECM will provide a foundation for understanding their function in topical products. This review will focus on collagens I, III, IV, V, and VII, as they are the most widely studied collagen components of the skin.
Figure 1.
Comparison of collagen I and IV structures. (A) The fibrillar structure of type I collagen provides the extraordinary tensile strength that is characteristic of skin. Type I collagen fibrils assemble side by side in parallel bundles that are interconnected with inter- and intra-molecular cross-links. The complex post-translational modifications that result in collagen fibril assembly are not easily reproduced in synthetic collagens (reprinted with permission from Fang et al.86). (B) In contrast to the amorphous lattice network of type I collagen, type IV collagen forms a complex branching network with the fibers linking head to head rather than in parallel. Triple helical segments are interrupted by long segments that cannot form a triple helix. The resulting two-dimensional network results in the formation of sheet-like structures (reprinted with permission from Kalluri87). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
As the most abundant and most readily available form of collagen, nearly every in vitro study related to wound healing has been performed with type I collagen (Col I). Due to the presence of the integrin recognition sequences RGD and GFOGER, Col I is known to control many cellular functions of fibroblasts and keratinocytes, including cell shape, adhesion, differentiation, and migration.14 In vitro, Col I has been shown to promote ECM deposition by dermal fibroblasts.15 In human dermal microvascular endothelial cells and in a mouse model of skin angiogenesis, an interaction between endothelial cells and three-dimensional Col I has been shown to induce activation of MAP kinase pathways that promote angiogenesis.16,17 Intact Col I has been shown to bind a number of proteases and inflammatory cytokines that are in excess in chronic wound fluid, including neutrophil elastase, MMP-2, interleukin (IL)-6, IL-8, and IL-1β as well as scavenging superoxide anion and peroxynitrate.18–20 These in vitro studies suggest that topical application of Col I should modulate the chronic wound environment and, in fact, a study of topically applied Col I to venous ulcers over a 2-week period with daily changes confirms reduction of MMP-2, -13, elastase, IL-1β, and IL-8 in the wound fluid.18
Collagen components in the ECM
Col I is a heterotrimer composed of two different α chains. In addition to the biological properties outlined earlier, the porous structure and capillary activity of intact fibrillar collagen allows it to absorb large amounts of fluid, forming a gel that maintains the moist wound interface.21 Fibrillar collagen is usually highly resistant to proteolytic degradation. However, in pathological processes, such as the chronic wound, fibrillar collagen is susceptible to degradation by multiple proteolytic enzymes, including MMP-1, -2, -8, -13, and -14.22,23 Initial cleavage of Col I results in two large fragments that can then spontaneously unwind into nonhelical gelatin derivatives which are susceptible to degradation by nonspecific proteases and gelatinases. Degradation of collagen into gelatin results in loss of the cellular binding capacity and ability to sequester inflammatory mediators. While remodeling of collagen is a fundamentally important physiologic function in wound healing, the development of collagen as a topically applied biomaterial requires enhanced stability. Cross-linking delays degradation but has been shown to decrease the bioavailability of the matrix and to reduce cellular engraftment.24,25
The next most prominent collagen in dermal wound healing is collagen III (Col III), accounting for ∼10% of collagen in skin. Col III is a homotrimeric fibrillar collagen that is predominant in early repair, providing initial support for cell migration and adherence. The increased expression of Col III in fetal tissues was originally thought to contribute to scarless fetal healing; however, there are multiple other factors involved, including the degree of cross-linking and differential expression of anti-inflammatory mediators.26–29 Using Col3-null mice, Liu et al. have shown that Col3 regulates fibril diameter and plays a critical role in maintaining cutaneous integrity; however, a specific role in wound repair has not been identified.30 Additional studies in the Col3-deficient murine model have shown that Col3, indeed, modulates scar formation via promotion of myofibroblast differentiation. Thus, complete absence of Col3 in Col3−/− mice leads to structural failure while relative deficiency (Col3+/− mice) leads to increased scarring and more rapid wound closure than in Col3+/+ mice.30,31 While these studies help define a role for Col3 in wound healing, they do not address whether addition of exogenous Col3 will modulate wound healing. Based on promising results in corneal implantation, Nuutila et al. examined whether topical recombinant Col3 gel would promote wound healing.32 Using a full-thickness porcine wound model, they evaluated the use of this gel as a carrier for keratinocytes and fibroblasts. They found that rhCol III gel promoted the formation of granulation tissue in the wound bed and was a suitable carrier for keratinocytes. An interesting aspect of this study was that when the fibroblasts were included the collagen deposition was equivalent to the control. This suggests that either the three-dimensional matrix or the combination with keratinocytes activated the fibroblasts, resulting in proteolytic degradation of the rhCol III carrier. MMP production was not measured in this study.32
Collagen IV is the main protein component of the BM and is, therefore, a major component of the ECM products used in wound healing. Lacking glycine, Col IV forms a pliable three-dimensional mesh rather than a tight helical structure.33,34 This makes it ideally suited as a platform for cell migration (Fig. 1). As a component of the vascular BM, Col IV plays a crucial role in endothelial cell adhesion, migration, and angiogenesis. Proteolytic cleavage of Col IV α chains generates NC1 fragments that have biological activity. Interestingly, many of the degradation products of Col IV α chain, including tumstatin, canstatin, and arresten, are antiangiogenic16,35 In addition, the biological activities of these NC1 fragments are opposite depending on whether they are immobilized or soluble. Immobilized NC1 domains from Col IV induce proliferation, survival, and migration of different cell types, while the soluble fragments negatively regulate proliferation and migration, induce apoptosis and ECM disorganization.35 The clinical relevance of this in wound healing is not known, but because MMP-9 and elastase are highly efficient at generating these fragments, it is likely that soluble NC1 fragments are prevalent in chronic wounds. This also suggests that topical addition of a Col IV-enriched matrix to the proteolytic mileau of chronic wounds may be counter-intuitive.
