Abstract
The objectives of this study were to determine the epidemiology and correlates of cardiovascular disease (CVD) risk among Ugandans on first-line antiretroviral therapy (ART). We conducted a cross-sectional study at an HIV clinic in southwestern Uganda. We enrolled adult patients on non-nucleoside-based ART regimens for a minimum of 2 years. We collected anthropometric and clinical measurements, smoking history, and blood for fasting lipid profile and blood sugar (FBS). Outcomes of interest were (1) presence of metabolic syndrome (at least two of the following: FBS >100 mg/dL, blood pressure of ≥130/85 mmHg, triglycerides ≥150 mg/dL, HDL <40 mg/DL, or waist circumference ≥94 cm in males or ≥80 cm in females); and (2) a Framingham score correlating to >5% 10-year CVD risk. Of the 250 participants enrolled, metabolic syndrome was detected in 145/250 (58%) of participants (62% in females and 50% in males). Forty-three participants (17%) had a Framingham risk correlating to a 5% or greater risk for CVD within 10 years (26% in males and 13% in females). In multivariate analyses, being female (AOR 3.13; 95% CI: 1.0–9.70; p = 0.04) and over 40 years of age (AOR 1.78; 95% CI: 1.00–3.17; p = 0.05) was independently associated with having metabolic syndrome. We found no independent risk factors for a Framingham risk score 10-year risk exceeding 5%, or associations between ART regimen and CVD risk profiles. We conclude that metabolic abnormalities are common among patients on first-line ART in rural Uganda, and appear to be more common in women than men.
Introduction
The expanded delivery of antiretroviral therapy (ART) in sub-Saharan Africa has increased life expectancy for people living with HIV (PLWH) in the region.1–3 Consequently, it is predicted that approximately 10 million people over the age of 50 will be living with HIV in the region by 2030.3,4 As such, an important public health priority is determining the primary causes of morbidity and mortality for this population.
In resource-rich settings, increasing availability of ART to HIV-infected populations has been associated with both a profound decline in overall mortality,5 and a shifting burden of morbidity from AIDS-related opportunistic infections, to chronic conditions of aging.6–10 In particular, multiple studies have demonstrated increased risk of myocardial infarction6,11 and stroke12–14 for PLWH compared to uninfected populations. This trend has also been noted in some resource poor settings.15 However, little data are available describing the primary causes of morbidity for aging people with HIV on ART in sub-Saharan Africa, where over 70% of the population of people living with HIV reside. As such, there is a critical need to assess the presence and extent of cardiovascular disease (CVD) risk in this population to plan the next generation of HIV care in the region.
We sought to determine the CVD risk profile in a population of older age individuals on ART at a public HIV clinic in southwestern Uganda. Our primary outcomes of interest were presence of metabolic syndrome and a Framingham score correlating to >5% 10-year CVD risk. Our overarching aim was to determine the epidemiology and correlates of CVD risk among Ugandans on first line ART.
Materials and Methods
Study site
The Mbarara Hospital Immune Suppression (ISS) is located within the premises of Mbarara Regional Referral Hospital (MRRH) in Southwestern Uganda. The total adult clinic enrolment is approximately 25,000 active patients, with over 9000 patients actively taking ART. Of these, 8858 (95%) are on first-line regimens.
Participants
We conducted a cross-sectional cohort study at the Mbarara Regional Referral Hospital Immune Suppression Syndrome clinic in southwestern Uganda. The study population consisted of adult patients on non-nucleoside-based (first-line) ART regimens for a minimum of 2 years with a self-reported adherence level of greater than 95%. Patients were excluded if they had a history of ART substitutions or were taking corticosteroids. Participants fulfilling the inclusion criteria were consented 5–7 days prior to an upcoming clinic appointment, and requested to fast for a minimum of 8 h prior to their visit.
