Abstract
Extracellular electron transfer in microorganisms has been applied for bioelectrochemical synthesis utilizing microbes to catalyze anodic and/or cathodic biochemical reactions. Anodic reactions (electron transfer from microbe to anode) are used for current production and cathodic reactions (electron transfer from cathode to microbe) have recently been applied for current consumption for valuable biochemical production. The extensively studied exoelectrogenic bacteria Shewanella and Geobacter showed that both directions for electron transfer would be possible. It was proposed that gram-positive bacteria, in the absence of cytochrome C, would accept electrons using a cascade of membrane-bound complexes such as membrane-bound Fe-S proteins, oxidoreductase, and periplasmic enzymes. Modification of the cathode with the addition of positive charged species such as chitosan or with an increase of the interfacial area using a porous three-dimensional scaffold electrode led to increased current consumption. The extracellular electron transfer from the cathode to the microbe could catalyze various bioelectrochemical reductions. Electrofermentation used electrons from the cathode as reducing power to produce more reduced compounds such as alcohols than acids, shifting the metabolic pathway. Electrofuel could be generated through artificial photosynthesis using electrical energy instead of solar energy in the process of carbon fixation.
Keywords: Bioelectrochemical synthesis, Extracellular electron transfer, Cathodic electron, Electrofuel
Background
An eventual replacement of fossil energy source with sustainable energy system is unavoidable. Biofuels have emerged as one of the sustainable fuels sources and it is considered as alternatives to petroleum. Biomass captured the energy from sunlight and stored it as high-energy chemical bonds, which is used for biofuels. More recently, electrofuels have been studied for liquid fuels as a means for intermittent electricity storage [1] using the energy of low-potential electrons such as hydrogen gas, reduced metal, or electricity [2]. It usually uses the interaction between microbes and electrode, through extracellular electron transfer.
Bioelectrochemical synthesis (BES) uses extracellular electron transfer of microorganisms catalyzing anodic and/or cathodic reactions. BES has two categories according to the direction of electron flow, microbial fuel cells (MFC, electricity production), and microbial electrosynthesis (MES, electricity consumption). A microbial fuel cell uses extracellular electron transfer to an electrode originating from organic compounds consumed by microorganisms. Microbial electrosynthesis uses electron transfer from an electrode to microorganisms producing reduced biochemical compounds. An electrode is thus used as an electron acceptor (MFC) or an electron donor (MES).
Extracellular electron transfer has been gaining wide interest in relation to microbial electrochemical synthesis [1, 3], interspecies electron transfer [4, 5], and microbial immobilization of heavy metals for bioremediation [6, 7] (Table 1). In particular, biofuels or biochemicals are reduced compounds and the reducing power is needed in microbial fermentation processes [8, 9]. An external supply of electrons using electricity enhances the reducing process in microbial metabolism. Direct electron transfer is ideal in extracellular electron transfer from a cathode to microbes.
Table 1.
The application of bioelectrochemical reduction
| Application | Product | Reaction conditions | Key outcomes | Ref. |
|---|---|---|---|---|
| Direct reduction | Cr6+ → Cr3+ | G. sulfurreducens, −600 mV vs. Ag/AgCl | U(VI) was removed and recovered using poised electrode | [19] |
| Shewanella oneidensis MR-1, −500 mV vs. Ag/AgCl | Lactate and the electrode as the electron donors for Cr(VI) reduction | [18] | ||
| Fumarate → succinate | G. sulfurreducens, −500 mV vs. Ag/AgCl | Fumarate reduction dependent on current supply | [48] | |
| Shewanella species in biocathode of microbial fuel cell | Similar comparison under chromate reducing condition | [102] | ||
| Nitrate reduction | Nitrifying and denitrifying microorganisms at +197 mV vs. SHE | Simultaneous occurrence of nitrification and denitrification at a biocathode | [49] | |
| Denitrifying microorganisms at −123 mV vs. SHE | Long-term stability, carbon-free operation | [51] | ||
| Indirect reduction | Caproate and caprylate production from acetate | Acetate fed at −0.9 V vs. NHE | In situ-produced hydrogen as electron donor, low concentration and reaction rates | [90] |
| Ethanol production from acetate | −550 mV vs. NHE, artificial mediator tested | Methyl viologen increased ethanol production but inhibited butyrate and methane formation, still hydrogen was coproduced at the cathode | [81] | |
| Alcohol formation from glycerol | Open circuit operation | Changes in microbial community and product outcomes after current supply | [87] | |
| Reduction of acetate and butyrate to mainly alcohols and acetone | −820 mV vs. Ag/AgCl | Halotolerant mixed sulfate-reducing bacteria culture | [92] | |
| Polyhydroxyalkanoates (PHA) from glucose | 512 mV, the biocathode coupled to a bioanode in an MEC | Microaerophilic microenvironment at cathode enhanced PHA synthesis as alternative pathway to re-oxidize the NADH | [94] | |
| Butyraldehyde to butanol | Immobilized alcohol dehydrogenase at −400 mV vs. Ag/AgCl | Reduction to alcohol by current without supplement of NADH | [88] | |
| Hydrogen production | −700 mV vs. Ag/AgCl | Increased cathodic hydrogen efficiency on microbial biocathode based on a naturally selected mixed culture | [103] | |
| 500 mV, the biocathode coupled to a bioanode in an MEC | Operated for a long period with high current density but phosphate precipitation on the biocathode | [104] | ||
| −700 mV vs. SHE | Desulfovibrio sp. as a dominant microorganism in the biocathode | [22] | ||
| Methane production | −700 mV vs. Ag/AgCl | Methane production directly from current | [53] | |
| −550 mV vs. NHE | CO2 reduction to CH4, need to reduce the internal resistance | [105] | ||
| Improved 1,3-propandiol production from glycerol | −900 mV vs. SHE | Electrical current as the driving force for a mixed population fermenting glycerol in the cathode | [93] | |
| Improved butanol production from glucose | +0.045 V vs. SHE | Increased alcohol production in electrofermentation with increased a ratio NADH/NAD+ | [24] | |
| Electrofuel from CO2 and electricity | Butyrate | −800 mV vs. SHE | Production of organic compounds from CO2 by hydrogen driven by a cathode | [100] |
| Acetate | −590 mV vs. SHE | Higher acetate production than on unmodified graphite | [99] | |
| Acetate, 2-oxobutyrate | −400 mV vs. SHE | The production of organic acids by current consumption | [106] |
The two mostly extensively studied microorganisms for extracellular electron transfer are Geobacter and Shewanella species. Geobacter and Shewanella are metal-reducing and gram-negative bacteria. Extracellular electron transfer in microorganisms is used in the metal reduction process by the microorganism and, in this case, the metal is used as an electron acceptor. When metal (hydr)oxides that are poorly soluble in water are present as electron acceptors, extracellular electron transfer occurs using multihaem c-type cytochromes in Geobacter and Shewanella [10]. Based on this phenomenon, the microorganisms are able to extracellularly transfer electrons and this can be applied for BES.
The mode of extracellular electron transfer is broadly divided into the following: (1) direct electron transfer: nanowire [11] or direct contact [12]; (2) mediators-shuttled: endogenous, exogenous as a redox compound or a by-product [13–15]; and (3) extracellular polymeric substances (EPS) of biofilms [16] (Fig. 1).
Fig. 1.