A quantitatively minor fibrillar collagen with broad distribution, Col V may have a role in angiogenesis and is essential for type I collagen fibril formation. The importance of Col V is illustrated in Ehlers–Danlos syndrome (EDS) in which the defective fibrillar network affects integrin binding.36 In vitro EDS fibroblasts had improved migratory capacity when supplemented with exogenous Col V.37 Type V collagen has been identified in vivo in vascular subendothelia and on the endothelial cell surface. Using an implanted sponge model, Inkinen et al. noted minimal staining for Col V in the loose connective tissue, but strong expression associated with blood vessel walls increased in parallel as the number of blood vessels increased. The Col V triple helical domain is cleaved by both MMP-2 and -9 but is resistant to digestion by collagenase.38,39
Collagen VII is the major component of anchoring fibrils that holds the epidermis and dermis together. Synthesized and secreted by epidermal keratinocytes and dermal fibroblasts, Col 7A1 is present at the provisional dermal-epidermal junction in early wounds and normal skin. Patients with recessive dystrophic epidermolysis bullosa (RDEB) have skin fragility, blistering, and wounds that heal with significant scarring due to complete absence of Col VII.40 Two different mouse models were used to show that Col7A1 is required for laminin-332/integrin α6β4-signaling which guides keratinocyte migration and that Col7A1 supports fibroblast migration and regulates fibroblast cytokine production in granulation tissue.41 The impact on granulation tissue was manifested by a prolonged myofibroblast phase and slower clearance of inflammatory cells, driven by increased TGF-β1. Importantly, these findings were confirmed in human chronic wounds (nonhealing venous ulcers). Acute surgical wounds were compared with nonhealing venous ulcers and wounds in patients with RDEB. The chronic wounds and RDEB wounds were shown to have similar defects with reduction or loss of COL7A1 under the healing epidermis, disorganized laminin-332; suprabasal expression of integrin α6; and upregulation of the downstream targets JNK and STAT3.41 In one of the few studies of its kind, topical recombinant Col VII was applied to a murine wound model and to an RDEB skin transplant model. The topical Col VII was shown to be stably incorporated into the wounds, to promote re-epithelization and to inhibit fibrosis through downregulation of CTGF and reduction of myofibroblasts.42 It remains to be seen whether these very important findings will translate to the human chronic wounds, although clearly that is the next step in this research.
Structure modification through processing
Collagen-based biomaterials fall primarily into two categories: decellularized matrices that retain the original tissue properties and ECM structure and collagen scaffolds derived through extraction, purification, and polymerization. The ideal biomaterial to promote tissue repair or regeneration within chronic wounds will (1) attract cells that are capable of synthesizing new tissue to the wound site; (2) promote cell proliferation; (3) provide a nonimmunogenic, resorbable scaffold for cellular migration and matrix deposition; (4) guide organization of new ECM deposition; (5) modulate proteolytic activity; and (6) adsorb and neutralize free radicals and/or excess metal ions. It is clear that collagen compounds meet many of these criteria and have several distinct advantages over other biomaterials in that they are biocompatible and nontoxic to multiple tissue types. Although the majority of collagen scaffolds are based on Col I, the descriptions cited earlier suggest that there may be selected indications for the use of alternative matrix components or composite materials. However, it is not clear whether the attributes of Col III, IV, V, or VII provide any true advantage and, in fact, it is likely that provision of Col I will promote synthesis of the other collagens within the wound bed.43 In addition to varying the composition, the performance of collagen biomaterials is affected by pore size and degradability. Porosity affects diffusion of oxygen and other nutrients as well as the ability of cells to migrate into the material. Porosity can be manipulated by mixing materials, changing the concentration, altering lyophilization techniques, and also varying source.15 While porcine and bovine sources are most common for Col I, recent studies have examined avian and piscine sources as being potentially more economical and environmentally sound with equivalent or improved properties.15,44 Recombinant technology with production of collagen from either plant or bacterial sources is currently under investigation and has been used in some animal studies.42,45,46 This would allow production of a non-animal collagen in an animal-free system, reducing variation of the final product with more control over purity and decreased risk of disease transmission. Currently, the recombinant technology is not as cost effective as the extraction methods and does not provide the necessary post-translational modifications required to recapitulate a stable ECM product, but may become more feasible in the future.13