Study procedures
Participants completed a study questionnaire to collect data on demographics, medication use, and past medical history. We collected serum for fasting lipid profile and fasting blood glucose. We recorded clinical measurements including anthropometric measurements and blood pressure. Blood pressures were measured on the day of enrollment by a single study staff member using an aneroid sphygmomanometer. Whole blood samples were immediately transported to the laboratory where serum was separated and lipid assays were batched and run by an automated enzyme-based chemistry analyzer (HUMASTAR® Random Access clinical chemistry analyzer, HUMAN, Wiesbaden, Germany).
Quality controls done at the beginning of each batched run. Serum LDL levels were measured directly. Fasting blood glucose (FBS) was obtained at the time of enrolment using a Bayer CONTOUR® blood glucose meter (Bayer, Ontario, Canada). Patients found to have a blood sugar above 7 mmol/L were asked to return for a repeat test at the next convenient date. We reviewed chart records to extract data on HIV diagnosis date, clinic enrollment data, ART initiation date, ART regimen history, CD4 count history, and weight history.
Statistical analysis
We first summarized participant characteristics including demographics, medical history, and HIV history, and calculated cohort prevalence of dyslipidemia, anthropomorphic measures, and metabolic syndrome. We defined hypertension in any study participant with a systolic blood pressure of ≥140 mmHg or diastolic BP of ≥90 mmHg.16 Our primary outcomes of interest were (1) presence of the metabolic syndrome (defined as having at least two of the following: a fasting glucose of >100 mg/dL, triglycerides >150 mg/dL, HDL-C <40 mg/dL, and waist circumference >102 cm in men and >88 cm in women17); and (2) a Framingham score correlating to >5% 10-year CVD risk.18
We used the LDL-based CHD risk score to calculate the Framingham risk score.18 We chose a cardiovascular disease risk of greater than 5% to denote moderate to high risk, unlike 10–20% as is the acceptable standard, because of the anticipated lower median age of the study participants. We fit univariable and multivariable logistic regression models for each outcome of interest.
Primary explanatory variables of interest included anti-retroviral therapy regimen, [defined as tenofovir, lamivudine, and efavirenz (TDF/3TC/EFV), zidovudine, lamivudine, and nevirapine (AZT/3TC/NVP), zidovudine, lamivudine, and efavirenz (AZT/3TC/EFV), or tenofovir, lamivudine, and nevirapine (TDF/3TC/NVP)], age, gender, body mass index (BMI), duration of ART use, family history of diabetes or hypertension, and nadir CD4 count. Variables with a p value <0.20 in univariable models were retained in multivariable models. Data analysis was performed with Stata version 11 (StataCorp, College Station, Texas, USA).
Ethics
Study procedures were reviewed and approved by the Mbarara University of Science and Technology and all participants signed informed consent.
Results
We screened a total of 271 patients. Of these, 21 were excluded, resulting in a total of 250 patients included in this analysis. Of the 21 who were excluded, 20 were on oral corticosteroids or oral contraceptive pills and one declined consent. Of the 250 patients enrolled, 68% (169/250) were female and the median age was 36 years [interquartile range (IQR) 30–43, Table 1].
Table 1.
Cohort Characteristics
| Characteristic | Total population (n = 250) |
|---|---|
| Age, median (IQR) | 36 (30–43) |
| Female, n (%) | 169 (68%) |
| Nadir CD4 count, median (IQR) | 224 (144–292) |
| Most recent CD4 count, median (IQR) | 466 (341–624) |
| Duration on ART in years, median (IQR) | 2.6 (2.5–2.7) |
| ART regimen | |
| TDF/3TC/EFV, n (%) | 129 (51.6) |
| AZT/3TC/NVP, n (%) | 68 (27.2) |
| AZT/3TC/EFV, n (%) | 48 (19.2) |
| TDF/3TC/NVP, n (%) | 5 (2.0) |
| Change in weight from ART initiation date in kilograms, mean (SD) | 1.56 (6.9) |
| Evidence of lipodystrophy, n (%) | 19 (7.6) |
3TC, lamivudine; ART, antiretroviral therapy; BMI, Body Mass Index; EFZ, efavirenz; IQR, inter quartile range; NVP, nevirapine; TDF, tenofovir.