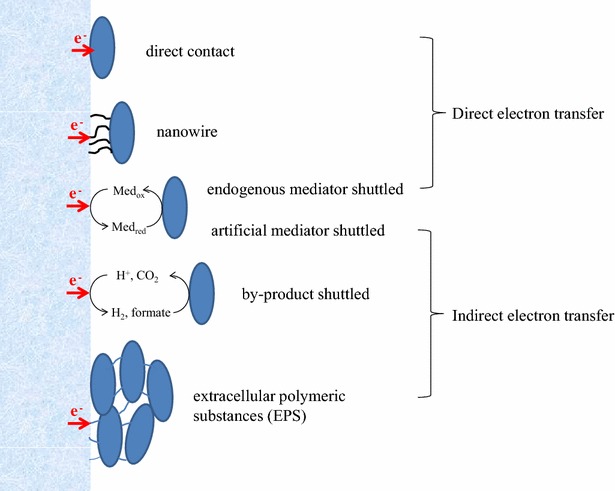
Cathodic electron transfer mode. Electrons from a cathode flow into a microbe directly, through direct contact, nanowire, and endogenous mediator; or indirectly, through an artificial mediator, by-product, or EPS
Electron transfer from a cathode to microbes
Multihame c-type cytochrome is a key component of the electron transfer channel in gram-negative bacteria [10]. Filamentous conductive pili are also involved in electron transfer in Shewanella [17] and Geobacter [11]. BES uses two directions, i.e., microbe → electrode (anode) in MFC and electrode (cathode) → microbe in MES, with the same or different mode. Electrons flow from an electron donor with a relatively lower redox potential to an electron acceptor with higher redox potential. In this light, in the present study we address the question that of whether it is possible to use the same electron transport chain for the opposite direction.
The redox tower in Fig. 2 shows the broad range of redox potential for MtrC (located on an extracellular site of the outer membrane), MrtA (a periplasmic c-type cytochrome), CymA (a link point between the inner membrane and the periplasm), and OmcA (anchored in the inner membrane), which were reported to play roles in electron transfer. It is proposed that reversible electron transfer within cytochrome c complex channels is feasible and the same electron transport chain can be used for the opposite direction.
Fig. 2.
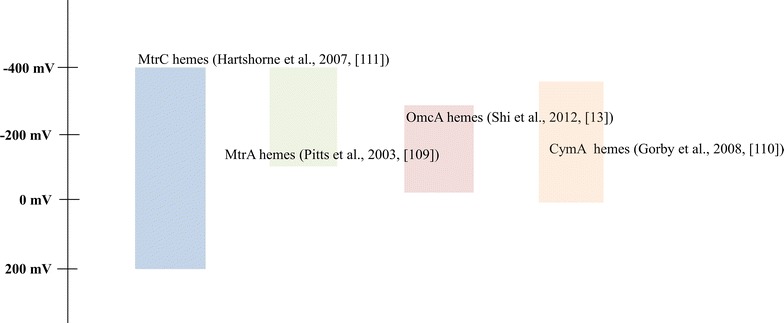
The broad range of redox potential in c-type cytochromes. Considering the possibility of bidirectional electron flow (cathodic, anodic), the broad redox potential suggests the direction of electron flow would be flexible in an electron transfer channel consisting of cytochrome C. The number in a square bracket represents the citation number in the reference list
Extensively studied in MFC as iron-reducing bacteria, Shewanella oneidensis MR-1 [18] and Geobacter spp. [19] were reported to reduce the highly toxic hexavalent chromium (Cr(VI)) using a cathode. This indicates that both directions for electron transfer would be possible in current-producing bacteria, i.e., microbe → anode and cathode → microbe. However, it was reported that Shewanella showed a reversed Mtr pathway [20] but Geobacter used a different mode in the opposite direction [21].
Direct electron transfer from a cathode to microbes has been observed in a biocathode for microbial communities including betaproteobacteria [22, 23] and firmicutes [22], in addition to Shewanella and Geobacter. The presence of other electroactive, electron endergonic strains thus should be possible. Clostridium pasteurianum increased butanol production using cathode electron transfer without any mediator [24]. Nevertheless, the precise electron transfer channel for acceptance of extracellular electrons has not been verified; the redox enzyme in the membrane, however, may be involved in electrochemical reduction. Ferredoxin extracted from C. pasteurianum previously showed direct electrochemical reduction [25], but there is still no evidence of direct electron transfer through ferredoxin in whole cells. Also, several other redox proteins could be candidates for extracellular electron transfer.
Predicted electron transfer proteins involved in extracellular electron transfer
For direct electron transfer, a membrane-bound redox protein is needed. However, there has been no study of redox proteins involved in direct electron transfer except periplasmic c-type cytochrome. Several studies have reported the possibility of direct electron transfer by microorganisms in the absence of c-type cytochrome, and here we present some possible redox proteins involved in electron transfer channels including cytochromes (Table 2).
Table 2.
Predicted electron transfer proteins associated in extracellular electron transfer
| Predicted electron transfer proteins | Active sites | Candidate microorganism associated in extracellular electron transfer | Microorganism used in bioelectrochemical system |
|---|---|---|---|
| Cytochrome C | Heme protein | Metal-reducing bacteria (Geobacter, Shewanella) | G. sulfurreducens [19, 48], Shewanella oneidensis [18] |
| Ferredoxin | Fe-S protein | Clostridia, acetogens, methanogens | Clostridium pasteurianum [24], Clostridium ljungdahlii, Clostridium aceticum, Moorella thermoacetica [106], Sporomusa ovata [106, 107], Methanothermobacter thermautotrophicus [108], Methanobacterium palustre, Methanococcus maripaludis [53, 54] |
| Rubredoxin | Fe-S protein without acid–labile sulfur | Sulfate-reducing bacteria | Desulfovibrio sp. [22, 70] |
| Hydrogenase | [NiFe] or [FeFe] or [Fe]-only | Sulfate-reducing bacteria, methanogen | Desulfovibrio sp. [22, 70], methanogens [52, 53] |
| Formate dehydrogenase | Molybdenum or tungsten | Sulfate-reducing bacteria | Desulfovibrio sp. [22, 70] |
Cytochrome
The heme in cytochrome participates in electron transfer processes. Cell surface-localized cytochromes (OmcE and OmcS in Geobacter sulfurreducens, MtrC and OmcA in Shewanella oneidensis MR-1) are important components for electron transfer [26]. A microarray analysis of G. sulfurreducens gene transcript abundance showed the c-type cytochrome was highly expressed in current-producing biofilms [21]. However, it was suggested that the mechanism of two opposite directions, i.e., microbe → electrode and electrode → microbe, would be significantly different in G. sulfurreducens. Recently, cytochrome PccH with a unusually low redox potential for cytochrome (−24 mV at pH 7) located in the periplasm was proposed as a candidate to provide electron transfer in G. sulfurreducens, even though PccH could not be involved in the first step of accepting electrons [27, 28]. It is meanwhile known that S. oneidensis has a similar mechanism in both directions mainly using flavins (flavin mononucleotide and riboflavin) with cytochrome C [29].
Ferredoxin: membrane-bound complex
Rnf complexes (a membrane-bound NADH:ferredoxin oxidoreductase) are redox-driven ion pumps and have a membrane-bound, proton-translocating ferredoxin: NAD+ oxidoreductase contributing to ATP synthesis (energy conservation) in acetogens such as Clostridium ljungdahlii. RnF is a multifunctional device with nitrogen fixation, proton translocation, and electron transport capabilities [30]. It is four flavin-containing cytoplasmatic multienzyme complexes from clostridia, acetogens, and methanogens [31] and so involved in flavin-based electron bifurcation (FBEB), which is regarded as a third mode of energy conservation in addition to substrate-level phosphorylation (SLP) and electron transport phosphorylation (ETP) [32]. Not all acetogens have rnf genes.