Although the native collagen triple helix structure is likely to be an ideal metalloprotease substrate with angiogenic and chemoattractant properties, it is rapidly degraded under physiologic conditions. The degradation rate and mechanical properties can be manipulated via cross-linking and sterilization methods. Chemical cross-linking may increase stability; however, the covalent bonds between the polymeric chains may be cytotoxic when the material is degraded.23,25 Alternative methods to increase stability include electrostatic cross-linking with chitosan or stabilization through hydrogen bonding with sugars or polyphenols.15,22 These methods stabilize the material structure and may increase the efficiency of chemical cross-linking without reducing the biological performance. Alternatively, physical or enzymatic cross-linking methods are available that are not cytotoxic.23 Common methods for sterilization include steam (autoclave), irradiation, and use of ethylene oxide, each of which may alter the physical or chemical properties of the material. In addition to cost, manufacturers should weigh the benefits of retaining the structure of the collagen-based biomaterial with retention of binding capacity for cytokines, proteases, and free radicals.19
Decellularized ECM
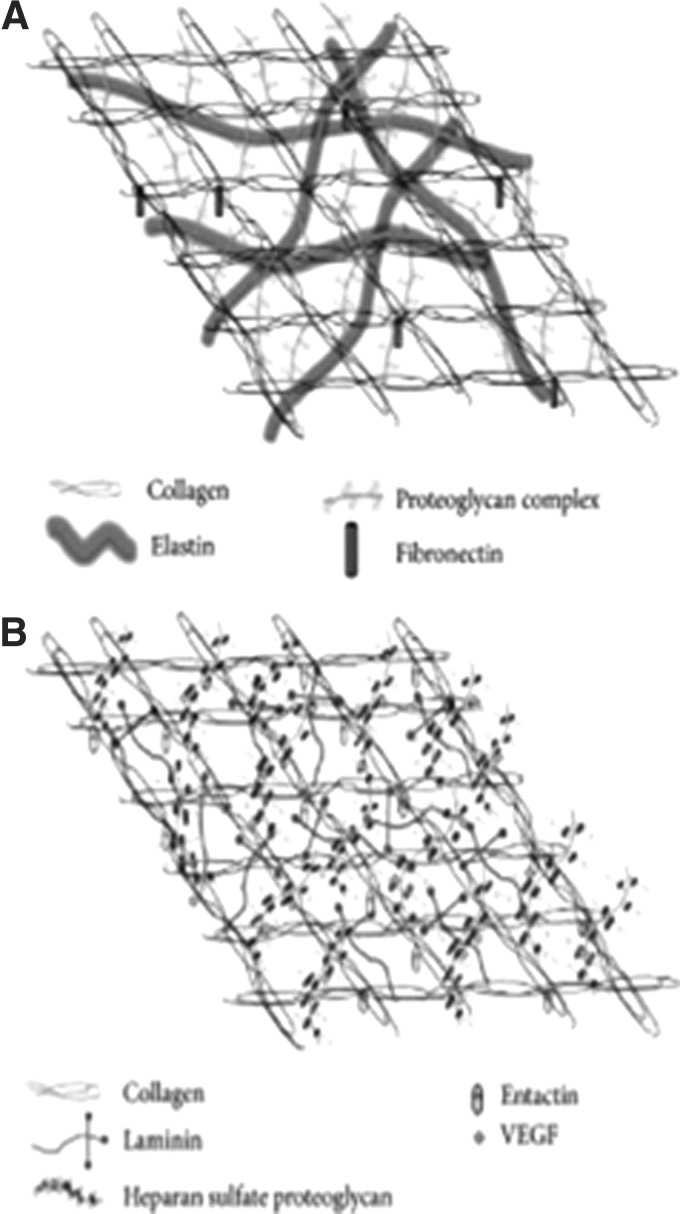
The ECM consists of two biochemically and morphologically distinct entities: the interstitial matrix with fibrillar and nonfibrillar collagens, elastins and glycosaminoglycans and the extracellular BMs composed of sheets with collagen IV, laminins, entactin, and heparin sulfate proteoglycans16 (Fig. 2). Through interactions with integrins, this highly dynamic structure orchestrates complex signaling cascades that regulate multiple cell functions, including cell shape, gene expression, proliferation, migration, and apoptosis (Fig. 3). The native ECM promotes intercellular crosstalk and stores mediators that regulate cellular processes which are important for wound healing. Because of its complex three-dimensional architecture augmented by structural and functional molecules which guide multiple aspects of the wound-healing cascade, one could argue that the ideal bioactive scaffold for chronic wounds should retain as many features of the native ECM as possible. However, chronic wounds are characterized by an imbalance of tissue deposition and tissue destruction, mediated by proteases. Thus, a critical evaluation of treatment modalities should consider whether topical matrices applied to chronic wounds primarily modulate the proteolytic microenvironment or whether they provide other regenerative, bio-inductive properties.
Figure 2.
ECM interactions and molecular organization. The ECM is composed of two distinct matrix entities. The protein composition of each leads to identifiable structural and functional differences; however, the two components interact to provide adhesive and structural support as well as influence cellular physiologic function. (A) The interstitial matrix, composed of fibrillar and nonfibrillar collagen, elastic fibers, and glycosaminoglycans, forms an amorphous structure that provides a repository for bioactive molecules. (B) With its high content of collagen IV, laminins, and heparin sulfate proteoglycans, the extracellular BM forms sheets that separate cells from the interstitial matrix (reprinted under CC BY license, Neve et al.16). BM, basement membrane; ECM, extracellular matrix.
Figure 3.
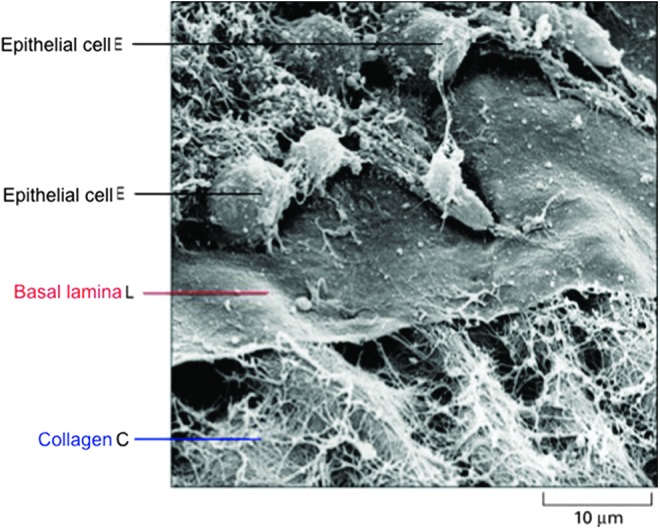
BM interactions. Scanning electron micrograph showing the relationship between the BM (basal lamina), collagen fibrils, and migrating epithelial cells. Some of the epithelial cells (E) have been removed to expose the upper surface of the basal lamina (L). A network of collagen fibrils (C) interacts with the lower face of the lamina (Credit: Courtesy of the late Robert Trelstad). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
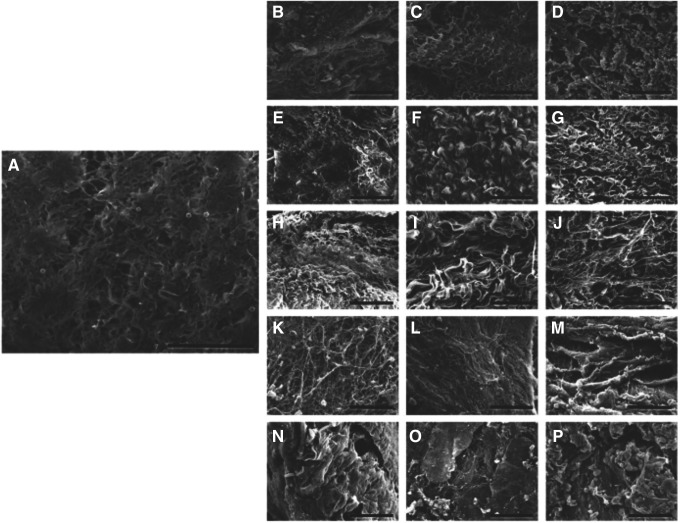
Derived from a variety of mammalian sources and anatomic locations, ECM for use as a biological scaffold is decellularized to reduce the negative host immune response. Decellularization may be accomplished by acid, detergent, enzymatic, and mechanical methods, all of which can affect the biochemical and structural characteristics47–49 (Fig. 4). Further processing, including lyophilization and the method of terminal sterilization, can impact the material's strength and availability of functional bioactive molecules.50 Nonetheless, the remaining matrix complexity far exceeds that of polymerized scaffold materials. The composition and ultrastructure of the ECM vary based on the source tissue and the methods used to decellularize the tissue. This influences the properties of the scaffold and may influence the degradation profile. The variables that affect outcome and how ECM induces constructive remodeling are only partially understood; however, the degradation rate and resulting degradation products may be as important as the structural attributes. Degradation of ECM by the host proteolytic enzymes, including the plasminogen activator/plasmin system and MMPs, results in cleavage of multiple ECM proteins, liberating growth factors and cytokines and exposing cryptic sites and proteins that are otherwise not available. Degradation products of ECM from several sources have been shown to have multiple biological properties, including angiogenic, chemotactic, and antibacterial activity.51–54 It is important to note that many of these degradation products were generated in vitro using chemical methods, and it is not known whether in vivo degradation will generate equivalent bioactivity.