The median current CD4 count was 466 cells/μL (IQR 341–624) and the median nadir CD4 count was 224 cells/μL (IQR 144–292). The median duration of ART was 2.6 years (IQR 2.5–2.7). One hundred and twenty nine participants (52%) were taking TDF/3TC/EFV, 68 (27%) were taking AZT/3TC/NVP, and 48(19%) were taking AZT/3TC/EFZ; while the remaining 2% were taking other regimens.
Table 2 shows cohort prevalence for various risk factors for cardiovascular disease. A majority of participants (86%, 214/250) had HDL <40 mg/dL, 12% (29/250) had high low density lipoprotein (LDL) ≥130 mg/dL, and 30% (74/250) triglycerides >150 mg/dL. Only 13 patients (5.2%) were found to be hypertensive, but 35% (85/250) had a BMI of greater than 25. Fifty patients (20%) were either current or past smokers and 17% (42/250) had a family history of diabetes or hypertension.
Table 2.
Cardiovascular Disease Risk Factors Among Patients on ART at an HIV Clinic in Rural Uganda
| Characteristic | Total cohort (n = 250) | Male (n = 81) | Female (n = 169) | p Value |
|---|---|---|---|---|
| Total cholesterol (TC) mg/dL, median (IQR) | 107 (77–139) | 100 (77–126) | 111 (81–142) | 0.18 |
| TC ≥200 mg/dL, n (%) | 16 (6.4) | 6 (7.4) | 10 (5.9) | 0.65 |
| Triglycerides, median (IQR) | 113 (74–157) | 119 (89–170) | 110 (62–154) | 0.09 |
| Triglycerides ≥150 mg/dL, n (%) | 74 (29.6) | 25 (30.9) | 49 (29.0) | 0.76 |
| HDL-cholesterol, median (IQR) | 30 (24–36) | 27 (21–32) | 30 (26–37) | 0.0004 |
| HDL-c ≤40 mg/dL, n (%) | 214 (85.6) | 73 (90.1) | 141 (83.4) | 0.16 |
| LDL-cholesterol, median (IQR) | 86 (68–106) | 82 (65–102) | 90 (69–110) | 0.06 |
| LDL-c ≥130 mg/dL, n (%) | 29 (11.6) | 5 (6.2) | 24 (14.2) | 0.06 |
| Total cholesterol/HDL ratio, median (IQR) | 3.5 (2.6–4.8) | 3.7 (2.7–5.1) | 3.4 (2.6–4.7) | 0.24 |
| TC/HDL-C ≥5, n (%) | 56 (22.4) | 21 (26) | 35 (20.7) | 0.35 |
| Hypertensiona, n (%) | 13 (5.2) | 3 (3.7) | 10 (5.9) | 0.46 |
| Body mass indexb | ||||
| BMI <18 | 13 (5.2) | 6 (7.4) | 7 (4.1) | 0.27 |
| BMI 18–24.9, n (%) | 150 (60.0) | 62 (76.5) | 88 (52.1) | <0.001 |
| BMI 25–29.9, n (%) | 57 (22.8) | 11 (13.6) | 46 (27.2) | 0.02 |
| BMI >30, n (%) | 30 (12.0) | 2 (2.4) | 28 (16.6) | 0.001 |
| Waist to hip ratio, median (IQR) | 0.83 (0.79–0.87) | 0.85 (0.82–0.90) | 0.82 (0.78–0.86) | <0.001 |
| High WHR^, n (%) | 74 (29.6) | 18 (22.2) | 56 (33.1) | 0.07 |
| Any current or former smoking, n (%) | 50 (20.0) | 36 (44.4) | 14 (8.3) | <0.001 |
| Current smoker, n (%) | 8 (3.2) | 5 (6.2) | 3 (1.8) | 0.06 |
| First degree relative with DM or HTN, n (%) | 42 (16.8) | 13 (16) | 29 (17.2) | 0.82 |
| Metabolic syndrome, any 3 of the following: (high WHR, high fasting glucose, HTN, high TG or LDL), n (%) | 145 (58) | 41 (50.6) | 104 (61.5) | 0.10 |
Hypertension defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Waist to hip ratio was categorized into abnormal if the value was >0.94 for males and >0.8 for females.
