Abstract
Phytonutrients have rapidly emerged as natural food chemicals possessing multifaceted biological actions that may support beneficial health outcomes. Among the vast array of phytonutrients currently being studied, sulforaphane, curcumin, quercetin, and resveratrol have been frequently reported to stimulate the expression of endogenous detoxification enzymes and may thereby facilitate the neutralization of otherwise harmful environmental agents. Some of these same phytonutrients, however, have also been implicated in disrupting normal cell proliferation and hence may possess toxic properties in and of themselves. In this study, we characterize the respective minimum threshold concentrations of the aforementioned phytonutrients in Hepa1c1c7 cells that stimulate NAD(P)H:quinone oxidoreductase (NQO1), a key enzyme in the hepatic neutralization of menadione, other biological oxidants, and some environmental carcinogens. Moreover, our findings demonstrate that relatively low concentrations of either sulforaphane or curcumin significantly (P < .05) increase NQO1 protein expression and activity without triggering G2/M cell cycle arrest or mitotic catastrophe. The minimal quercetin concentration inducing NQO1, however, was 100-fold higher than that which disrupted mitosis. Also, while resveratrol modestly stimulated NQO1, the minimally effective resveratrol concentration concomitantly induced evidence of cellular apoptosis. Taken together, these findings indicate that only particular phytonutrients are likely efficacious in upregulating NQO1 activity without also leading to hepatic cytotoxicity.
Key Words: : cell cycle, curcumin, quercetin, resveratrol, sulforaphane
Introduction
Phytonutrients are bioactive chemical constituents of plant-based foods that are thought to promote human health.1 Unlike classical nutrients, which are essential to life and required for the growth and repair of biological tissues,2–4 phytonutrients appear to support human health by protecting against diseases that could develop as a consequence of exposure to toxic environmental agents.5 A growing literature describes a common mechanism by which several phytonutrients stimulate the expression of Phase II hepatic detoxification enzymes and thereby enhance the neutralization of environmental carcinogens and biological oxidants that could otherwise lead to the development of cancer or cardiovascular disease.6,7 The phytonutrients sulforaphane (isothiocyanate from broccoli and other cruciferous vegetables), curcumin (phenolic from turmeric spice), quercetin (flavonoid from apple, onion, and berries), and resveratrol (phenolic from grape and red wine), each stimulates the cellular Nrf2/ARE pathway, which regulates Phase II enzyme expression.8–12 In the cytoplasm, these electrophilic phytonutrients are thought to bind directly with the sulfhydryl-rich Keap1 protein, which exists in complex with the Nrf2 transcription factor. Upon binding of phytonutrients to Keap1, Nrf2 then dissociates and freely translocates into the nucleus, where it activates the antioxidant response element (ARE). Indeed, substantial evidence now supports a role for sulforaphane, curcumin, quercetin, and resveratrol in upregulating Phase II detoxification enzymes, to include hepatic NAD(P)H:quinone oxidoreductase (NQO1).13–15
Among the Phase II detoxification enzymes, expression of NQO1 is commonly used as a biomarker for effective hepatic Nrf2/ARE pathway stimulation.16 NQO1 has also been shown to neutralize (by chemical reduction) the active centers of biological oxidants, such as menadione derived from environmental exposures to petroleum fuel chemicals.17–19 Organic hydrocarbon chemical constituents of refined petroleum-based fuels (e.g., naphthalene, 2-methylnaphthalene) are capable of entering the human circulation20–22 and may increase risk for developing cancer or other pathologies in humans who are occupationally exposed.23 The 2-methylnaphthalene chemical constituent, in particular, is subject to Phase I detoxification in the liver, yet the resulting menadione product remains toxic if not adequately modulated by subsequent Phase II reactions, such as chemical reduction by NQO1.17,24–26 Hence, if particular phytonutrients are indeed capable of stimulating NQO1 expression (and overall NQO1 activity) at physiological concentrations and without their own inherent toxicity, this would provide an intriguing mechanism by which diet could enhance human resilience against disease-causing environmental agents.
While phytonutrients are thought to be responsible for many of the health-promoting effects of diets rich in vegetables and fruits,1 it is important to consider their respective dose–response relationships and whether a potential for phytonutrient toxicity may also exist. The interaction of phytonutrients with sulfhydryl groups (i.e., within Keap1) is believed to be essential for ARE activation and ultimate stimulation of Phase II detoxification enzymes.13 In light of this mechanism, questions arise as to possible phytonutrient interactions with additional off-target proteins.27 Sulforaphane, for example, has been shown to bind directly with tubulin protein,28 depolymerize microtubules,29 and lead to mitotic catastrophe.30,31 Evidence of similar toxicity exists for curcumin, quercetin, and resveratrol, where these phytonutrients have each been reported to impact cell cycle regulation (and lead to biomarkers of cell death) in various experimental models.32–34 However, rigorous dose–response comparisons identifying specific outcomes of phytonutrient efficacy versus toxicity within a common experimental model have not yet been described. Given that phytonutrients may bind with proteins other than Keap1,28 cells could be more sensitive to phytonutrient-induced cell cycle disruptions, compared with the respective minimum threshold concentrations triggering Nrf2/ARE pathway activation. Conversely, certain phytonutrients may be safer (or more appropriate) for use in upregulating hepatic NQO1, provided that substantially higher concentrations of those phytonutrients are necessary to disrupt cell cycle progression.