Figure 4.
Effect of processing on ECM structure. Scanning electron microscopy images of control small intestine samples (A), and tissues decellularized with 1.5 M NaCl (B), 3 M NaCl (C), 5 M NaCl (D), 0.1% SDS (E), 0.3% SDS (F), 0.6% SDS (G), 0.1% triton X-100 (H), 0.3% triton X-100 (I), 0.6% triton X-100 (J), 10 min SC (K), 20 min SC (L), 30 min SC (M), 10 min UV (N), 20 min UV (O), and 30 min UV (P). Scale bar represents 50 μm (reprinted under CC BY license, Oliveira et al.88).
The source of material for ECM biological scaffolds is continuing to expand. Common source materials include human, fetal, and porcine skin, equine and bovine pericardium, porcine small intestinal submucosa, and ovine forestomach.49 With initial research starting in the 1990s, porcine small intestine submucosa (SIS) is one of the most well-studied sources of ECM. Its composition, macro and microstructure, biomechanical properties, in vivo degradation rate, cell-matrix interactions, and use as a graft for multiple indications have been exhaustively explored.49,51,53,55,56 Recent studies have identified variability even within this material based on the age of the animal and whether the material was obtained from the distal or proximal jejunum.50 This may account for some of the variability in outcomes and provides some rationale for use of tissue from young or fetal sources.57,58 Several commercially available ECM scaffolds are derived from fetal tissue, which is rich in glycosaminoglycans and Col III with less cross-linking. These properties provide a more “open” matrix that facilitates migration and proliferation of a number of cell types as well as facilitates ECM turnover and remodeling. Degradation products of human fetal skin-derived ECM were shown to have stronger chemoattractant activity for skin-specific, lineage-directed stem and progenitor cells than human adult skin-derived ECM. Degradation products of porcine adult skin-derived ECM had stronger chemoattractant activity than human adult skin-derived ECM, confirming that both age and tissue source are important factors in chemoattractant activity.52 An acellular dermal substrate would seem to be the ideal matrix for wound healing, and dermis is one of the common source tissues for ECM products. Dermis has a dense and compact architecture with collagen fibers that are thick, coarse, and woven in a unique, nonuniaxial fiber orientation. Since it is so dense, decellularization of the dermal ECM requires a combination of detergents and enzymatic agents, which disrupts the ECM ultrastructure, cross-links proteins, and removes some ECM constituents. In contrast, SIS is composed of thin, laminated layers that can be decellularized by more gentle measures with less disruption of the natural ECM architecture.59 In a rat partial-thickness wound model comparing dermal ECM with SIS-ECM, cellular infiltration into dermal ECM at 4 weeks was ∼10-fold less than in SIS-ECM, limited to the margins, and degradation was much slower (20% SIS-ECM vs. 80% dermal-ECM remaining after 4 weeks and 50% of the dermal ECM remaining at 6 months in this model).55 In another study comparing synthetic PLGA yarn, collagen scaffold, and acellular dermal matrix (ADM) in a rat subcutaneous implantation model, it was noted that the dense structure of the ADM with its small pore size did not support vascularization or cellular ingrowth and that the ADM was still visible in the wound bed at 8 weeks.60 These studies illustrate the importance of the three-dimensional architecture on tissue regeneration and vascularization and highlight the need to choose the material based on the intended use (structural support vs. rapid tissue regeneration). As with most studies, they were performed in acute animal models that do not simulate chronic wound conditions.
Clinical application of collagen-based biomaterials
The array of commercially available collagen-based biomaterials for wound care is in constant flux, primarily based on economics. Multiple systematic reviews have evaluated the evidence for use of collagen and ECM products for chronic wounds.61–67 Despite the scientific foundation outlined earlier, the support for use of these products in chronic wounds is quite sparse. It is clear from a review of reimbursement policies that rigorous evidence, primarily in the form of randomized controlled trials (RCTs), is expected for these materials to be considered “medically necessary.” There are some RCTs that compare a single product to a “standard of care” control. Most of these are small, industry-sponsored trials and use moist gauze dressings as the standard of care. There are a few RCTs that compare products, although the majority of these have compared cellular with acellular matrices. Well-designed, investigator-initiated, comparative effectiveness trials such as those proposed by Lev-Tov et al. are the ideal.68 While it would be cost prohibitive to compare the wide array of currently available materials, well-powered, comparative effectiveness trials with appropriate controls and rigorous outcome measures may set the standard for bringing new products to the field.
Three products that stand out in terms of clinical trial data are oxidized regenerated cellulose (ORC)/collagen with and without added silver, acellular human dermal matrix (Graftjacket), and porcine SIS, all of which show some improvement in healing but have variable results (Table 1).
Table 1.
