Abstract
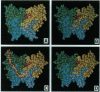
B3(dsFv)-PE38KDEL is a recombinant immunotoxin composed of the Fv region of monoclonal antibody B3 connected to a truncated form of Pseudomonas exotoxin (PE38KDEL), in which the unstable Fv heterodimer (composed of heavy- and light-chain variable regions) is held together and stabilized by a disulfide bond [termed disulfide-stabilized Fv (dsFV)]. A computer modeled structure of the B3(Fv), made by mutating and energy minimizing the amino acid sequence and structure of McPC603, enabled us to identify positions in conserved framework regions that "hypothetically" could be used for disulfide stabilization without changing the structure or affecting antigen binding. This prediction was evaluated experimentally by constructing a disulfide-linked two-chain dsFv-immunotoxin that was produced in Escherichia coli. The activity and specificity of this immunotoxin was indistinguishable from its single-chain Fv (scFv) counterpart, indicating that, as in B3(scFv), the structure of the binding region is retained in B3(dsFv). Because we introduced the stabilizing disulfide bond in between two framework residues in a position that is conserved in most Fv molecules, this method of linkage between the heavy- and light-chain variable regions should be generally applicable to construct immunotoxins and dsFv molecules using other antibodies. Furthermore, the finding that B3(dsFv) was much more stable at 37 degrees C in human plasma than B3(scFv) indicates that dsFvs are possibly more versatile for therapeutic application than scFvs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batra J. K., Kasprzyk P. G., Bird R. E., Pastan I., King C. R. Recombinant anti-erbB2 immunotoxins containing Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5867–5871. doi: 10.1073/pnas.89.13.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
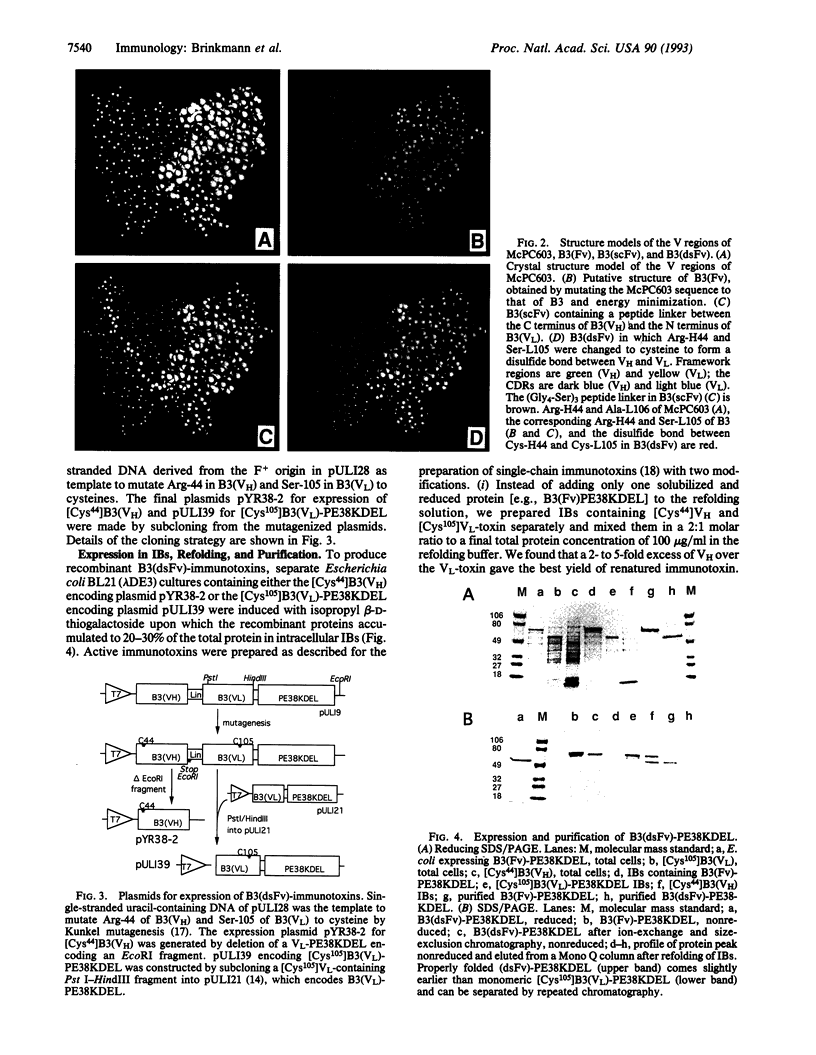
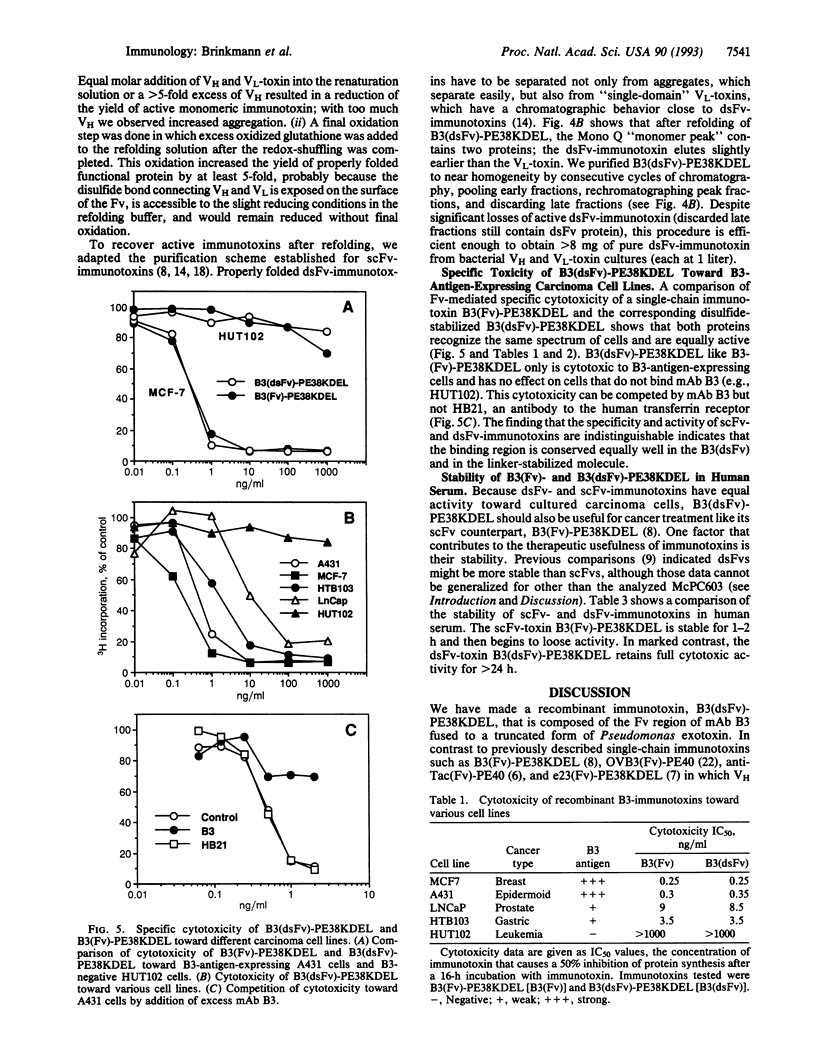
- Brinkmann U., Lee B. K., Pastan I. Recombinant immunotoxins containing the VH or VL domain of monoclonal antibody B3 fused to Pseudomonas exotoxin. J Immunol. 1993 Apr 1;150(7):2774–2782. [PubMed] [Google Scholar]