BMI, Body Mass Index; DM, diabetes mellitus; HDL, high density lipoprotein; HTN, hypertension; LDL, low density lipoprotein; WHR, waist to hip ratio.
Over half of participants (58%, 145/250) had metabolic syndrome. Fifty-one percent (41/81) of the males and 62% (104/169) of females met the definition of metabolic syndrome. In multivariate models, age over 40 (AOR 3.22, 95% CI 1.06–9.82, p = 0.04) and female gender (AOR 1.81, 95% CI 1.02–3.23, p = 0.04; Table 3) were both independently associated with the presence of the metabolic syndrome.
Table 3.
Factors Associated with Presence of Metabolic Syndromea
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | Odds ratio | Confidence interval | p Value | Adjusted odds ratio | Confidence interval | p Value |
| Age | ||||||
| 20–25 | REF | REF | REF | REF | REF | REF |
| 26–40 | 1.32 | 0.48–3.62 | 0.58 | 1.54 | 0.56–4.31 | 0.40 |
| >40 | 2.44 | 0.54–7.09 | 0.10 | 3.22 | 1.06–9.82 | 0.04 |
| Sex, female | 1.56 | 0.91–2.66 | 0.10 | 1.81 | 1.02–3.23 | 0.04 |
| Duration on HAART | 2.08 | 0.58–7.43 | 0.30 | N/A | – | – |
| Nadir CD4 count, | 1.00 | 0.99–1.00 | 0.73 | N/A | – | – |
| Current CD4 count | 1.00 | 1.00–1.00 | 0.81 | |||
| ART regimen | ||||||
| TDF/3TC/EFV | REF | – | – | REF | – | – |
| AZT/3TC/NVP | 1.76 | 0.95–3.25 | 0.07 | 1.67 | 0.89–3.14 | 0.11 |
| AZT/3TC/EFV | 1.00 | 0.51–1.94 | 0.99 | 1.05 | 0.53–2.08 | 0.88 |
| TDF/3TC/NVP | 1.26 | 0.20–7.82 | 0.80 | 1.23 | 0.19–7.96 | 0.83 |
| Family history of diabetes or hypertension | 0.96 | 0.49–1.87 | 0.90 | N/A | ||
| Current or former smoker | 0.82 | 0.44–1.52 | 0.52 | N/A | ||
Metabolic syndrome was defined as having two or more of the following: a fasting glucose of >100 mg/dL, triglycerides >150 mg/dL, HDL-C <40 mg/dL; waist circumference >102 cm in men and >88 cm in women.17
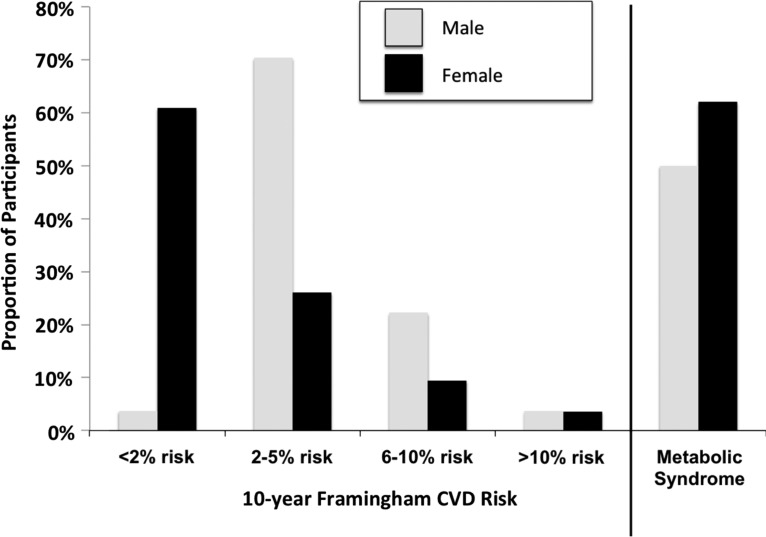
Using the Framingham risk score for assessing 10-year cardiovascular risk,17% (43/250) of participants had a 10-year cardiovascular risk exceeding 5%, with the proportion in males being double that of females (26% versus 13%, p = 0.01) (Fig. 1). In the overall cohort, 4% (9/250) had a 10-year cardiovascular risk exceeding 10% (Table 4). In multivariable regression models, we found no independent risk factors for a Framingham risk score corresponding to a 10-year risk exceeding 5% (Table 5).