Randomized controlled trials with collagen biomaterials
| Reference | Intervention | Wound Type | Number in Study | Outcome |
|---|---|---|---|---|
| DiDomenico et al.89 | Apligraf vs. Theraskin | DFU | 28 | 12 week healed: 42.3% Apligraf vs. 66.7% Theraskin |
| Reyzelman et al.90 | Graftjacket vs. moist wound therapy | DFU | 86 | 12 week healed: 69.6% Graftjacket vs. 46.2% control |
| Brigido et al.91 | Graftjacket vs. hydrogel | DFU | 28 | 16 week healed: 86% Graftjacket vs. 28% control |
| Brigido et al.92 | Graftjacket vs. debridement, curasol and gauze | DFU | 40 | 12 week healed: 85% Graftjacket vs. 5% control |
| Romanelli et al.93 | Oasis vs. petrolatum gauze | Mixed leg ulcers | 50 | 8 week healed: 80% Oasis vs. 65% control |
| Romanelli et al.94 | Oasis vs. hyaluronic acid | Mixed leg ulcers | 54 | 16 week healed: 82.6% Oasis vs. 46.2% hyaluronic acid |
| Mostow et al.95 | Oasis/compression vs. compression | VLU | 120 | 12 week healed: 55% Oasis vs. 34% control |
| Niezgoda et al.96 | Oasis vs. Regranex gel | VLU | 73 | 12 week healed: 49% Oasis vs. 28% Regranex |
| Gottrup et al.97 | ORC/collagen/silver vs. saline moistened gauze | DFU | 39 | 14 week healed: 52% ORC/collagen/silver vs. 31% control |
| Veves et al.98 | ORC/collagen vs. saline moistened gauze | DFU | 276 | 12 week healed: 37% ORC/collagen vs. 28.3% control |
| Vin et al.99 | ORC/collagen vs. nonadherent | VLU | 29 | 12 week healed: 41% ORC/collagen vs. 31% control |
| Donaghue et al.100 | Collagen/alginate vs. saline moistened gauze | DFU | 75 | 8 week healed: 48% collagen vs. 36% control |
DFU, diabetic foot ulcer; ORC, oxidized regenerated cellulose; VLU, venous leg ulcer.
In addition to clinical trial data, both ORC/collagen and porcine SIS have mechanistic data from in vitro, animal and human studies that suggest they may be beneficial in modulating the chronic wound environment. ORC/collagen is a mixture of 45% ORC with 55% bovine collagen type I. ORC is created by controlled milling of the hemostat SURGICEL®. It is a bioresorbable, biocompatible, and negatively charged polymer that binds positively charged molecules such as metal ions which are necessary for protease activity and has proven free radical absorption.69,70 The ORC and collagen components have been shown to bind MMPs, but maximal binding is achieved with the combined material.71 In its initial polymer form, ORC/collagen may act to provide both a matrix, for the binding and stabilization of growth factor and chemokine gradients, and a hydrated scaffold for cellular migration. Breakdown products may stimulate macrophage influx when ORC is implanted.70,72
ORC/collagen significantly reduced all key proteases in diabetic foot ulcer wound fluid via physical binding4 and was shown to increase fibroblast chemotaxis and proliferation in a dose-dependent fashion. It is hypothesized that these effects are secondary to the effects of peptides released from collagen and hydrolyzed breakdown products of ORC. In vivo, ORC/collagen normalized healing in diabetic mice compared with controls and increased granulation tissue deposition.70 Clinical studies have shown a significant reduction of MMP, elastase, and gelatinase content in diabetic foot ulcers with either ORC/collagen or ORC/collagen/silver.73,74 Although not proof of cause and effect, when combined with the evidence that elevated inflammatory protease activity in the wound is a robust indicator of nonhealing,75 the portfolio of studies strongly supports the fact that ORC/collagen will modulate the chronic wound microenvironment.
The basic and preclinical studies that support use of porcine SIS in chronic wounds are summarized in the discussion of ECM (above). In addition to modulation of proteolytic activity,56 SIS provides a structural matrix and growth factors that may promote angiogenesis and cell migration.76 The ECM research focus has recently swung heavily toward regenerative medicine with an emphasis on organ replacement. ECM modulates a wide variety of cell responses which are tissue dependent, that is, based on the derivation of the ECM (organ vs. organism), and cell dependent, that is, based on the cells expected to grow into the matrix (endothelial cells, fibroblasts, etc.). Although not directed specifically at chronic wounds, the proteomic analyses, both in vitro and in vivo studies of cellular interactions with the wide variety of scaffolds being used for tissue regeneration will provide an opportunity to better understand the composition of the ECM and may lead to development of customized tissue-engineered products that are targeted at specific deficits.47,77,78
Future Directions
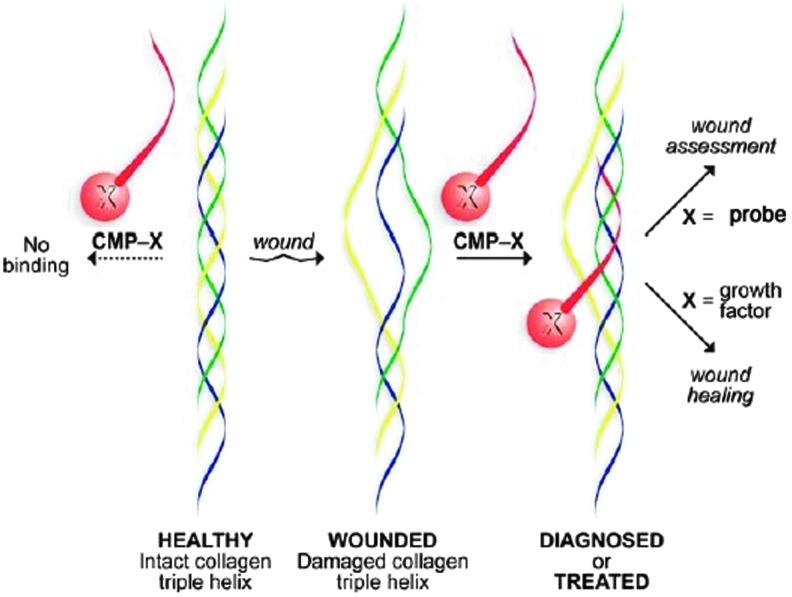
The holy grail of wound care is a cost-effective, topically applied material with a long shelf life which can sense and deliver, that is, assess the wound environment and deliver the appropriate bioactive cells or drugs to promote healing. That product does not exist, but tissue engineering is moving forward at a rapid pace due to improvements in nanotechnology. In the short term, we can anticipate a movement away from mammalian sources of ECM components due to the potential for disease transmission, religious preferences, immune reactions, and the search for more ecological sources. The use of discarded or waste materials and by-products from other industries is promising.15,44,79 A shift toward synthetics that mimic binding properties of Col I, provide strength, and decrease cost is already underway. However, the current synthetic biopolymers do not recapitulate the complexity of the natural ECM and have limited biological functionality. On the other hand, decellularized ECM scaffolds, which have demonstrated efficacy in large animal and human trials of tissue replacement but not chronic wounds, are limited by material properties such as size, shape, degradation rate, physical form, or mechanical strength, defined by tissue source.49 Hybrids that combine synthetic and purified biological materials or decellularized ECM in electrospun polymer nanofibers may be the solution to customized, tunable materials with improved bioactivity.80,81 These composite materials have the potential to be highly versatile, providing a platform for cell-based therapy, controlled release drug delivery, and incorporation of sensor technology.82,83 Electrospun customized ECM component scaffolds have been developed for therapeutic stem cell delivery. These bioengineering techniques provide the ability to customize the mechanical characteristics, elasticity, and rates of degradation and to guide stem cell lineage differentiation.84 Matrikines and matricryptins are bioactive fragments released from the ECM by enzymatic degradation. Some of these fragments have been tested in experimental models of wound healing and fibrosis and show potential as therapeutic molecules either individually, as combinatory peptides, or linked to scaffolds.85 Finally, the combination of a synthetic or composite surface with collagen mimetic peptides may provide the potential for concurrent wound assessment and targeted delivery of therapeutic molecules directly to the wound bed23 (Fig. 5).