- Brinkmann U., Pai L. H., FitzGerald D. J., Willingham M., Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Pastan I., Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992 Sep;205(2):263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd, Persson M. A., Koenig S., Chanock R. M., Lerner R. A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Batra J. K., Gallo M. G., Willingham M. C., FitzGerald D. J., Pastan I. A rapid method of cloning functional variable-region antibody genes in Escherichia coli as single-chain immunotoxins. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1066–1070. doi: 10.1073/pnas.87.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Queen C., Junghans R. P., Waldmann T. A., FitzGerald D. J., Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989 Jun 1;339(6223):394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- Glockshuber R., Malia M., Pfitzinger I., Plückthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990 Feb 13;29(6):1362–1367. doi: 10.1021/bi00458a002. [DOI] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenic D. E., Yokota T., Filpula D. R., Finkelman M. A., Dodd S. W., Wood J. F., Whitlow M., Snoy P., Schlom J. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991 Dec 1;51(23 Pt 1):6363–6371. [PubMed] [Google Scholar]
- Pai L. H., Batra J. K., FitzGerald D. J., Willingham M. C., Pastan I. Anti-tumor activities of immunotoxins made of monoclonal antibody B3 and various forms of Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3358–3362. doi: 10.1073/pnas.88.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoliano M. W., Bird R. E., Johnson S., Asel E. D., Dodd S. W., Wood J. F., Hardman K. D. Conformational stability, folding, and ligand-binding affinity of single-chain Fv immunoglobulin fragments expressed in Escherichia coli. Biochemistry. 1991 Oct 22;30(42):10117–10125. doi: 10.1021/bi00106a007. [DOI] [PubMed] [Google Scholar]
- Pastan I., FitzGerald D. Recombinant toxins for cancer treatment. Science. 1991 Nov 22;254(5035):1173–1177. doi: 10.1126/science.1683495. [DOI] [PubMed] [Google Scholar]
- Pastan I., Lovelace E. T., Gallo M. G., Rutherford A. V., Magnani J. L., Willingham M. C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991 Jul 15;51(14):3781–3787. [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Stemmer W. P., Morris S. K., Wilson B. S. Selection of an active single chain Fv antibody from a protein linker library prepared by enzymatic inverse PCR. Biotechniques. 1993 Feb;14(2):256–265. [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]