FIG. 1.
Ten-year Framingham cardiovascular disease risk and metabolic syndrome among a cohort of HIV individuals on stable antiretroviral therapy in southwestern Uganda.
Table 4.
Ten-Year Cardiovascular Disease Risk Using the Framingham Risk Score
| Framingham risk (10-year CHD risk using LDL score) | <2% | 2–5% | 6–10% | >10% |
|---|---|---|---|---|
| Total population (n = 250), n (%) | 106 (42.4) | 101 (40.4) | 34 (13.6) | 9 (3.6) |
| Men (n = 81), n (%) | 3 (3.7) | 57 (70.4) | 18 (22.2) | 3 (3.7) |
| Women (n = 169), n (%) | 103 (61.0) | 44 (26.0) | 16 (9.5) | 6 (3.6) |
CVD, cardiovascular disease.
Table 5.
Factors Associated with 10-Year Framingham Risk Score Corresponding to Greater than 5% Risk of Cardiovascular Event
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | Odds ratio | Confidence interval | p Value | Adjusted odds ratio | Confidence interval | p Value |
| Sex, female | 0.57 | 0.25–1.33 | 0.20 | 0.56 | 0.24–1.33 | 0.19 |
| Duration on HAART | 0.29 | 0.04–1.91 | 0.20 | 0.23 | 0.03–1.77 | 0.13 |
| Nadir CD4 count, | 1.00 | 0.99–1.00 | 0.87 | |||
| Current CD4 count | 1.00 | 1.00–1.00 | 0.34 | |||
| ART regimen | ||||||
| TDF/3TC/EFV | REF | – | – | REF | – | – |
| AZT/3TC/NVP | 1.25 | 0.51–3.06 | 0.62 | 1.71 | 0.65–4.53 | 0.28 |
| AZT/3TC/EFV | 0.36 | 0.08–1.63 | 0.18 | 0.46 | 0.10–2.18 | 0.32 |
| TDF/3TC/NVP | 0.00 | N/A | N/A | |||
| Weight change | 0.98 | 0.92–1.04 | 0.60 | |||
| Family history of diabetes or hypertension | 0.65 | 0.18–2.28 | 0.50 | |||
Discussion
In a cohort of relatively young people with HIV infection stably on ART from a largely rural setting in southwestern Uganda, over half of study participants had evidence of the metabolic syndrome, and nearly 1 in 5 had a 10-year CVD risk of >5%. Rates of metabolic syndrome were higher in females, whereas males more commonly had elevated cardiovascular disease risk scores. Given the relative paucity of health systems infrastructure targeted to cardiovascular disease prevention and treatment in the region,19 there is an important need to expand the focus of HIV care in the region to non-AIDS defining conditions.