In the present study, we sought to determine whether sulforaphane, curcumin, quercetin, or resveratrol might be effective in stimulating NQO1 without disrupting the cell cycle or otherwise impacting liver cell proliferation or mitotic progression. All experiments were carried out in the Hepa1c1c7 cell line, and dose–response comparisons among the four phytonutrients were executed concurrently using cells of the same passage number. The Hepa1c1c7 cell line was selected due to its frequently reported use in the literature as a model for measuring Nrf2/ARE pathway stimulation and the activity of Phase II detoxification enzymes, to include NQO1.16,35 Also, evidence describing the direct binding of phytonutrients with ubiquitous tubulin protein28 strongly suggests that observations of ensuing mitotic disruption would be similarly apparent, irrespective of cell lineage, provided those phytonutrients come into contact with the dynamic M-phase microtubules of proliferating cells.36 While nonhepatic tissues may be exposed to phytonutrients within the systemic blood circulation,37 the liver would be expected to experience relatively high physiological phytonutrient concentrations subsequent to dietary consumption, yet before conjugation reactions associated with first-pass metabolism.24 Hence, in experiments using Hepa1c1c7 liver cell culture, we endeavored to discern the relative potencies of phytonutrients in stimulating hepatic NQO1, while identifying the respective minimum phytonutrient concentrations resulting in biomarkers of mitotic catastrophe.
Materials and Methods
Cell culture
Mouse immortalized Hepa1c1c7 hepatocytes, available from ATCC (Manassas, VA, USA), were used to assess the efficacy of phytonutrients to upregulate NQO1, in light of potential phytonutrient impacts to cell cycle progression. Stock Hepa1c1c7 cells were routinely cultured in an alpha minimum essential medium containing 10% fetal bovine serum. Cultures were maintained at 37°C under a 5% CO2 atmosphere, and the medium was changed every 48 h. One day after seeding, proliferating cultures received fresh medium replacement containing individual phytonutrients or dimethyl sulfoxide (vehicle, 0.1% final concentration across all samples). The following phytonutrients, obtained from LKT Laboratories (St. Paul, MN, USA), were evaluated in cell culture: sulforaphane (S8046), curcumin (C8069), quercetin (Q8016), and resveratrol (R1776).
NQO1 activity assay
Hepa1c1c7 cells were seeded at a density of 1 × 104 cells/cm2 in 21 cm2 Corning tissue culture dishes. One day after seeding, cultures (n = 5/group) were exposed to increasing phytonutrient concentrations for 24 h. All samples were then washed twice with Tris-buffered saline, harvested in ice-cold lysis buffer (0.8% Digitonin, 50 mM Tris, 10 mM NaF, 10 mM NaPPi, 1 mM Na3VO4, 2 mM EDTA, 250 nM Aprotinin, 20 μM Leupeptin, 1 μM Pepstatin A), centrifuged (15,000 g for 15 min at 4°C), and assayed for total protein content. The NQO1 activity was then measured as previously described.16,38 Briefly, cell lysate aliquots of equal protein were added to the reaction mixture (25 mM Tris, 0.18 mg/mL bovine serum albumin, 0.01% Tween 20, 5 μM FAD, 200 μM NADPH, 40 μM 2,6-dichlorophenolindophenol), with or without 10 μM dicumarol, yielding a final concentration of 35 μg total protein per 1 mL reaction mixture across all samples. The reduction of 2,6-dichlorophenolindophenol was measured in a Beckman Coulter DU730 spectrophotometer at 600 nm, with the portion of activity responding to dicumarol indicating the NQO1 activity.
Western blot analysis
Hepa1c1c7 cell lysates were obtained as indicated in the above NQO1 activity assay procedure. Cell lysate aliquots of equal protein were then fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked and then incubated with primary antibody to NQO1 (ab2346; Abcam, Cambridge, MA, USA). Following subsequent incubation with a horseradish peroxidase-conjugated secondary antibody from the appropriate species (sc-2020; Santa Cruz Biotechnology, Santa Cruz, CA, USA), immunodetection was carried out using enhanced chemiluminescence (34080; Pierce Biotechnology, Rockford, IL, USA). Membranes were also reprobed for actin (sc-1616; Santa Cruz Biotechnology) to confirm equal loading of total protein. Finally, the X-ray film was scanned into Adobe Photoshop and densitometric analysis performed using the public domain ImageJ program (developed at the U.S. National Institutes of Health and is available on the Internet [http://rsb.info.nih.gov/ij/]).