Figure 5.
Novel use of collagen mimetic peptides for sensing and delivery. CMP (red) can be engineered to bind to or displace weak or damaged collagen, increasing the stability of the triple helix. A probe that fluoresces, luminesces, or has a color reaction or a growth factor can be attached as a “pendant” to facilitate wound assessment or bioactive molecule delivery (reprinted with permission from Chattopadhyay and Raines23). CMP, collagen mimetic peptides. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Summary
Intact ECM from different tissue types and individual ECM components vary in their ability to promote cellular ingrowth, gene expression, and induction of new tissue. Whether these properties can be harnessed and customized to promote chronic wound healing remains to be seen. Although the ECM is clearly bioactive and bioinductive in a number of situations, the majority of studies have been performed in vitro or in acute tissue defects, with minimal data supporting bioactivity in the chronic wound bed. New tissue-engineered materials are on the horizon that may provide customized platforms for cell and bioactive molecular delivery. Successful incorporation into clinical practice will require that these materials undergo rigorous clinical testing with demonstrable outcomes accompanied by cost-efficacious protocols.
Take-Home Messages.
• The theoretical properties of collagen scaffolds and ECM devices are primarily based on experimental models of acute tissue repair rather than on chronic wounds.
• The ECM contains multiple types of collagen; however, no studies have definitively shown improved healing to be based on the type or source of collagen.
• ORC/collagen, porcine SIS, and human ADM have been shown to improve healing of chronic wounds in randomized clinical trials. The variability of results, use of different controls, and small sample sizes prevent a comparison between studies.
• Tissue-engineered devices with customized approaches to deliver drugs, bioactive molecules, or stem cells that target the deficiencies of the chronic wound hold promise for the future.
Abbreviations and Acronyms
- ADM
acellular dermal matrix
- BM
basement membrane
- Col
collagen
- CTGF
connective tissue growth factor
- DFU
diabetic foot ulcer
- ECM
extracellular matrix
- EDS
Ehlers–Danlos syndrome
- GFOGER
integrin binding sequence Gly-Phe-Hyp-Gly-Glu-Arg
- IL
interleukin
- JNK
c-Jun-N-terminal kinase
- MMPs
matrix metalloproteinases
- NC1
noncollagenous C-terminal domain
- ORC
oxidized regenerated cellulose
- RCT
randomized controlled trials
- RDEB
recessive dystrophic epidermolysis bullosa
- RGD
tripeptide Arg-Gly-Asp
- SIS
small intestine submucosa
- STAT3
signal transducer and activator of transcription 3
- TGF-β
transforming growth factor beta
- TIMPs
tissue-derived inhibitors of metalloproteinases
- VLU
venous leg ulcer
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author. No ghostwriters were used to write this article.
About the Author
Lisa J. Gould, MD, PhD, is the Medical Director of the Kent Hospital Wound Recovery and Hyperbaric Medicine Center and an Affiliate Professor in the Department of Molecular Pharmacology and Physiology at the University of South Florida. Her clinical and basic science research focuses on ischemic and chronic wounds in older adults.