An energy-conserving hydrogenase (Ech) also plays a role in reducing ferredoxin with proton motive force [33]. It involves a coupling mechanism: an exergonic process attributes to coupled endergonic process; ferredoxin reduction with low potential as an exergonic reaction is coupled with H2 or NADH, a high-potential acceptor as endergonic reduction [34]. In methanogens, in the absence of cytochromes, methyltransferase is involved in a exergonic reaction to drive the extrusion of ions (Na+ or H+) across the membrane [35]. In the context energy conservation in a bioelectrochemical system, electron supplementation from cathode would lead to FBEB. Electron bifurcating ferredoxin reduction H+ gradient (for C. ljungdahlii) or Na+ (for Acetobacterium woodii) via membrane-bound Rnf complex was supposed as key components in electron transport chain [36].
Rubredoxin
Rubredoxin (Rub) is also an electron transfer protein having a Fe-S cluster with relatively small molecules (about 55 amino acids) [37, 38]. It is one of the electron transfer components of sulfate-reducing bacteria (SRB) [39] and is also detected in Clostridium pasteurianum [37]. In Desulfovibrio vulgaris, Rub reduces hydrogen peroxide and superoxide [40]. Rub showed an electrochemical response with electrodes [41, 42]. Detailed roles of Rub in microorganisms have not been found but it is expected to be involved in an electron transfer channel.
Hydrogenase and formate dehydrogenase
It was recently reported that a hydrogenase and formate dehydrogenase, which are released from cells, are adsorbed onto electrodes to accept electrons in biocorrosion and bioelectrosynthesis [43]. Methyl viologen-mediated electron transfer to hydrogenase from cathodes and mediatorless H2 production using cathodic electron transfer were previously suggested as electron transfer modes [44]. Formate dehydrogenase also showed direct electron transfer from cathodes [43, 45]. The periplasmic formate dehydrogenase transfers electrons to cytochrome C in D. desulfuricans [46]. The combination of periplasmic enzyme and c-type cytochrome likely provides the electrical wiring [44]. Several membrane-bound enzymes such as fumarate reductase [47, 48] and a denitrification enzyme [49–51] led to bioelectrochemical reduction. Therefore, a periplasmic enzyme could be involved in an electron transfer channel in bioelectrochemical systems.
Electroactive microorganisms
Methanogens and acetogens
The conversion of CO2 to CH4 was reported in a biocathode consisting of a methanogen via direct or indirect (H2 mediator) channels [52–54]. The electron donor for methanogenesis is H2 for autotrophic methanogens or acetate for acetoclastic methanogens. It is supposed that, as in metal-reducing bacteria, the specific electron transfer channel in methanogens plays a role in extracellular electron transfer. Abiotically produced hydrogen is also used by methanogens in indirect electromethanogenesis, instead of direct cathodic electrons [55]. While no electron transfer channel involved in electron transfer from a cathode in methanogens has been identified, energy conservation by bifurcated electron transfer in methanogens could still potentially be found [56].
The study of enzyme purification and protein identification using mass spectroscopy in an acetotrophic methanogen, Methanosarcina acetivorans, showed that ferredoxin reduced membrane-associated multi-heme cytochrome c in Rnf [57, 58]. Methanogens have membrane-associated hydrogenases using ferredoxin or methanophenazine as redox partners [59]. It was reported that hydrogenase and formate dehydrogenase released out of cells mediate electron transfer between a cathode and Methanococcus maripaludis [43]. Also, interspecies electron transfer was shown through flagellum-like appendages between Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus in the form of aggregates [5].
Several acetogenic bacteria (acetate production from CO2 and H2) including Sporomusa ovata, Sporomusa silvacetica, Sporomusa sphaeroides,Clostridium ljungdahlii, Clostridium aceticum, and Moorella thermoacetica consumed electrons from a cathode to reduce CO2 to acetate [60]. Recently, an acetogen closely related with Sporomusa sphaeroides was isolated and showed acetogenic growth using Fe(O) as a sole electron donor [61]. The acetogens Moorella thermoacetica and C. formicoaceticum reduced CO2 to formate, consuming electricity at the cathode compartment [62]. Although the mode of electron transfer to an electroactive acetogen from a cathode is still not known, the membrane-bound cytochromes and cobalt-containing corrinoids were suggested as candidates for an electron transfer channels [63]. Also, cytochrome-b enzymes (membrane-integral b-type cytochromes, −0.215 V vs. SHE) were suggested to be involved in the electron transfer process of acetogens [64].
Metal-oxidizing bacteria and sulfur-utilizing bacteria
The ability of iron-reducing bacteria to give electrons anodes gave rise to the hypothesis that iron-oxidizing bacteria (FeOB) could accept electrons from cathodes in two FeOB, Mariprofundus ferrooxydans and Rhodopseudomonas palustris, in recent studies [65–67] (Fig. 3). The marine isolate Mariprofundus ferrooxydans PV-1 used a cathode as a sole electron donor, generated ATP, and fixed CO2 [67]. Rhodopseudomonas palustris TIE-1 accepted electrons from a cathode, independent of photosynthesis. The dark current indicated extracellular electron uptake uncoupled from the cyclic photosynthetic apparatus and the pioABC operon influenced electron uptake [65]. Rhodopseudomonas palustris TIE-1 increased electron uptake rate 56-fold with unlimited Fe(II) supplementation in a photobioelectrochemical system [67].
Fig. 3.
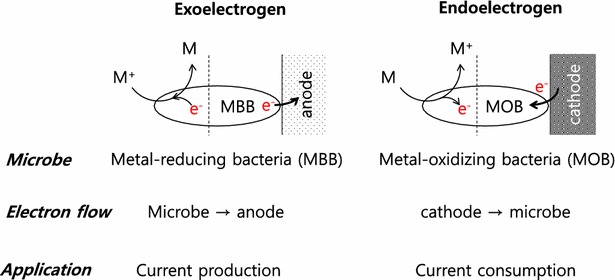
Analogy between metal-utilizing bacteria and direct extracellular electron transfer in a bioelectrochemical system. The left side of the dotted line shows the electron flow with metals in metal-utilizing bacteria and the right side indicates interaction with the electrode
It was reported that isolated marine corrosive delta proteobacterial SRB used elemental iron as the sole electron donor and reduced sulfate, showing the possibility of extracellular electron transfer [68]. Recently, a sulfide-oxidizing bacteria, Desulfobulbaceae, was reported to reduce oxygen in the upper layers of marine sediments using centimeter-long filaments [69]. For removal of H2S, the product of sulfate reduction and a toxic gas to oxygen-consuming organisms, sulfide-oxidizing bacteria used oxygen as an electron acceptor using filaments as electrical cables for H2S oxidation to S [69].
The SRB reduced acetate, butyrate to ethanol, butanol, respectively, using electrons through direct electron transfer from a cathode [70]. It was suggested that the direct electron transfer could take place via a redox enzyme such as cytochrome-b in SRB [70]. The SRB Desulfopila corrodens strain IS4 showed direct electron transfer affecting iron corrosion. Electrochemical and infrared spectroelectrochemical analyses indicated c-type cytochromes were involved in electron transfer [71]. Acidithiobacillus ferrooxidans, Desulfosporosinus orientis,Thiobacillus denitrificans, Sulfurimonas denitrificans, and Desulfovibrio piger also showed electroactivity to accept electrons from a cathode in pure cultures [72].
Cathode modification for enhanced performance of bioelectrochemical reduction
Efforts to improve the efficiency of electron transfer between a cathode and microorganisms have focused on increasing of the interfacial area and interfacial interactions. Nanoparticle attachment on a cathode was attempted with nano-nickel [73], carbon nanotubes [74, 75], conjugated oligoelectrolytes (COEs) [76], and carbon nanotubes on reticulated vitreous carbon (NanoWeb-RVC) [74, 77]. Also, a graphene-modified biocathode enhanced bioelectrochemical production of hydrogen in a MES system [78].