[3H]thymidine incorporation assay of DNA synthesis
Hepa1c1c7 cells were seeded at a density of 1 × 104 cells/cm2 in Costar 48-well plates. One day after seeding, cultures (n = 5/group) were exposed to increasing phytonutrient concentrations, together with [3H] thymidine (1 μCi), for 24 h. The medium was then removed and the cells assayed for tritium incorporation as previously described.28
Cell cycle analysis
Hepa1c1c7 cells were seeded at a density of 1 × 104 cells/cm2 in 25 cm2 Corning tissue culture flasks. One day after seeding, cultures (n = 4/group) were exposed to increasing phytonutrient concentrations and, after 24 h, harvested by trypsinization. The cells were prepared for analysis using a Becton Dickinson FACSCalibur Flow Cytometer as previously described.28
Hepatic cytology
Hepa1c1c7 cells were seeded at a density of 1 × 104 cells/cm2 in 25 cm2 Corning tissue culture flasks. One day after seeding, cultures were exposed to increasing phytonutrient concentrations and, after 24 h, harvested by trypsinization. Samples were then spun onto slides, stained with Wright–Giemsa dye, and examined for evidence of mitotic catastrophe as previously described.32
Statistical analysis
Data derived from experiments comparing two means were analyzed by independent t-test and the accompanying test for homogeneity of variance. In the case of experiments requiring the comparison of more than two means, ANOVA was performed followed by Tukey's Studentized Range Honest Significant Difference (HSD) for the pair-wise comparisons. All calculations were completed using JMP IN (SAS Institute, Cary, NC, USA), with statistically significant differences established at P < .05.
Results
Sulforaphane potently stimulates NQO1
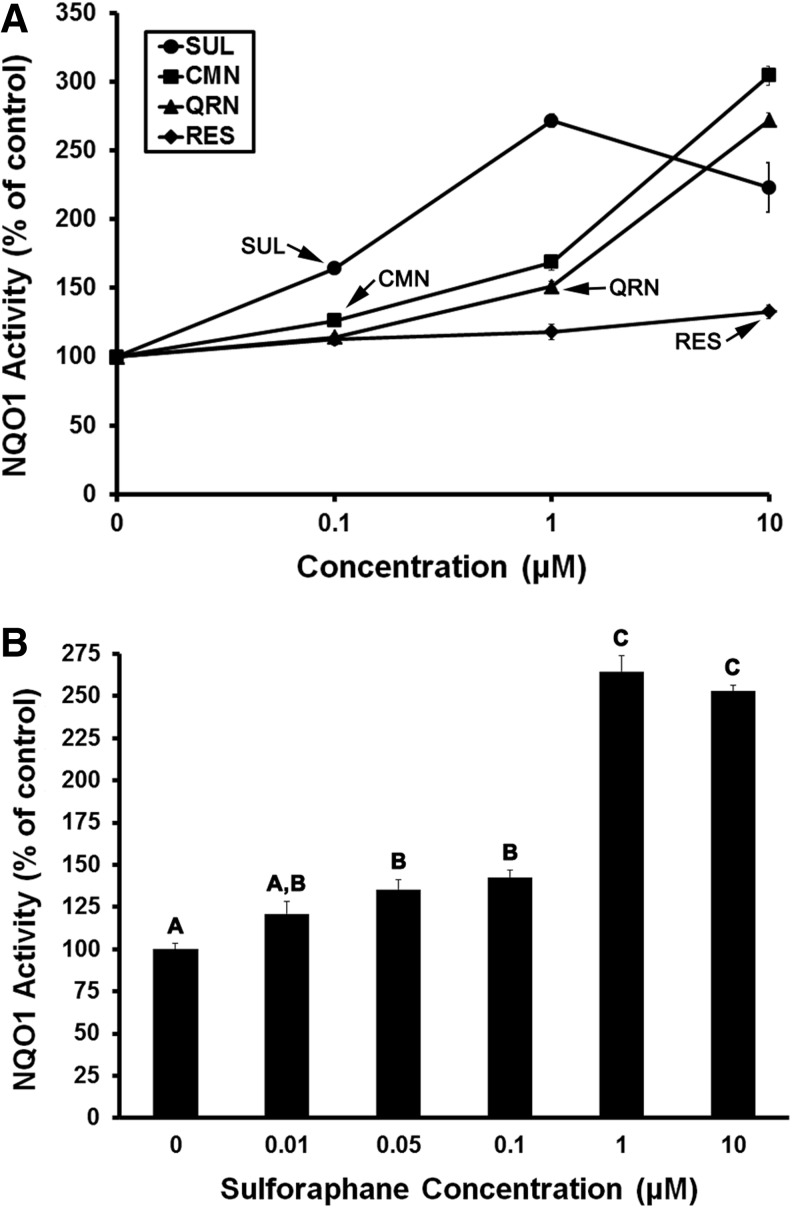
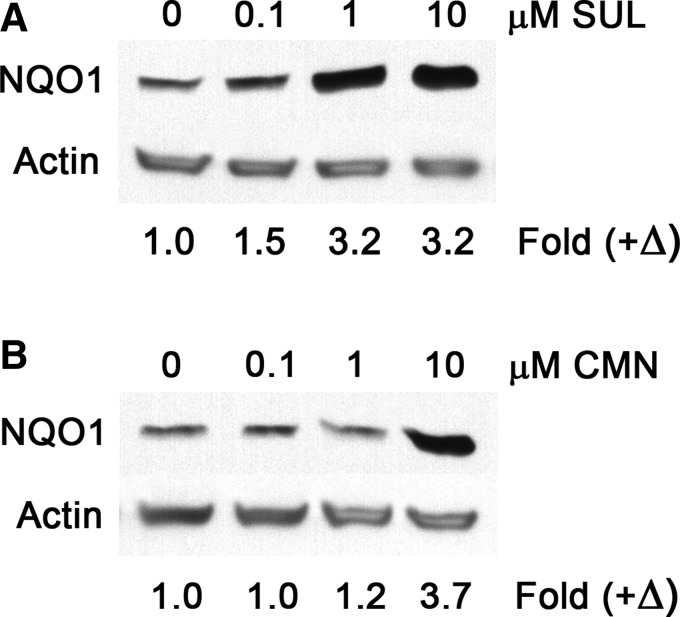
Sulforaphane, curcumin, quercetin, and resveratrol were each found to stimulate the activity of NQO1 in Hepa1c1c7 cells, yet at varying concentrations. In concurrent experiments, sulforaphane and curcumin each significantly (P < .05) increased the NQO1 activity within 24 h, at concentrations as low as 0.1 μM. However, the magnitude of NQO1 activation by 0.1 μM sulforaphane was clearly greater compared with curcumin or the other phytonutrients (Fig. 1A). Sulforaphane at a 10-fold