Our work is consistent with preliminary data from other resource poor settings. A recent study investigating the prevalence of metabolic syndrome among ART naïve and ART-experienced HIV infected Thai adults found an overall prevalence of metabolic syndrome of 22.2%. Among the ART-experienced, a prevalence of 24.9% was detected. As in our study, female gender was cited among the risk factors independently associated with metabolic syndrome.15
Within the sub-Saharan African region, a recent study investigating the prevalence of hypertension and Framingham risk score stratification in a large HIV-positive cohort in Uganda found that 20% of men were at least 10% or more long-term risk of acute cardiovascular disease.20 Similar to our study, the risk appeared to be gender dependent with nearly all women in the 10% or less 10-year Framingham risk score category.
The differences in CVD risk between that study and the current one could be attributed to social or environmental factors. For example, our study population was derived from the semi-rural environment in the Ankole region of southwestern Uganda. This differs from the prior study in Uganda, whose participants were from in and around Kampala, the urban capital city of the country. Further work will be needed to further clarify why CVD risk might differ in these sub-populations.
Another study investigating the incidence of metabolic disorders and cardiovascular risk in treatment-naive HIV-infected patients of sub-Saharan origin found a relatively high cumulative incidence of both metabolic syndrome and insulin resistance, at 14.4% and 19.2%, respectively.21 Similar to our setting, being female was significantly associated with developing metabolic syndrome. Unlike our study, they found relatively low rates of CVD risk using the Framingham risk calculator. Importantly, that study was conducted among Africans, but in France, raising the possibility of an environmental interaction with HIV and CVD risk.
Our study also found significantly higher rates of obesity among women than men, including a nearly 8-fold increase prevalence of morbid obesity (16% vs. 2%). Indeed, the relatively high rate of metabolic syndrome we found in women versus men appears to be at least partially driven by anthropomorphic measurements. This finding has been demonstrated elsewhere in sub-Saharan Africa. For example, a study investigating obesity in treated female HIV patients from sub-Saharan Africa, found an overall prevalence of 28%. Women of African origin were most affected, 49% being obese, with a further 32% overweight.22
Another study conducted in South Africa found that the prevalence of obesity in women was 6.5 times higher than in men.23 But associations between HIV infection, ART, and obesity appear to be region specific, because gender-based differences in obesity have not been reported to the same extent in other locations.24 A study from the United States documented greater increases in weight both in women and in those of African American descent.25 Future research priorities include identification of appropriate thresholds for overweight and obesity in the sub-Saharan population that predict morbidity,26 and discerning the impact of such high rates of obesity on the long-term health of this population.
While it is well established that higher CVD profiles also correlate with higher risk of stroke and myocardial infarction among PLWH in the United States and Europe,11,14,27–30 whether this same relationship exists in Saharan Africa remains to be elucidated. Much of the data on CVD outcomes in the region are limited to cross-sectional studies and are focused on hypertension prevalence,31,32 with studies from South African being the primary exception.