Another attempt involved positively charged surface modification. Extracellular electron transfer from a cathode to a microbe was increased using a positively charged functional group on the surface of a cathode [74]. Negatively charged S. ovate preferred to attach on a cathode and enhanced acceptance of electrons from the cathode for the reduction of CO2 to acetate [74]. The positively charged anode led to an enriched biofilm on an anode but the negatively charged cathode has a repulsive interaction with microorganisms because the cell walls of most bacteria have an overall net negative charge. Therefore, attachment with microorganisms on a cathode has a charge barrier and one study showed that both the zeta potential and the hydrophobicity of cells increased in a current-consuming biofilm [24]. Modification should be tried according to the changes of cell surface characteristics on a cathode, in contrast with on an anode.
Application for valuable biofuel production
A study of the life cycle assessment (LCA) showed MFCs do not give environmental benefit relative to the conventional anaerobic treatment [79]. The development of the MEC system connected with valuable product formation was suggested for positive energy gain [79, 80]. Thus, the product developments using bioelectrochemical reaction between microbe-cathode are promising research directions.
Metabolic shift to reduced compound production (electrofermentation)
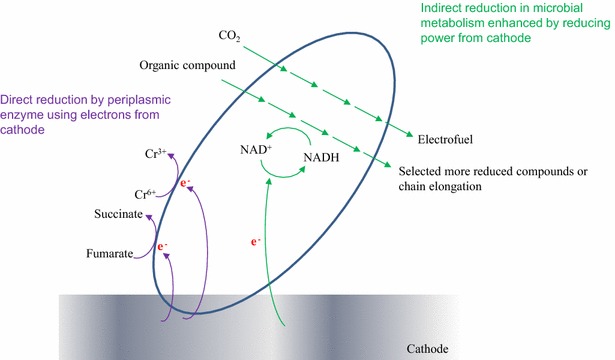
Electron transfer via an artificial mediator from a cathode has been applied in several studies and it showed an increase of reduced compound production [15, 81–85]. The distribution of final products would be determined by the electron and carbon flow in the fermentation process. Therefore, it is important to control the electron/carbon flow accordingly for production of the targeted bioproduct. Recently, an increase of butanol production in C. pasteurianum in a bioelectrochemical system showed the reducing power from a cathode could shift the metabolic pathway to solvent production [24]. The supplement of electrons via the cathode into a microbe led to enhanced reduction reaction directly (working on surface-associated redox enzymes, such as hydrogenases and presumably dehydrogenases [43]) or indirectly (increasing a reduced cofactor such as NADH, Fig. 4). The direct reduction process was studied in fumarate reduction to succinate [47], nitrate reduction to nitrite [48], nitrobenzene reduction to aniline [86], and hexavalent chromium reduction [18]. The indirect reduction process includes ethanol production from acetate [81], alcohol formation from glycerol [87], and butyraldehyde to butanol [88].
Fig. 4.
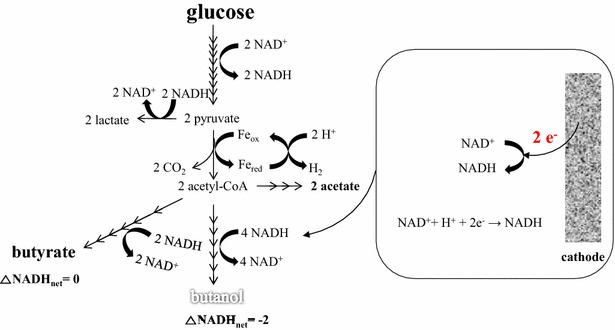
The microbial metabolic pathway of NADH-consuming compound production. One arrow indicates one step of reaction. The butyrate is NADH-balanced and generally produced more than butanol. The NADH reduction (inset) by electricity increases the flux of electron for butanol formation, more NADH-consuming pathway. Fe ox oxidized form of ferredoxin, Fe red reduced form of ferredoxin
Reduction for value-added bioproducts: chain elongation
The interaction between a cathode and microbes led to reverse β oxidation [89] and reduced propionate (C3) to valerate (C5) in a glycerol-fed bioelectrochemical system [87]. Without fermentable substrates, the reduction of acetate (C2) to caproate (C6) and caprylate (C8) took place in a Clostridium kluyveri-predominant mixed culture in a bioelectrochemical system at −0.9 V vs. NHE cathode potential using in-situ produced hydrogen as an electron donor [90]. The reduction of acetate (C2) and butyrate (C4) into alcohols (C1 ~ C4), acetone (C2) and caproate (C6) occurred in a mixed culture of SRB at a potential of −0.85 V vs. Ag/AgCl via direct electron transfer [70].
The application of a cathode for additional reducing power can improve low-grade chemicals to valuable biofuels with energy supplement through the reduction process of an acid to alcohol or by chain elongation. In particular, landfill leachate, which contains acetate, propionate, and butyrate as main components [91], could be used as feed stocks in bioelectrochemical systems to upgrade waste to value-added biofuels, for examples, acetate to butanol [92] (Fig. 5), glycerol to 1,3-propandiol [93], glucose to polyhydroxyalkanoates (PHA) [92].
Fig. 5.
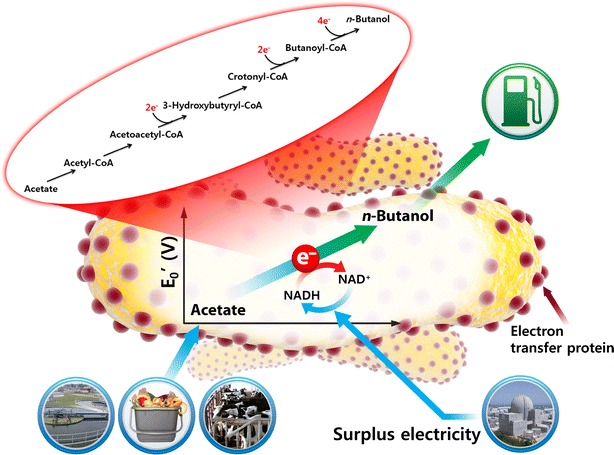
The upgrade of waste into value-added biofuels. The acetate from wastes, such as waste activated sludge, food waste, and animal manure, was feed stocks for biofuel production by electroactive microorganisms. The extracellular electron transfer from cathode to microbe via electron transfer protein could be used for the reduction of acetate to butanol. NADH the reduced form, NAD+ the oxidized form of nicotinamide adenine dinucleotide, respectively
Electrofuel production (CO2 fixation)
Various electron sources can be used as electron donors (organic compounds, H2, H2O, etc.) or acceptors (O2, metal, CO2, etc.) by microbial organisms, whereas humans only use organic carbon as an electron donor and O2 as an electron acceptor. BES uses an electrode as an electron donor (cathode) or an electron acceptor (anode). In particular, electrofuel is a carbon fixation process using a cathode as an electron donor and CO2 as a carbon source, and this process mimics natural photosynthesis in plants [95–97].
Electrofuel has several advantages: (1) the CO2 greenhouse gas can be used as a substrate, and the efficiency of the electricity to chemical commodities is relatively high (80 ~ 90 %), i.e., more efficient than photosynthesis; (2) the electricity can be from many renewable sources; and (3) it has good specificity to produce desired chemical commodities. However, research in this ara is an early stage and the final titer is low and the CO2 reduction rate is slow [98].
An acetogen used an electrode as an electron source to produce 2-oxobutyrate as well as acetate [60]. The long-term operation of a bioelectrochemical system with CO2 produced acetate at a level of 10.5 g/L over 20 days [99]. However, the concentration of other carbon compounds was still small, such as butyrate 35 mg/L [100], isobutanol 846 mg/L, and 3-methyl-a-butanol 570 mg/L [13].