higher concentration (i.e., 1 μM) produced maximal stimulation of NQO1. Upon further examination, 0.05 μM sulforaphane was found to be a minimum threshold concentration triggering significant (P < .05) NQO1 activation. The magnitude of NQO1 activation in response to either 0.05 or 0.1 μM sulforaphane was 40–50% greater than the basal NQO1 activity observed in vehicle-administered controls (Fig. 1B). NQO1 protein expression was similarly upregulated by 50% in response to 0.1 μM sulforaphane (Fig. 2A). Conversely, in response to curcumin, NQO1 protein upregulation was observed only at concentrations in excess of 0.1 μM (Fig. 2B).
FIG. 1.
Phytonutrients differentially stimulate NQO1 activity. Hepa1c1c7 cell cultures were treated with increasing concentrations of phytonutrients for 24 h, before assay of NQO1 activity. SUL, CMN, QRN, and RES each stimulated NQO1 activity over a range of concentrations, with SUL being most efficacious at concentrations ≤1 μM (A). SUL triggered significant (P < .05) NQO1 activation at concentrations as low as 0.05 μM (B). Values are mean ± SEM, n = 5. Arrows indicate the lowest respective phytonutrient concentrations stimulating NQO1 activity, P < .05 (A). Means lacking a common letter differ, P < .05 (B). CMN, curcumin; NQO1, NAD(P)H:quinone oxidoreductase; QRN, quercetin; RES, resveratrol; SUL, sulforaphane.
FIG. 2.
SUL and CMN upregulate NQO1 protein expression. Hepa1c1c7 cell cultures were treated with increasing concentrations of phytonutrients for 24 h, before Western blot analysis. Exposure to 0.1 μM SUL triggered a 50% increase in NQO1 (A), while 1 μM CMN was needed to detect minimal NQO1 upregulation (B). Values are positive fold changes (+Δ) in NQO1 protein expression, calculated from the respective ratios of NQO1:actin band densities.
Sulforaphane, curcumin, and quercetin inhibit DNA synthesis and induce G2/M arrest
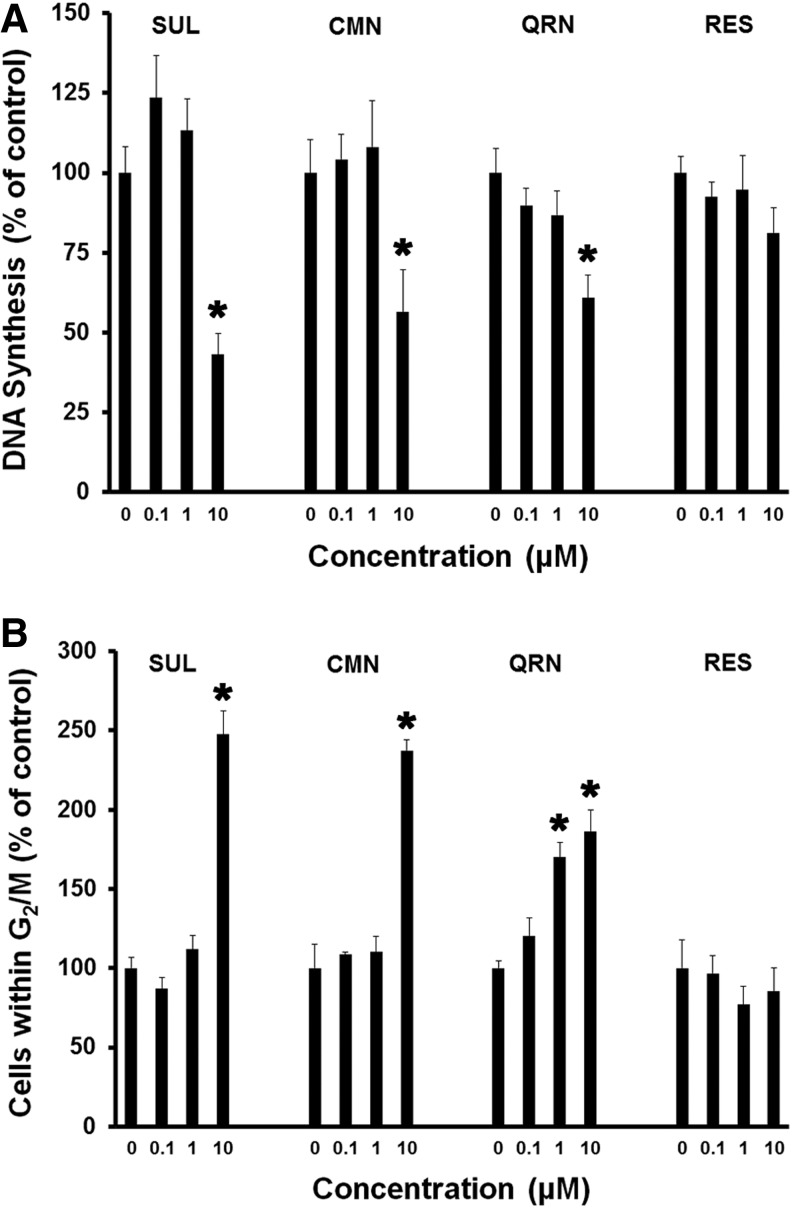
Exposure to 10 μM sulforaphane, curcumin, or quercetin led to significant (P < .05) suppression of Hepa1c1c7 cell DNA synthesis (Fig. 3A), as well as G2/M cell cycle arrest (Fig. 3B), within 24 h. Although not statistically significant, 0.1 μM sulforaphane led to modest stimulation of DNA synthesis without any increase in the proportion of cells within G2/M. Moreover, only