References
- 1.Fife CE, Carter MJ, Walker D, Thomson B. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds 2012;24:10–17 [PubMed] [Google Scholar]
- 2.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–162 [DOI] [PubMed] [Google Scholar]
- 3.Black E, et al. . Decrease of collagen deposition in wound repair in type 1 diabetes independent of glycemic control. Arch Surg 2003;138:34–40 [DOI] [PubMed] [Google Scholar]
- 4.Cullen B, Smith R, Mcculloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10:16–25 [DOI] [PubMed] [Google Scholar]
- 5.Bermudez DM, et al. . Impaired biomechanical properties of diabetic skin. Am J Pathol 2011;178:2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 2003;162:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loot MAM, et al. . Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol 2002;81:153–160 [DOI] [PubMed] [Google Scholar]
- 8.Cook H, Stephens P, Davies KJ, Harding KG, Thomas DW. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP-1, TIMP-2, and MMP-2 activity. J Invest Dermatol 2000;115:225–233 [DOI] [PubMed] [Google Scholar]
- 9.Pastar I, et al. . Epithelialization in wound healing: a comprehensive review. Adv Wound Care 2014;3:445–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volk SW, Iqbal SA, Bayat A. Interactions of the extracellular matrix and progenitor cells in cutaneous wound healing. Adv Wound Care 2013;2:261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stojadinovic O, et al. . Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med 2008;12:2675–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem 2008;56:687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem 2009;78:929–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautam S, Chou C-F, Dinda AK, Potdar PD, Mishra NC. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater Sci Eng C 2014;34:402–409 [DOI] [PubMed] [Google Scholar]
- 15.Parenteau-Bareil R, et al. . Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater 2011;7:3757–3765 [DOI] [PubMed] [Google Scholar]
- 16.Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. BioMed Res Int 2014;2014:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whelan MC. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem 2002;278:327–334 [DOI] [PubMed] [Google Scholar]
- 18.Wiegand C, et al. . Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res 2010;302:419–428 [DOI] [PubMed] [Google Scholar]
- 19.Wiegand C, et al. . Effect of the sterilization method on the performance of collagen type I on chronic wound parameters in vitro. J Biomed Mater Res B Appl Biomater 2009;90B:710–719 [DOI] [PubMed] [Google Scholar]
- 20.Schönfelder U, et al. . Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials 2005;26:6664–6673 [DOI] [PubMed] [Google Scholar]
- 21.Friess W. Collagen—biomaterial for drug delivery. Eur J Pharm 1998;45:113–136 [DOI] [PubMed] [Google Scholar]
- 22.Goo HC, Hwang Y-S, Choi YR, Cho HN, Suh H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials 2003;24:5099–5113 [DOI] [PubMed] [Google Scholar]
- 23.Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing: collagen-Based Biomaterials. Biopolymers 2014;101:821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg 2002;183:445–456 [DOI] [PubMed] [Google Scholar]
- 25.Harriger MD, Supp AP, Warden GD, Boyce ST. Glutaraldehyde crosslinking of collagen substrates inhibits degradation in skin substitutes grafted to athymic mice. J Biomed Mater Res 1997;35:137–145 [DOI] [PubMed] [Google Scholar]
- 26.Cuttle L, et al. . Collagen in the scarless fetal skin wound: Detection with Picrosirius-polarization. Wound Repair Regen 2005;13:198–204 [DOI] [PubMed] [Google Scholar]
- 27.Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol 2012;2012:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zgheib C, Xu J, Liechty KW. Targeting inflammatory cytokines and extracellular matrix composition to promote wound regeneration. Adv Wound Care 2014;3:344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg 2010;126:1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A 1997;94:1852–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 2011;194:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuutila K, et al. . Recombinant human collagen III gel for transplantation of autologous skin cells in porcine full-thickness wounds: rhCol-III gel for wound healing. J Tissue Eng Regen Med [Epub ahead of print]; DOI: 10.1002/term.1691 [DOI] [PubMed] [Google Scholar]
- 33.KüHn K, et al. . The structure of type IV collagen. Ann N Y Acad Sci 1985;460:14–24 [DOI] [PubMed] [Google Scholar]
- 34.Abreu-Velez A, Howard M. Collagen IV in normal skin and in pathological processes. North Am J Med Sci 2012;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega N. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci 2002;115:4201–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viglio S, et al. . Wound repair capability in EDS fibroblasts can be retrieved by exogenous type V collagen. Sci World J 2008;8:956–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viglio S, et al. . Rescue of migratory defects of Ehlers–Danlos syndrome fibroblasts in vitro by type v collagen but not insulin-like binding protein-1. J Invest Dermatol 2008;128:1915–1919 [DOI] [PubMed] [Google Scholar]
- 38.Inkinen K, Turakainen H, Wolff H, Ahonen J. Cloning of cDNA for Rat Pro α1(V) Collagen mRNA. Expression patterns of type I, type III and type V collagen genes in experimental granulation tissue. Connect Tissue Res 1999;40:209–220 [DOI] [PubMed] [Google Scholar]
- 39.Inkinen K, Wolff H, Von Boguslawski K, Ahonen J. Type V collagen in experimental granulation tissue. Connect Tissue Res 1998;39:281–294 [DOI] [PubMed] [Google Scholar]
- 40.Bruckner-Tuderman L. Dystrophic epidermolysis bullosa: pathogenesis and clinical features. Dermatol Clin 2010;28:107–114 [DOI] [PubMed] [Google Scholar]
- 41.Nyström A, et al. . Collagen VII plays a dual role in wound healing. J Clin Invest 2013;123:3498–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, et al. . Topical application of recombinant type VII collagen incorporates into the dermal–epidermal junction and promotes wound closure. Mol Ther 2013;21:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev 2003;55:1595–1611 [DOI] [PubMed] [Google Scholar]
- 44.Westgate S, Cutting K, DeLuca G, Asaad K. Collagen dressings made easy. Wounds UK 2012;8 [Google Scholar]
- 45.Peng YY, et al. . Towards scalable production of a collagen-like protein from Streptococcus pyogenes for biomedical applications. Microb Cell Factories 2012;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shilo S, et al. . Cutaneous wound healing after treatment with plant-derived human recombinant collagen flowable gel. Tissue Eng Part A 2013;19:1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lun S, et al. . A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials 2010;31:4517–4529 [DOI] [PubMed] [Google Scholar]
- 48.Youngstrom DW, Barrett JG, Jose RR, Kaplan DL. Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS One 2013;8:e64151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials 2007;28:3587–3593 [DOI] [PubMed] [Google Scholar]
- 50.Lin H-K, et al. . Understanding roles of porcine small intestinal submucosa in urinary bladder regeneration: identification of variable regenerative characteristics of small intestinal submucosa. Tissue Eng Part B Rev 2014;20:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brennan EP, et al. . Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng 2006;12:2949–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brennan EP, Tang X-H, Stewart-Akers AM, Gudas LJ, Badylak SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med 2008;2:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarikaya A, et al. . Antimicrobial activity associated with extracellular matrices. Tissue Eng 2002;8:63–71 [DOI] [PubMed] [Google Scholar]