In the absence of direct electron transfer, hydrogen led the reduction process with a hybrid microbial–water-splitting catalyst system [13, 101]. The hydrogen from water splitting was used to reduce carbon dioxide to produce liquid fuels and engineered Ralstonia eutropha produced isopropanol up to 216 mg/L [101]. Fermentative hydrogen production enhanced at −0.6 V vs. SHE led to increased 1,3-propandiol production [93]. Electrochemical generation of formate also mediated electron supplementation to microbes from a cathode in BES [13].
Conclusions
The cathodic reaction in BES is of increasing concern in the context of producing alternative fuels. Beginning with metal-utilizing bacteria, several electroactive bacteria were found and applied for the conversion of electrical to chemical energy as biofuels or biotransformation (Fig. 6). Nonetheless, many technical challenges must still be addressed and the titer of final product is also low. However, research is still in an early stage and efforts such as cell membrane modification and cathode surface modification would enhance the efficiency of BES, as shown in previous studies on MFC.
Fig. 6.
The application of bioelectrochemical reduction for cathodic electron transfer from a cathode to a microbe
Authors’ contributions
OC and BIS conceptualized the manuscript. OC was responsible for literature review, data acquisition and analysis, and initial writing. BIS contributed with review and editing. Both authors read and approved the final manuscript.
Acknowledgements
This work was supported by the research fund of the Korean Ministry of Environment as “Converging Technology Project (202–101–006)” and the Korea Government Ministry of Trade, Industry and Energy as “the New & Renewable Energy of the Korea Institute of Energy Technology Evaluation and Planning (KETEP, No. 20133030000300).”
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- BES
bioelectrochemical synthesis
- COEs
conjugated oligoelectrolytes
- Ech
energy-conserving hydrogenase
- EPS
extracellular polymeric substances
- ETP
electron transport phosphorylation
- FBEB
flavin-based electron bifurcation
- FeOB
iron-oxidizing bacteria
- MES
microbial electrosynthesis
- MFC
microbial fuel cells
- Rub
Rubredoxin
- RVC
reticulated vitreous carbon
- SHE
standard hydrogen electrode
- SLP
substrate-level phosphorylation
- SRB
sulfate-reducing bacteria
Contributor Information
Okkyoung Choi, Email: okgii77@hanyang.ac.kr.
Byoung-In Sang, Phone: +82. 2. 2220. 2328, Email: biosang@hanyang.ac.kr.
References
- 1.Li H, Liao JC. Biological conversion of carbon dioxide to photosynthetic fuels and electrofuels. Energ Environ Sci. 2013;6(10):2892–2899. doi: 10.1039/c3ee41847b. [DOI] [Google Scholar]
- 2.Hawkins AS, Han Y, Lian H, Loder AJ, Menon AL, Iwuchukwu IJ, et al. Extremely thermophilic routes to microbial electrofuels. Acs Catalysis. 2011;1(9):1043–1050. doi: 10.1021/cs2003017. [DOI] [Google Scholar]
- 3.Schroder U, Harnisch F, Angenent LT. Microbial electrochemistry and technology: terminology and classification. Energ Environ Sci. 2015;8(2):513–519. doi: 10.1039/C4EE03359K. [DOI] [Google Scholar]
- 4.Smith JA, Nevin KP, Lovley DR. Syntrophic growth via quinone-mediated interspecies electron transfer. Front in Microbiol. 2015;6:121. doi: 10.3389/fmicb.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Si Ishii, Kosaka T, Hori K, Hotta Y, Watanabe K. Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl Environ Microbiol. 2005;71(12):7838–7845. doi: 10.1128/AEM.71.12.7838-7845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pous N, Casentini B, Rossetti S, Fazi S, Puig S, Aulenta F. Anaerobic arsenite oxidation with an electrode serving as the sole electron acceptor: a novel approach to the bioremediation of arsenic-polluted groundwater. J Hazard Mater. 2015;283:617–622. doi: 10.1016/j.jhazmat.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Stams AJM, De Bok FAM, Plugge CM, Van Eekert MHA, Dolfing J, Schraa G. Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol. 2006;8(3):371–382. doi: 10.1111/j.1462-2920.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim T, Kim B. Electron flow shift in Clostridium acetobutylicum fermentation by electrochemically introduced reducing equivalent. Biotechnol Lett. 1988;10(2):123–128. doi: 10.1007/BF01024638. [DOI] [Google Scholar]
- 9.Vasconcelos I, Girbal L, Soucaille P. Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J Bacteriol. 1994;176(5):1443–1450. doi: 10.1128/jb.176.5.1443-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi L, Squier TC, Zachara JM, Fredrickson JK. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol. 2007;65(1):12–20. doi: 10.1111/j.1365-2958.2007.05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435(7045):1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 12.Myers CR, Myers JM. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992;174(11):3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi L, Rosso KM, Clarke TA, Richardson DJ, Zachara JM, Fredrickson JK. Molecular Underpinnings of Fe(III) Oxide Reduction by Shewanella Oneidensis MR-1. Front Microbiol. 2012;3:50. doi: 10.3389/fmicb.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci. 2008;105(10):3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi O, Um Y, Sang B-I. Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol Bioeng. 2012;109(10):2494–2502. doi: 10.1002/bit.24520. [DOI] [PubMed] [Google Scholar]
- 16.Cao B, Shi L, Brown RN, Xiong Y, Fredrickson JK, Romine MF, et al. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: characterization by infrared spectroscopy and proteomics. Environ Microbiol. 2011;13(4):1018–1031. doi: 10.1111/j.1462-2920.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 17.Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci. 2006;103(30):11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xafenias N, Zhang Y, Banks CJ. Enhanced performance of hexavalent chromium reducing cathodes in the presence of Shewanella oneidensis MR-1 and lactate. Environ Sci Technol. 2013;47(9):4512–4520. doi: 10.1021/es304606u. [DOI] [PubMed] [Google Scholar]
- 19.Gregory KB, Lovley DR. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ Sci Technol. 2005;39(22):8943–8947. doi: 10.1021/es050457e. [DOI] [PubMed] [Google Scholar]
- 20.Ross DE, Flynn JM, Baron DB, Gralnick JA, Bond DR. Towards electrosynthesis in Shewanella: energetics of reversing the Mtr pathway for reductive metabolism. PLoS One. 2011;6(2):e16649. doi: 10.1371/journal.pone.0016649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strycharz SM, Glaven RH, Coppi MV, Gannon SM, Perpetua LA, Liu A, et al. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry. 2011;80(2):142–150. doi: 10.1016/j.bioelechem.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Croese E, Pereira M, Euverink G-J, Stams AM, Geelhoed J. Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell. Appl Microbiol Biotechnol. 2011;92(5):1083–1093. doi: 10.1007/s00253-011-3583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G-W, Choi S-J, Lee T-H, Lee G-Y, Cha J-H, Kim C-W. Application of biocathode in microbial fuel cells: cell performance and microbial community. Appl Microbiol Biotechnol. 2008;79(3):379–388. doi: 10.1007/s00253-008-1451-0. [DOI] [PubMed] [Google Scholar]