quercetin induced a clear dose-dependent trend of inhibited DNA synthesis across the entire 0–10 μM range of treatments. Quercetin similarly triggered a dose-dependent accumulation of Hepa1c1c7 cells within G2/M, achieving statistical significance (P < .05) at 1 μM concentration. At the higher 10 μM concentration, quercetin (as well as sulforaphane and curcumin) approximately doubled the number of Hepa1c1c7 cells within G2/M. Resveratrol, on the other hand, was unlike the other three phytonutrients, in that, it failed to significantly impact Hepa1c1c7 cell cycle phase distributions at any concentration examined. Mean DNA synthesis was, however, suppressed by ∼20% in response to 10 μM resveratrol.
FIG. 3.
SUL, CMN, and QRN each inhibit DNA synthesis, while inducing G2/M cell cycle arrest. Hepa1c1c7 cell cultures were treated with increasing concentrations of phytonutrients for 24 h, before measurement of [3H] thymidine incorporation (A) or analysis by flow cytometry (B). Mean values (±SEM) are expressed as the percentage of control [3H] thymidine incorporation (A, n = 5) and percentage of control cells within G2/M cell cycle phases (B, n = 4). Asterisks (*) indicate significant differences (P < .05) compared to the respective control groups.
Lower concentrations of sulforaphane, curcumin, and quercetin trigger mitotic catastrophe
Hallmark evidence of mitotic catastrophe was observed in response to varying concentrations of sulforaphane, curcumin, or quercetin in Hepa1c1c7 cells. As compared with vehicle-administered controls, exposure to 1 μM and greater concentrations of sulforaphane or curcumin resulted in aberrant mitotic figures displaying disproportionate DNA segregation and lagging chromosomes, as well as cellular micronuclei (Fig. 4A–D). Lower concentrations of sulforaphane or curcumin (i.e., ≤ 0.1 μM) produced no apparent alteration to the mitotic or interphase Hepa1c1c7 cell phenotype. Disproportionate DNA segregation was, however, observed in response to quercetin concentrations as low as 0.01 μM (Fig. 4E, F). Resveratrol treatments in the 0–10 μM range did not lead to evidence of mitotic catastrophe, yet exposure to the relatively high 10 μM concentration produced interphase cells containing multiple apoptotic bodies (Fig. 4G, H).
FIG. 4.