A prospective analysis characterizing stroke in black South African HIV-positive patients found that people with HIV suffered strokes at relatively young ages (median 32 years old).29 Another study of chronic co-morbidities among individuals aged 50 years and older in South Africa found over twice the prevalence of angina among PLWH compared to HIV-uninfected controls (13.7% vs. 5.8%).33 In contrast, a recent study comparing carotid intima media thickness measurements between PLWH and HIV uninfected controls found no difference between groups, although the uninfected population was slightly older and a higher proportion were male.34
Nevertheless, another recent study noted strong associations between persistent immune activation after ART and carotid atherosclerosis among HIV-infected persons in Uganda, suggesting a role of HIV-associated inflammation in increasing CVD risk in this population.35 As outcome data begin to accrue, important questions for the field include whether the increased risk of CVD seen in relatively young people with HIV in sub-Saharan Africa will translate into CVD morbidity and mortality, and whether current risk scores accurately predict risk in this population.36
A number of limitations to this work should be considered. First, the study was cross sectional in design, limiting our ability to identify predictors and causative mechanisms of cardiovascular disease risk in the study population. Second, the Framingham risk scores calculated in our study were developed and validated on western populations. HIV infection,37 as well as other regional variations in risk,38 are likely to cause divergence from the Framingham estimator. For example, our study population was of a lower age than the age distribution in the populations for whom conventional CVD risk prediction models were developed.37 We expect that we are likely to be under-estimating CVD risk, given the known increased risk attributable to HIV infection that can be under-estimated by current risk profiles.39
Third, we were unable to measure duration of HIV infection or its impact on CVD risk because it was unknown in most study participants. To account for this limitation, we used CD4 nadir as a surrogate for HIV disease duration and severity. Fourth, we were unable to measure associations between specific ART medicines and our CVD outcomes, because the majority of patients were on one of two regimens (TDF/3TC/EFV or AZT/3TC/NVP), preventing us from comparing subclasses of drugs. Lastly, we did not have an HIV-uninfected group in our study, which limits our ability to assess the relative contribution of HIV infection to CVD risk.
In summary, we found a high prevalence of metabolic abnormalities among people living with HIV on first-line ART in rural, southwestern Uganda. Our data add to a growing body of information supporting an epidemiologic shift among people with HIV infection with access to ART, even in low-income and remote settings.3 Our finding of higher rates of metabolic syndrome in females is keeping with other data from the region and suggests the potential for a different CVD risk profile in this population. Additional data are needed to further identify the contribution of HIV infection to CVD risk and the impact of cardiovascular disease on morbidity and mortality in this population. The use of statins has been shown to reduce cardiovascular risk potentially in HIV-infected patients elsewhere.40–42 Further studies are required to assess the feasibility of this and other scalable interventions that may decrease morbidity and mortality for this population.
Acknowledgments
The source of funding for the study was the Joint Clinical Research Center in Kampala Uganda. MJS receives research funding from the NIH (K23MH 099916) and the Harvard Center for AIDS Research.
Author Disclosure Statement
All authors declare no financial conflicts of interest.
References
- 1.Calza L, Manfredi R, Chiodo F. Hyperlipidaemia in patients with HIV-1 infection receiving highly active antiretroviral therapy: Epidemiology, pathogenesis, clinical course and management. Intl J Antimicrob Agents 2003;22:89–99 [DOI] [PubMed] [Google Scholar]
- 2.Quinn TC. HIV epidemiology and the effects of antiviral therapy on long-term consequences. AIDS (London, England) 2008;22:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills EJ, Barnighausen T, Negin J. HIV and aging—Preparing for the challenges ahead. N Engl J Med 2012;366:1270–1273 [DOI] [PubMed] [Google Scholar]
- 4.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. JAIDS 2006;41:194–200 [DOI] [PubMed] [Google Scholar]
- 6.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. New Engl J Med 2007;356:1723–1735 [DOI] [PubMed] [Google Scholar]
- 7.El-Sadr W, Lundgren JD, Neaton J, et al. CD4+ count-guided interruption of antiretroviral treatment. New Engl J Med 2006;355:2283–2296 [DOI] [PubMed] [Google Scholar]
- 8.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Int Med 2014;160:458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: The latest epidemic. AIDS Patient Care STDs 2008;22:925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crum-Cianflone NF, Weekes J, Bavaro M. Review: Thromboses among HIV-infected patients during the highly active antiretroviral therapy era. AIDS Patient Care STDs 2008;22:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: Evidence and implications. Therapeut Adv Chronic Dis 2013;4:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke. AIDS 2014;28:1911–1919 [DOI] [PubMed] [Google Scholar]