- 54.Li F, et al. . Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium 2004;11:199–206 [DOI] [PubMed] [Google Scholar]
- 55.Carey LE, et al. . In vivo degradation of 14C-labeled porcine dermis biologic scaffold. Biomaterials 2014;35:8297–8304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi L, Ronfard V. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): a mini review. Int J Burns Trauma 2013;3:173–179 [PMC free article] [PubMed] [Google Scholar]
- 57.Sicari BM, et al. . The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 2012;33:5524–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tottey S, et al. . The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 2011;32:128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011;32:3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.You C, Wang X, Zheng Y, Han C. Three types of dermal grafts in rats: the importance of mechanical property and structural design. Biomed Eng OnLine 2013;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greer N, et al. . Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med 2013;159:532. [DOI] [PubMed] [Google Scholar]
- 62.Holmes C, Wrobel J, Mac Eachern MP, Boles BR. Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab Syndr Obes Targets Ther 2013;6:17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valle MF, et al. . Comparative effectiveness of advanced wound dressings for patients with chronic venous leg ulcers: a systematic review. Wound Repair Regen 2014;22:193–204 [DOI] [PubMed] [Google Scholar]
- 64.Harding KG, Kirsner RS, Lee D, Mulder G, Serena T. International consensus. Acellular matrices for the treatment of wounds. An expert working group review. Wounds Int 2010. www.woundsinternational.com/pdf/content_9732.pdf (last accessed October11, 2014)
- 65.Snyder DL, Sullivan N, Schoelles KM. Skin substitutes for treating chronic wounds technology assessment report. (2012). www.ahrq.gov/research/findings/ta/skinsubs/HCPRO610-skinsubst-final.pdf (last accessed October11, 2014)
- 66.Hankin CS, Knispel J, Lopes M, Bronstone A, Maus E. Clinical and cost efficacy of advanced wound care matrices for venous ulcers. J Manag Care Pharm 2012;18:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Donnell TF, Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. J Vasc Surg 2006;44:1118–1125 [DOI] [PubMed] [Google Scholar]
- 68.Lev-Tov H, Li C-S, Dahle S, Isseroff RR. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials 2013;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dimitrijevich SD, et al. . In vivo degradation of oxidized, regenerated cellulose. Carbohydr Res 1990;198:331–341 [DOI] [PubMed] [Google Scholar]
- 70.Hart J, et al. . The role of oxidised regenerated cellulose/collagen in wound repair: effects in vitro on fibroblast biology and in vivo in a model of compromised healing. Int J Biochem Cell Biol 2002;34:1557–1570 [DOI] [PubMed] [Google Scholar]
- 71.Cullen B, et al. . The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol 2002;34:1544–1556 [DOI] [PubMed] [Google Scholar]
- 72.Pierce AM, Wiebkin OW, Wilson DF. Surgicel: its fate following implantation. J Oral Pathol 1984;13:661–670 [DOI] [PubMed] [Google Scholar]
- 73.Gottrup F, et al. . Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment: RCT on collagen/ORC/silver in diabetic foot ulcers. Wound Repair Regen 2013;21:216–225 [DOI] [PubMed] [Google Scholar]
- 74.Ulrich D, Smeets R, Unglaub F, Wöltje M, Pallua N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J Wound Ostomy Continence Nurs 2011;38:522–528 [DOI] [PubMed] [Google Scholar]
- 75.McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care 2013;2:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoganson DM, et al. . The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials 2010;31:6730–6737 [DOI] [PubMed] [Google Scholar]
- 77.Sicari BM, et al. . An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med 2014;6:234ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pashuck ET, Stevens MM. Designing regenerative biomaterial therapies for the clinic. Sci Transl Med 2012;4:160sr4. [DOI] [PubMed] [Google Scholar]
- 79.Muthukumar T, Prabu P, Ghosh K, Sastry TP. Fish scale collagen sponge incorporated with Macrotyloma uniflorum plant extract as a possible wound/burn dressing material. Colloids Surf B Biointerfaces 2014;113:207–212 [DOI] [PubMed] [Google Scholar]
- 80.Stankus JJ, Freytes DO, Badylak SF, Wagner WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed 2008;19:635–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jayarama Reddy V, et al. . Nanofibrous structured biomimetic strategies for skin tissue regeneration: nanofibrous structures for wound healing. Wound Repair Regen 2013;21:1–16 [DOI] [PubMed] [Google Scholar]
- 82.Gibson M, et al. . Tissue extracellular matrix nanoparticle presentation in electrospun nanofibers. BioMed Res Int 2014;2014:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhat S, Kumar A. Biomaterials and bioengineering tomorrow's healthcare. Biomatter 2013;3:e24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Machula H, Ensley B, Kellar R. Electrospun tropoelastin for delivery of therapeutic adipose-derived stem cells to full-thickness dermal wounds. Adv Wound Care 2014;3:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol 2014;23:457–463 [DOI] [PubMed] [Google Scholar]
- 86.Fang M, et al. . Type I collagen D-spacing in fibril bundles of dermis, tendon, and bone: bridging between nano- and micro-level tissue hierarchy. ACS Nano 2012;6:9503–9514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalluri R. Angiogenesis: Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 2003;3:422–433 [DOI] [PubMed] [Google Scholar]
- 88.Oliveira AC, et al. . Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS One 2013;8:e66538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DiDomenico L, Emch KJ, Landsman AR, Landsman AA. Prospective comparison of diabetic foot ulcers treated with either a cryopreserved skin allograft or a bioengineered skin substitute. Wounds 2011;23:184–189 [PubMed] [Google Scholar]
- 90.Reyzelman A, et al. . Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. J Int Wound 2009;6:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. J Int Wound 2006;3:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics 2004;27:145–149 [DOI] [PubMed] [Google Scholar]
- 93.Romanelli M, Dini V, Bertone MS. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Adv Skin Wound Care 2010;23:34–38 [DOI] [PubMed] [Google Scholar]
- 94.Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus hyaloskin in the treatment of difficult-to-heal wounds of mixed arterial/venous aetiology. J Int Wound 2007;4:3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 2005;41:837–843 [DOI] [PubMed] [Google Scholar]
- 96.Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS wound matrix to regranex gel for diabetic ulcers. Adv Skin Wound Care 2005;18:258–266 [DOI] [PubMed] [Google Scholar]
- 97.Gottrup F, et al. . Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment: RCT on collagen/ORC/silver in diabetic foot ulcers. Wound Repair Regen 2013;21:216–225 [DOI] [PubMed] [Google Scholar]
- 98.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822–827 [DOI] [PubMed] [Google Scholar]
- 99.Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care 2002;11:335–341 [DOI] [PubMed] [Google Scholar]
- 100.Donaghue VM, et al. . Evaluation of a collagen-alginate wound dressing in the management of diabetic foot ulcers. Adv Wound Care 1998;11:114–119 [PubMed] [Google Scholar]