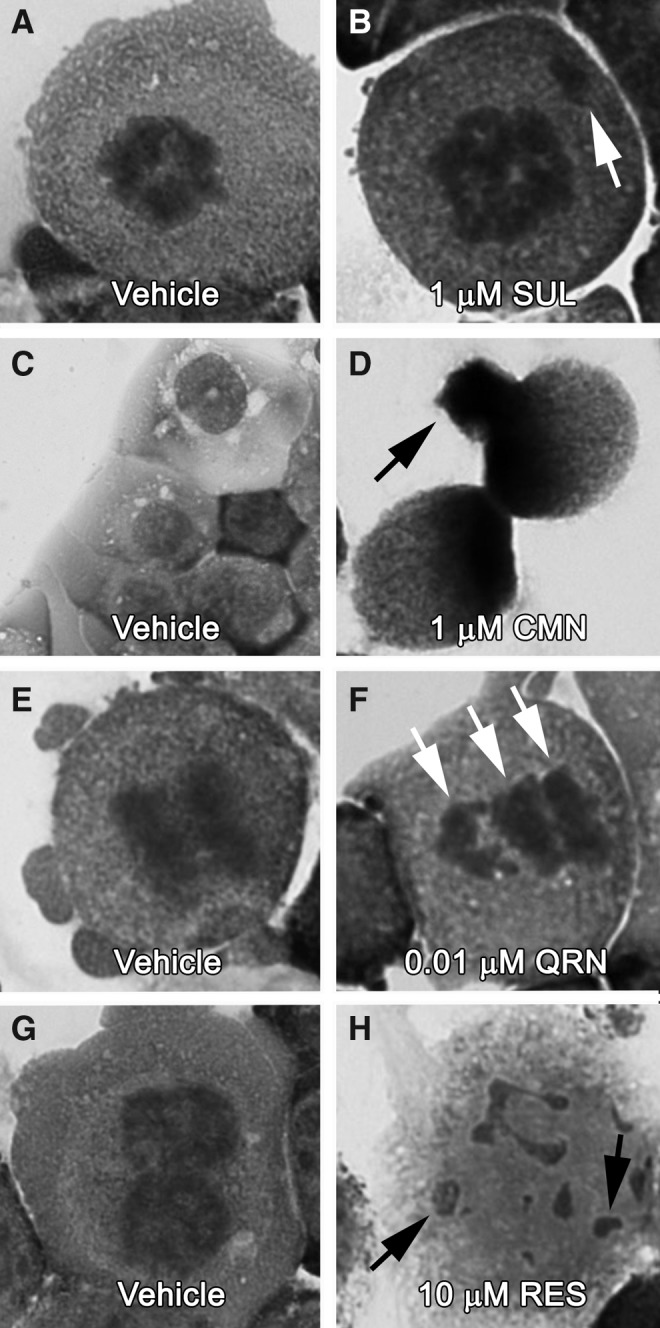
SUL, CMN, and QRN trigger mitotic catastrophe, at relatively low concentrations. Hepa1c1c7 cell cultures were treated with increasing concentrations of phytonutrients for 24 h, before Wright–Giemsa staining and examination under light microscopy. Concentrations as low as 1 μM SUL, 1 μM CMN, and 0.01 μM QRN produced aberrant mitotic figures (see arrows, B and F) and micronucleation (see arrow, D). The higher 10 μM RES concentration produced only apoptotic bodies (see arrows, H).
Discussion
Our experiments have confirmed the actions of sulforaphane, curcumin, quercetin, and resveratrol in stimulating the hepatic NQO1 activity, yet these phytonutrients were vastly different in their minimum effective concentrations and potencies. Moreover, we have provided evidence of an additional mechanism by which sulforaphane, curcumin, and quercetin are each capable of perturbing mitotic cell cycle progression. Resveratrol, on the other hand, triggered evidence of apoptotic cell death, but only at a relatively high concentration associated with very modest NQO1 stimulation. These findings strongly suggest that particular phytonutrients may be better suited than others, and perhaps safer, in upregulating NQO1 and other detoxification pathways thought to afford protection against otherwise toxic agents from the environment.
Sulforaphane was clearly the most potent of the four phytonutrients in stimulating hepatic NQO1 activity (Fig. 1), which was similarly observed in the level of NQO1 protein expression (Fig. 2). More importantly, however, sulforaphane and curcumin stimulated the NQO1 activity without disrupting hepatic cell cycle progression and at concentrations likely achievable in plasma or serum following oral administration.39,40 Furthermore, in light of our recent report describing the impact of relatively low curcumin concentrations on endothelial cell mitotic progression,32 sulforaphane is now emerging as perhaps a superior phytonutrient with regard to safe stimulation of Phase II detoxification. Sulforaphane clearly induces hepatic NQO1 at concentrations far below those shown to bind tubulin protein,28 depolymerize cellular microtubules,29 or otherwise lead to biomarkers of mitotic catastrophe.30,31 Our findings from liver cell culture are especially encouraging in light of a recent clinical trial describing efficacy of a broccoli sprout beverage to rapidly increase urinary excretion of conjugated industrial chemicals in humans chronically exposed to air pollution. This study by Egner et al. further showed sulforaphane bioavailability to be consistent over 12 weeks of daily consumption, suggesting that a broccoli sprout beverage (or broccoli itself) could serve as an inexpensive means of protecting large populations around the world.41 Such clinical studies are inherently limited, however, to merely describing particular outcomes in question, and may overlook possible treatment-induced mechanisms of toxicity that are not detectable as a function of the experimental design. Interestingly, our results add credence to the earlier clinical trial by providing mechanistic biochemical evidence of sulforaphane's ability to safely stimulate NQO1 without concomitant mitotic disruption.
Our findings suggest the opposite scenario for the phytonutrient quercetin. We found 1 μM quercetin to be the minimum concentration inducing significant (P < .05) stimulation of NQO1 activity (Fig. 1A), while this same quercetin concentration triggered significant (P < .05) G2/M cell cycle arrest (Fig. 3B). Moreover, a 100-fold lower quercetin concentration produced aberrant mitotic figures, a finding that inspired us to consider the possibility that phytonutrients (or other exogenous substances) may impact cell cycle characteristics at concentrations lower than those generally acknowledged following results from flow cytometry analysis and/or mitotic index calculations. Indeed, concentrations of sulforaphane at or below 1 μM produced no appreciable changes in cell cycle phase distributions measured by flow cytometry, while 10 μM sulforaphane led to dramatic G2/M accumulation (Fig. 3B). Upon cytological examination, however, 1 μM sulforaphane produced classical evidence of mitotic catastrophe (Fig. 4), similar to that previously reported only in response to higher concentrations.29,30 These findings indicate that relatively low phytonutrient concentrations may not lead to overt G2/M block, but could still have more subtle effects within M-phase itself, analogous to the actions of some tubulin-binding drugs in suppressing mitotic microtubule dynamics without necessarily leading to gross microtubule depolymerization.36 Hence, future studies aiming to describe chemical dose–response phenomena on G2/M phase characteristics should be carefully designed, in light of potential M-phase effects that may not be readily detectable by flow cytometry.