- 14.Chow F. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. JAIDS 2012;60:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jantarapakde J, Phanuphak N, Chaturawit C, et al. Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDs 2014;28:331–340 [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on Detection, Evaluation, and Treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation 2004;109:433–438 [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 19.Peck R, Mghamba J, Vanobberghen F, et al. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: A cross-sectional survey. Lancet Global Health 2014;2:e285–e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mateen FJ, Kanters S, Kalyesubula R, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertension 2013;31:1372–1378 [DOI] [PubMed] [Google Scholar]
- 21.Eholié SP, Lacombe K, Krain A, et al. Metabolic disorders and cardiovascular risk in treatment-naïve HIV-infected patients of Sub-Saharan origin starting antiretrovirals: Impact of westernized lifestyle. AIDS Res Human Retroviruses 2015;31:384–392 [DOI] [PubMed] [Google Scholar]
- 22.McCormick CL, Francis AM, Iliffe K, et al. Increasing obesity in treated female HIV patients from Sub-Saharan Africa: Potential causes and possible targets for intervention. Frontiers Immunol 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malaza A, Mossong J, Bärnighausen T, Newell M-L. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PloS One 2012;7:e47761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alencastro PR, Fuchs SC, Wolff FH, Ikeda ML, Brandão AB, Barcellos NT. Independent predictors of metabolic syndrome in HIV-infected patients. AIDS Patient Care STDs 2011;25:627–634 [DOI] [PubMed] [Google Scholar]
- 25.Lakey W, Yang L-Y, Yancy W, Chow S-C, Hicks C. Short communication: From wasting to obesity: Initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Human Retroviruses 2013;29:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowther NJ, Norris SA. The current waist circumference cut point used for the diagnosis of metabolic syndrome in sub-Saharan African women is not appropriate. PloS One 2012;7:48883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Int Med 2013;173:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Althoff KN, McGinnis KA, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015;60:627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mochan A, Modi M, Modi G. Stroke in Black South African HIV-Positive patients: A prospective analysis. Stroke 2003;34:10–15 [DOI] [PubMed] [Google Scholar]
- 30.Triant VA. Epidemiology of coronary heart disease in HIV patients. Rev Cardiovasc Med 2014;15:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okello S, Kanyesigye M, Muyindike WR, et al. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in south-western Uganda. J Hypertens 2015;33:2039–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peck RN, Shedafa R, Kalluvya S, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: A cross-sectional study. BMC Med 2014;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotwani P, Kwarisiima D, Clark TD, et al. Epidemiology and awareness of hypertension in a rural Ugandan community: A cross-sectional study. BMC Pub Health 2013;13:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fourie C, Schutte A, Smith W, Kruger A, van Rooyen J. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis 2015;240:154–160 [DOI] [PubMed] [Google Scholar]
- 35.Siedner MJ, Kim J-H, Nakku RS, et al. Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis 2015:jiv450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knobel H, Jericó C, Montero M, et al. Global cardiovascular risk in patients with HIV infection: Concordance and differences in estimates according to three risk equations (Framingham, SCORE, and PROCAM). AIDS Patient Care STDs 2007;21:452–457 [DOI] [PubMed] [Google Scholar]
- 37.Friis-Møller N, Thiébaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehab 2010;17:491–501 [DOI] [PubMed] [Google Scholar]
- 38.Hajifathalian K, Ueda P, Lu Y, et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): A pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol 2015;3:339–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosepele M, Regan S, Meigs JB, Grinspoon S, Triant VA. Application of new ACC/AHA cholesterol guidelines to an HIV clinical care cohort. Conference on Retorviruses and Opportunistic Infections, February23–26, 2015 Seattle, WA, USA 2015 [Google Scholar]
- 40.Krsak M, Kent DM, Terrin N, Holcroft C, Skinner SC, Wanke C. Myocardial infarction, stroke, and mortality in cART-treated HIV patients on statins. AIDS Patient Care STDs 2015;29:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52–e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitka M. Exploring statins to decrease HIV-related heart disease risk. JAMA 2015;314:657–659 [DOI] [PubMed] [Google Scholar]