- 24.Choi O, Kim T, Woo HM, Um Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Sci Rep. 2014 doi: 10.1038/srep06961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong FA, Hill HAO, Walton NJ. Direct electrochemical reduction of ferredoxin promoted by Mg2+ FEBS Lett. 1982;145(2):241–244. doi: 10.1016/0014-5793(82)80175-0. [DOI] [Google Scholar]
- 26.Shi L, Richardson DJ, Wang Z, Kerisit SN, Rosso KM, Zachara JM, et al. The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer. Environ Microbiol Rep. 2009;1(4):220–227. doi: 10.1111/j.1758-2229.2009.00035.x. [DOI] [PubMed] [Google Scholar]
- 27.Dantas JM, Campelo LM, Duke NEC, Salgueiro CA, Pokkuluri PR. The structure of PccH from Geobacter sulfurreducens: A novel low reduction potential monoheme cytochrome essential for accepting electrons from an electrode. FEBS J. 2015:n/a-n/a. doi:10.1111/febs.13269. [DOI] [PubMed]
- 28.Dantas JM, Tomaz DM, Morgado L, Salgueiro CA. Functional characterization of PccH, a key cytochrome for electron transfer from electrodes to the bacterium Geobacter sulfurreducens. FEBS Lett. 2013;587(16):2662–2668. doi: 10.1016/j.febslet.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Sydow A, Krieg T, Mayer F, Schrader J, Holtmann D. Electroactive bacteria—molecular mechanisms and genetic tools. Appl Microbiol Biotechnol. 2014;98(20):8481–8495. doi: 10.1007/s00253-014-6005-z. [DOI] [PubMed] [Google Scholar]
- 30.Tremblay PL, Zhang T, Dar SA, Leang C, Lovley DR. The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:nAD+ oxidoreductase essential for autotrophic growth. MBio. 2012;4(1):e00406–e00412. doi: 10.1128/mBio.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhury NP, Mowafy AM, Demmer JK, Upadhyay V, Koelzer S, Jayamani E, et al. Studies on the mechanism of electron bifurcation catalyzed by electron transferring flavoprotein (Etf) and butyryl-CoA dehydrogenase (Bcd) of Acidaminococcus fermentans. J Biol Chem. 2014;289(8):5145–5157. doi: 10.1074/jbc.M113.521013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckel W, Thauer RK. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation. Biochim Biophys Acta. 2013;1827(2):94–113. doi: 10.1016/j.bbabio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Hess V, Poehlein A, Weghoff MC, Daniel R, Muller V. A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genom. 2014;15:1139. doi: 10.1186/1471-2164-15-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane N, Martin William F. The origin of membrane bioenergetics. Cell. 2012;151(7):1406–1416. doi: 10.1016/j.cell.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 35.Bertsch J, Oppinger C, Hess V, Langer JD, Muller V. Heterotrimeric NADH-oxidizing methylenetetrahydrofolate reductase from the acetogenic bacterium Acetobacterium woodii. J Bacteriol. 2015;197(9):1681–1689. doi: 10.1128/JB.00048-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kracke F, Vassilev I, Krömer JO. Microbial electron transport and energy conservation—the foundation for optimizing bioelectrochemical systems. Front Microbiol. 2015 doi: 10.3389/fmicb.2015.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovenberg W, Sobel BE. Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. P Natl Acad Sci USA. 1965;54(1):193–199. doi: 10.1073/pnas.54.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park I, Youn B, Harley J, Eidsness M, Smith E, Ichiye T, et al. The unique hydrogen bonded water in the reduced form of Clostridium pasteurianum rubredoxin and its possible role in electron transfer. JBIC, J Biol Inorg Chem. 2004;9(4):423–428. doi: 10.1007/s00775-004-0542-3. [DOI] [PubMed] [Google Scholar]
- 39.Bruschi M, Hatchikian CE, Golovleva LA, Gall JL. Purification and characterization of cytochrome c3, ferredoxin, and rubredoxin isolated from Desulfovibrio desulfuricans Norway. J Bacteriol. 1977;129(1):30–38. doi: 10.1128/jb.129.1.30-38.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coulter ED, Kurtz DM., Jr A role for rubredoxin in oxidative stress protection in Desulfovibrio vulgaris: catalytic electron transfer to rubrerythrin and two-iron superoxide reductase. Arch Biochem Biophys. 2001;394(1):76–86. doi: 10.1006/abbi.2001.2531. [DOI] [PubMed] [Google Scholar]
- 41.Correia dos Santos MM, Paes de Sousa PM, Simões Gonçalves ML, Krippahl L, Moura JJG, Lojou É, et al. Electrochemical studies on small electron transfer proteins using membrane electrodes. J Electroanal Chem. 2003;541:153–162. doi: 10.1016/S0022-0728(02)01427-4. [DOI] [Google Scholar]
- 42.dos Correia Santos MM, de Paes Sousa PM, Simões Gonçalves ML, Ascenso C, Moura I, Moura JJG. Electrochemical studies of rubredoxin from Desulfovibrio vulgaris at modified electrodes. J Electroanal Chem. 2001;501(1–2):173–179. doi: 10.1016/S0022-0728(00)00521-0. [DOI] [Google Scholar]
- 43.Deutzmann JS, Sahin M, Spormann AM. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio. 2015;6(2). doi:10.1128/mBio.00496-15. [DOI] [PMC free article] [PubMed]
- 44.Rosenbaum M, Aulenta F, Villano M, Angenent LT. Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Biores Technol. 2011;102(1):324–333. doi: 10.1016/j.biortech.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Srikanth S, Maesen M, Dominguez-Benetton X, Vanbroekhoven K, Pant D. Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES) Biores Technol. 2014;165:350–354. doi: 10.1016/j.biortech.2014.01.129. [DOI] [PubMed] [Google Scholar]
- 46.da Silva SM, Pacheco I, Pereira IA. Electron transfer between periplasmic formate dehydrogenase and cytochromes c in Desulfovibrio desulfuricans ATCC 27774. J Biol Inorg Chem. 2012;17(5):831–838. doi: 10.1007/s00775-012-0900-5. [DOI] [PubMed] [Google Scholar]
- 47.Dumas C, Basseguy R, Bergel A. Microbial electrocatalysis with Geobacter sulfurreducens biofilm on stainless steel cathodes. Electrochim Acta. 2008;53(5):2494–2500. doi: 10.1016/j.electacta.2007.10.018. [DOI] [Google Scholar]
- 48.Gregory KB, Bond DR, Lovley DR. Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol. 2004;6(6):596–604. doi: 10.1111/j.1462-2920.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- 49.Virdis B, Read ST, Rabaey K, Rozendal RA, Yuan Z, Keller J. Biofilm stratification during simultaneous nitrification and denitrification (SND) at a biocathode. Biores Technol. 2011;102(1):334–341. doi: 10.1016/j.biortech.2010.06.155. [DOI] [PubMed] [Google Scholar]
- 50.Desloover J, Puig S, Virdis B, Clauwaert P, Boeckx P, Verstraete W, et al. Biocathodic nitrous oxide removal in bioelectrochemical systems. Environ Sci Technol. 2011;45(24):10557–10566. doi: 10.1021/es202047x. [DOI] [PubMed] [Google Scholar]
- 51.Pous N, Puig S, Dolors Balaguer M, Colprim J. Cathode potential and anode electron donor evaluation for a suitable treatment of nitrate-contaminated groundwater in bioelectrochemical systems. Chem. Eng J. 2015;263:151–159. [Google Scholar]