In conclusion, among the four phytonutrients examined, sulforaphane was clearly most potent in stimulating hepatic NQO1 and possessed a relatively low minimum effective concentration, likely achievable in body fluids subsequent to dietary intakes.39 Moreover, the extensive range of efficacious sulforaphane concentrations allowed for the widest window of safety between the minimum effective concentration and the lowest concentration triggering mitotic disruption (Table 1). Our findings, coupled with results from recent clinical trials,41,42 strongly suggest that sulforaphane-containing foods may offer exceptional promise as modalities for safely protecting populations exposed to environmental carcinogens. Future studies should focus on specific high-risk groups, with an eye toward identifying stable nutrient delivery platforms affording optimal sulforaphane bioavailability.
Table 1.
Summary of Phytonutrient Treatment Effects in Hepa1c1c7 Cells
| Phytonutrient | NQO1 activation (μM) | Growth inhibition (μM) | G2/M accretion (μM) | Mitotic disruption (μM) |
|---|---|---|---|---|
| Sulforaphane (SUL)a | 0.05 | 10.00 | 10.00 | 1.00 |
| Curcumin (CMN)a | 0.10 | 10.00 | 10.00 | 1.00 |
| Quercetin (QRN) | 1.00 | 10.00 | 1.00 | 0.01 |
| Resveratrol (RES) | 10.00 | — | — | — |
Values indicate the minimum phytonutrient concentrations (μM) triggering the respective cellular outcomes. Bold highlights the margin of safety.
SUL and CMN activate NQO1 at concentrations that do not inhibit cell growth or lead to G2/M cell cycle phase accumulation or aberrant mitosis.
CMN, curcumin; NQO1, NAD(P)H:quinone oxidoreductase; QRN, quercetin; RES, resveratrol; SUL, sulforaphane.
Acknowledgments
This study was supported by the U.S. Army Medical Research and Materiel Command, In-house Laboratory Independent Research Program (ILIR).
Disclaimer
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or the Department of Defense.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Beecher GR: Phytonutrients' role in metabolism: Effects on resistance to degenerative processes. Nutr Rev 1999;57:S3–S6 [DOI] [PubMed] [Google Scholar]
- 2.Zeisel SH, Blusztajn JK: Choline and human nutrition. Annu Rev Nutr 1994;14:269–296 [DOI] [PubMed] [Google Scholar]
- 3.Prado EL, Dewey KG: Nutrition and brain development in early life. Nutr Rev 2014;72:267–284 [DOI] [PubMed] [Google Scholar]
- 4.Wan JM, Haw MP, Blackburn GL: Nutrition, immune function, and inflammation: An overview. Proc Nutr Soc 1989;48:315–335 [DOI] [PubMed] [Google Scholar]
- 5.Sciullo EM, Vogel CF, Wu D, Murakami A, Ohigashi H, Matsumura F: Effects of selected food phytochemicals in reducing toxic actions of TCDD and p,p′-DDT in U937 macrophages. Arch Toxicol 2010;84:957–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeffery EH: Diet and detoxification enzymes. Altern Ther Health Med 2007;13:S98–S99 [PubMed] [Google Scholar]
- 7.Riccioni G, Speranza L, Pesce M, Cusenza S, D'Orazio N, Glade MJ: Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 2012;28:605–610 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Talalay P, Cho CG, Posner GH: A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc Natl Acad Sci USA 1992;89:2399–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishinaka T, Ichijo Y, Ito M, Kimura M, Katsuyama M, Iwata K, Miura T, Terada T, Yabe-Nishimura C: Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol Lett 2007;170:238–247 [DOI] [PubMed] [Google Scholar]
- 10.Tanigawa S, Fujii M, Hou DX: Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med 2007;42:1690–1703 [DOI] [PubMed] [Google Scholar]
- 11.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A: Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 2010;299:H18–H24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turpaev KT: Keap1-Nrf2 signaling pathway: Mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc) 2013;78:111–126 [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Surh YJ: Nrf2 as a novel molecular target for chemoprevention. Cancer Lett 2005;224:171–184 [DOI] [PubMed] [Google Scholar]
- 14.Wu RP, Hayashi T, Cottam HB, Jin G, Yao S, Wu CC, Rosenbach MD, Corr M, Schwab RB, Carson DA: Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2010;107:7479–7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanson AL, Bakovic M: Dietary regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived compounds and trace minerals. Nutrients 2014;6:3777–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang ES, Jeffery EH: Induction of quinone reductase by sulforaphane and sulforaphane N-acetylcysteine conjugate in murine hepatoma cells. J Med Food 2005;8:198–203 [DOI] [PubMed] [Google Scholar]