- 52.Villano M, Aulenta F, Ciucci C, Ferri T, Giuliano A, Majone M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Biores Technol. 2010;101(9):3085–3090. doi: 10.1016/j.biortech.2009.12.077. [DOI] [PubMed] [Google Scholar]
- 53.Cheng S, Xing D, Call DF, Logan BE. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol. 2009;43(10):3953–3958. doi: 10.1021/es803531g. [DOI] [PubMed] [Google Scholar]
- 54.Lohner ST, Deutzmann JS, Logan BE, Leigh J, Spormann AM. Hydrogenase-independent uptake and metabolism of electrons by the archaeon Methanococcus maripaludis. ISME J. 2014;8(8):1673–1681. doi: 10.1038/ismej.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hara M, Onaka Y, Kobayashi H, Fu Q, Kawaguchi H, Vilcaez J, et al. Mechanism of electromethanogenic reduction of CO2 by a thermophilic methanogen. Energy Procedia. 2013;37:7021–7028. doi: 10.1016/j.egypro.2013.06.637. [DOI] [Google Scholar]
- 56.Costa KC, Wong PM, Wang T, Lie TJ, Dodsworth JA, Swanson I, et al. Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase. Proc Natl Acad Sci. 2010;107(24):11050–11055. doi: 10.1073/pnas.1003653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang M, Tomb J-F, Ferry J. Electron transport in acetate-grown Methanosarcina acetivorans. BMC Microbiol. 2011;11(1):165. doi: 10.1186/1471-2180-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlegel K, Welte C, Deppenmeier U, Müller V. Electron transport during aceticlastic methanogenesis by Methanosarcinaacetivorans involves a sodium-translocating Rnf complex. FEBS J. 2012;279(24):4444–4452. doi: 10.1111/febs.12031. [DOI] [PubMed] [Google Scholar]
- 59.Thauer RK, Kaster A-K, Goenrich M, Schick M, Hiromoto T, Shima S. Hydrogenases from methanogenic Archaea, nickel, a novel cofactor, and H2 Storage. Annu Rev Biochem. 2010;79(1):507–536. doi: 10.1146/annurev.biochem.030508.152103. [DOI] [PubMed] [Google Scholar]
- 60.Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, et al. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microbiol. 2011;77(9):2882–2886. doi: 10.1128/AEM.02642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato S, Yumoto I, Kamagata Y. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl Environ Microbiol. 2015;81(1):67–73. doi: 10.1128/AEM.02767-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song J, Kim Y, Lim M, Lee H, Lee JI, Shin W. Microbes as electrochemical CO2 conversion catalysts. ChemSusChem. 2011;4(5):587–590. doi: 10.1002/cssc.201100107. [DOI] [PubMed] [Google Scholar]
- 63.Xafenias N, Mapelli V. Performance and bacterial enrichment of bioelectrochemical systems during methane and acetate production. Int J Hydrogen Energ. 2014;39(36):21864–21875. doi: 10.1016/j.ijhydene.2014.05.038. [DOI] [Google Scholar]
- 64.Müller V. Energy conservation in acetogenic bacteria. Appl Environ Microbiol. 2003;69(11):6345–6353. doi: 10.1128/AEM.69.11.6345-6353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bose A, Gardel EJ, Vidoudez C, Parra EA, Girguis PR. Electron uptake by iron-oxidizing phototrophic bacteria. Nat Commun. 2014 doi: 10.1038/ncomms4391. [DOI] [PubMed] [Google Scholar]
- 66.Doud DFR, Angenent LT. Toward electrosynthesis with uncoupled extracellular electron uptake and metabolic growth: enhancing current uptake with Rhodopseudomonas palustris. Environ Sci Technol Lett. 2014;1(9):351–355. doi: 10.1021/ez500244n. [DOI] [Google Scholar]
- 67.Summers ZM, Gralnick JA, Bond DR. Cultivation of an obligate fe(ii)-oxidizing lithoautotrophic bacterium using electrodes. mBio. 2013;4(1). doi:10.1128/mBio.00420-12. [DOI] [PMC free article] [PubMed]
- 68.Dinh HT, Kuever J, Muszmann M, Hassel AW, Stratmann M, Widdel F. Iron corrosion by novel anaerobic microorganisms. Nature. 2004;427(6977):829–832. doi: 10.1038/nature02321. [DOI] [PubMed] [Google Scholar]
- 69.Pfeffer C, Larsen S, Song J, Dong M, Besenbacher F, Meyer RL, et al. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491(7423):218–221. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- 70.Sharma M, Aryal N, Sarma PM, Vanbroekhoven K, Lal B, Benetton XD, et al. Bioelectrocatalyzed reduction of acetic and butyric acids via direct electron transfer using a mixed culture of sulfate-reducers drives electrosynthesis of alcohols and acetone. Chem Commun. 2013;49(58):6495–6497. doi: 10.1039/c3cc42570c. [DOI] [PubMed] [Google Scholar]
- 71.Beese-Vasbender PF, Nayak S, Erbe A, Stratmann M, Mayrhofer KJJ. Electrochemical characterization of direct electron uptake in electrical microbially influenced corrosion of iron by the lithoautotrophic SRB Desulfopila corrodens strain IS4. Electrochim Acta. 2015;167:321–329. doi: 10.1016/j.electacta.2015.03.184. [DOI] [Google Scholar]
- 72.Rodrigues TdC, Rosenbaum MA. Microbial electroreduction: screening for new cathodic biocatalysts. ChemElectroChem. 2014;1(11):1916–1922. doi: 10.1002/celc.201402239. [DOI] [Google Scholar]
- 73.Nie H, Zhang T, Cui M, Lu H, Lovley DR, Russell TP. Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys Chem Chem Phys. 2013;15(34):14290–14294. doi: 10.1039/c3cp52697f. [DOI] [PubMed] [Google Scholar]
- 74.Jourdin L, Freguia S, Donose BC, Chen J, Wallace GG, Keller J, et al. A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis. J Mater Chem A. 2014;2(32):13093–13102. doi: 10.1039/C4TA03101F. [DOI] [Google Scholar]
- 75.Flexer V, Chen J, Donose BC, Sherrell P, Wallace GG, Keller J. The nanostructure of three-dimensional scaffolds enhances the current density of microbial bioelectrochemical systems. Energ Environ Sci. 2013;6(4):1291–1298. doi: 10.1039/c3ee00052d. [DOI] [Google Scholar]
- 76.Yan H, Catania C, Bazan GC. Membrane-intercalating conjugated oligoelectrolytes: Impact on bioelectrochemical systems. Adv Mater. 2015:n/a-n/a. doi:10.1002/adma.201500487. [DOI] [PubMed]
- 77.Jourdin L, Grieger T, Monetti J, Flexer V, Freguia S, Lu Y, et al. High acetic acid production rate obtained by microbial electrosynthesis from carbon dioxide. Environ Sci Technol. 2015 doi: 10.1021/acs.est.5b03821. [DOI] [PubMed] [Google Scholar]
- 78.Su M, Wei L, Qiu Z, Jia Q, Shen J. A graphene modified biocathode for enhancing hydrogen production. RSC Advances. 2015;5(41):32609–32614. doi: 10.1039/C5RA02695D. [DOI] [Google Scholar]
- 79.Foley JM, Rozendal RA, Hertle CK, Lant PA, Rabaey K. Life cycle assessment of high-rate anaerobic treatment, microbial fuel cells, and microbial electrolysis cells. Environ Sci Technol. 2010;44(9):3629–3637. doi: 10.1021/es100125h. [DOI] [PubMed] [Google Scholar]
- 80.Pant D, Singh A, Van Bogaert G, Gallego YA, Diels L, Vanbroekhoven K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: relevance and key aspects. Renew Sust Energ Rev. 2011;15(2):1305–1313. doi: 10.1016/j.rser.2010.10.005. [DOI] [Google Scholar]
- 81.Steinbusch KJJ, Hamelers HVM, Schaap JD, Kampman C, Buisman CJN. Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environ Sci Technol. 2010;44(1):513–517. doi: 10.1021/es902371e. [DOI] [PubMed] [Google Scholar]