- 17.Newsome JJ, Hassani M, Swann E, Bibby JM, Beall HD, Moody CJ: Benzofuran-, benzothiophene-, indazole- and benzisoxazole-quinones: Excellent substrates for NAD(P)H:quinone oxidoreductase 1. Bioorg Med Chem 2013;21:2999–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stohs SJ, Ohia S, Bagchi D: Naphthalene toxicity and antioxidant nutrients. Toxicology 2002;180:97–105 [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Labor, Occupational Health & Safety Administration: www.osha.gov/dts/chemicalsampling/data/CH_250460.html (accessed March2015)
- 20.Pleil JD, Smith LB, Zelnick SD: Personal exposure to JP-8 jet fuel vapors and exhaust at air force Bases. Environ Health Perspect 2000;108:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egeghy PP, Hauf-Cabalo L, Gibson R, Rappaport SM: Benzene and naphthalene in air and breath as indicators of exposure to jet fuel. Occup Environ Med 2003;60:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao YE, Kupper LL, Serdar B, Egeghy PP, Rappaport SM, Nylander-French LA: Dermal exposure to jet fuel JP-8 significantly contributes to the production of urinary naphthols in fuel-cell maintenance workers. Environ Health Perspect 2006;114:182–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council, Committee on Toxicology, Subcommittee on Jet-Propulsion Fuel 8: Toxicologic Assessment of Jet-Propulsion Fuel 8. The National Academies Press, Washington, DC, 2003 [PubMed] [Google Scholar]
- 24.Timbrell JA: Factors affecting toxic responses: Metabolism. In: Principles of Biochemical Toxicology, 2nd edition. Taylor & Francis, London, 1991, pp. 73–124 [Google Scholar]
- 25.Witschi HR, Pinkerton KE, Van Winkle LS, Last JA: Toxic responses of the respiratory system. In: Casarett & Doull's Toxicology—The Basic Science of Poisons, 7th edition (Klaasen CD, ed.). McGraw Hill, New York, NY, 2008, pp. 609–630 [Google Scholar]
- 26.U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Toxicological Profile for Naphthalene, 1-Methylnaphthalene, and 2-Methylnaphthalene. August 1995. www.bvsde.paho.org/bvsapud/i/fulltext/profile/naththalene.pdf (accessed March2015)
- 27.Murakami A, Ohnishi K: Target molecules of food phytochemicals: Food science bound for the next dimension. Food Funct 2012;3:462–476 [DOI] [PubMed] [Google Scholar]
- 28.Jackson SJ, Singletary KW: Sulforaphane: A naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis 2004;25:219–227 [DOI] [PubMed] [Google Scholar]
- 29.Jackson SJ, Singletary KW: Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J Nutr 2004;134:2229–2236 [DOI] [PubMed] [Google Scholar]
- 30.Jackson SJ, Singletary KW, Venema RC: Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vasc Pharmacol 2007;46:77–84 [DOI] [PubMed] [Google Scholar]
- 31.Azarenko O, Okouneva T, Singletary KW, Jordan MA, Wilson L: Suppression of microtubule dynamic instability and turnover in MCF7 breast cancer cells by sulforaphane. Carcinogenesis 2008;29:2360–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson SJ, Murphy LL, Venema RC, Singletary KW, Young AJ: Curcumin binds tubulin, induces mitotic catastrophe, and impedes normal endothelial cell proliferation. Food Chem Toxicol 2013;60:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson SJ, Venema RC: Quercetin inhibits eNOS, microtubule polymerization, and mitotic progression in bovine aortic endothelial cells. J Nutr 2006;136:1178–1184 [DOI] [PubMed] [Google Scholar]
- 34.Delmas D, Solary E, Latruffe N: Resveratrol, a phytochemical inducer of multiple cell death pathways: Apoptosis, autophagy and mitotic catastrophe. Curr Med Chem 2011;18:1100–1121 [DOI] [PubMed] [Google Scholar]
- 35.Ushida Y, Talalay P: Sulforaphane accelerates acetaldehyde metabolism by inducing aldehyde dehydrogenases: Relevance to ethanol intolerance. Alcohol Alcohol 2013;48:526–534 [DOI] [PubMed] [Google Scholar]
- 36.Jordan MA: Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents 2002;2:1–17 [DOI] [PubMed] [Google Scholar]
- 37.Nallasamy P, Si H, Babu PV, Pan D, Fu Y, Brooke EA, Shah H, Zhen W, Zhu H, Liu D, Li Y, Jia Z: Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J Nutr Biochem 2014;25:824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiou TJ, Wang YT, Tzeng WF: DT-diaphorase protects against menadione-induced oxidative stress. Toxicology 1999;139:103–110 [DOI] [PubMed] [Google Scholar]
- 39.Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E: Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res 2011;64:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY: Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001;21:2895–2900 [PubMed] [Google Scholar]
- 41.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, Kensler TW: Rapid and sustainable detoxification of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prev Res (Phila) 2014;7:813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TY, Thames G, Krak M, Zhang Y, Nel A: Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct 2013;5:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]