- 82.Park DH, Zeikus JG. Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J Bacteriol. 1999;181(8):2403–2410. doi: 10.1128/jb.181.8.2403-2410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park DH, Laivenieks M, Guettler MV, Jain MK, Zeikus JG. Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl Environ Microbiol. 1999;65(7):2912–2917. doi: 10.1128/aem.65.7.2912-2917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emde R, Schink B. Enhanced propionate formation by Propionibacterium freudenreichii subsp. freudenreichii in a three-electrode amperometric culture system. Appl Environ Microbiol. 1990;56(9):2771–2776. doi: 10.1128/aem.56.9.2771-2776.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kracke F, Krömer JO. Identifying target processes for microbial electrosynthesis by elementary mode analysis. BMC Bioinform. 2014;15(1):410. doi: 10.1186/s12859-014-0410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang A-J, Cheng H-Y, Liang B, Ren N-Q, Cui D, Lin N, et al. Efficient reduction of nitrobenzene to aniline with a biocatalyzed cathode. Environ Sci Technol. 2011;45(23):10186–10193. doi: 10.1021/es202356w. [DOI] [PubMed] [Google Scholar]
- 87.Dennis PG, Harnisch F, Yeoh YK, Tyson GW, Rabaey K. Dynamics of cathode-associated microbial communities and metabolite profiles in a glycerol-fed bioelectrochemical system. Appl Environ Microbiol. 2013;79(13):4008–4014. doi: 10.1128/AEM.00569-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schlager S, Neugebauer H, Haberbauer M, Hinterberger G, Sariciftci NS. Direct electrochemical addressing of immobilized alcohol dehydrogenase for the heterogeneous bioelectrocatalytic reduction of butyraldehyde to butanol. ChemCatChem. 2015;7(6):967–971. doi: 10.1002/cctc.201402932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature. 2011;476(7360):355–359. doi: 10.1038/nature10333. [DOI] [PubMed] [Google Scholar]
- 90.Van Eerten-Jansen MCAA, Ter Heijne A, Grootscholten TIM, Steinbusch KJJ, Sleutels THJA, Hamelers HVM, et al. Bioelectrochemical production of caproate and caprylate from acetate by mixed cultures. ACS Sustain Chem Eng. 2013;1(5):513–518. doi: 10.1021/sc300168z. [DOI] [Google Scholar]
- 91.Lozecznik S, Sparling R, Clark SP, VanGulck JF, Oleszkiewicz JA. Acetate and propionate impact on the methanogenesis of landfill leachate and the reduction of clogging components. Biores Technol. 2012;104:37–43. doi: 10.1016/j.biortech.2011.09.123. [DOI] [PubMed] [Google Scholar]
- 92.Sharma M, Aryal N, Sarma PM, Vanbroekhoven K, Lal B, Benetton XD, et al. Bioelectrocatalyzed reduction of acetic and butyric acids via direct electron transfer using a mixed culture of sulfate-reducers drives electrosynthesis of alcohols and acetone. Chem Commun. 2013;49(58):6495–6497. doi: 10.1039/c3cc42570c. [DOI] [PubMed] [Google Scholar]
- 93.Zhou M, Chen J, Freguia S, Rabaey K, Keller J. Carbon and electron fluxes during the electricity driven 1,3-propanediol biosynthesis from glycerol. Environ Sci Technol. 2013;47(19):11199–11205. doi: 10.1021/es402132r. [DOI] [PubMed] [Google Scholar]
- 94.Srikanth S, Venkateswar Reddy M, Venkata Mohan S. Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Biores Technol. 2012;125:291–9. doi:10.1016/j.biortech.2012.08.060. [DOI] [PubMed]
- 95.Hawkins AS, Han Y, Lian H, Loder AJ, Menon AL, Iwuchukwu IJ, et al. Extremely thermophilic routes to microbial electrofuels. ACS Catal. 2011;1(9):1043–1050. doi: 10.1021/cs2003017. [DOI] [Google Scholar]
- 96.Conrado R, Haynes C, Haendler B, Toone E. Electrofuels: a new paradigm for renewable fuels. Advanced Biofuels and Bioproducts. New York: Springer; 2013. [Google Scholar]
- 97.Hawkins AS, McTernan PM, Lian H, Kelly RM, Adams MWW. Biological conversion of carbon dioxide and hydrogen into liquid fuels and industrial chemicals. Curr Opin Biotech. 2013;24(3):376–384. doi: 10.1016/j.copbio.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 98.Tremblay P-L, Zhang T. Electrifying microbes for the production of chemicals. Front Microbiol. 2015 doi: 10.3389/fmicb.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marshall CW, Ross DE, Fichot EB, Norman RS, May HD. Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes. Environ Sci Technol. 2013;47(11):6023–6029. doi: 10.1021/es400341b. [DOI] [PubMed] [Google Scholar]
- 100.Ganigue R, Puig S, Batlle-Vilanova P, Balaguer MD, Colprim J. Microbial electrosynthesis of butyrate from carbon dioxide. Chem Commun. 2015;51(15):3235–3238. doi: 10.1039/C4CC10121A. [DOI] [PubMed] [Google Scholar]
- 101.Torella JP, Gagliardi CJ, Chen JS, Bediako DK, Colón B, Way JC, et al. Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system. Proc Natl Acad Sci. 2015;112(8):2337–2342. doi: 10.1073/pnas.1424872112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsu L, Masuda SA, Nealson KH, Pirbazari M. Evaluation of microbial fuel cell Shewanella biocathodes for treatment of chromate contamination. RSC Adv. 2012;2(13):5844–5855. doi: 10.1039/c2ra20478a. [DOI] [Google Scholar]
- 103.Rozendal RA, Jeremiasse AW, Hamelers HVM, Buisman CJN. Hydrogen production with a microbial biocathode. Environ Sci Technol. 2008;42(2):629–634. doi: 10.1021/es071720+. [DOI] [PubMed] [Google Scholar]
- 104.Jeremiasse AW, Hamelers HVM, Buisman CJN. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry. 2010;78(1):39–43. doi: 10.1016/j.bioelechem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Van Eerten-Jansen MCAA, Heijne AT, Buisman CJN, Hamelers HVM. Microbial electrolysis cells for production of methane from CO2: long-term performance and perspectives. Int J Energy Res. 2012;36(6):809–819. doi: 10.1002/er.1954. [DOI] [Google Scholar]
- 106.Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, et al. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microb. 2011;77(9):2882–2886. doi: 10.1128/AEM.02642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nevin KP, Woodard TL, Franks AE, Summers ZM, Lovley DR. Microbial electrosynthesis: Feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio. 2010;1(2). doi:10.1128/mBio.00103-10. [DOI] [PMC free article] [PubMed]
- 108.Sato K, Kawaguchi H, Kobayashi H. Bio-electrochemical conversion of carbon dioxide to methane in geological storage reservoirs. Energy Convers Manag. 2013;66:343–350. doi: 10.1016/j.enconman.2012.12.008. [DOI] [Google Scholar]
- 109.Pitts KE, Dobbin PS, Reyes-Ramirez F, Thomson AJ, Richardson DJ, Seward HE. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA: expression in Escherichia coli confers the ability to reduce soluble Fe(III) chelates. J Biol Chem. 2003;278(30):27758–27765. doi: 10.1074/jbc.M302582200. [DOI] [PubMed] [Google Scholar]
- 110.Gorby Y, McLean J, Korenevsky A, Rosso K, El-Naggar MY, Beveridge TJ. Redox-reactive membrane vesicles produced by Shewanella. Geobiology. 2008;6(3):232–241. doi: 10.1111/j.1472-4669.2008.00158.x. [DOI] [PubMed] [Google Scholar]
- 111.Hartshorne R, Jepson B, Clarke T, Field S, Fredrickson J, Zachara J, et al. Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. JBIC, J Biol Inorg Chem. 2007;12(7):1083–1094. doi: 10.1007/s00775-007-0278-y. [DOI] [PubMed] [Google Scholar]


