Abstract
Obesity is a well-known risk factor for colorectal cancer but precisely how it influences risks of malignancy remain unclear. During colon cancer development in humans or animals, attenuation of the colonic cell surface receptor guanylyl cyclase C (GUCY2C) that occurs due to loss of its paracrine hormone ligand guanylin contributes universally to malignant progression. In this study, we explored a link between obesity and GUCY2C silencing in colorectal cancer. Using genetically engineered mice on different diets, we found that diet-induced obesity caused a loss of guanylin expression in the colon with subsequent GUCY2C silencing, epithelial dysfunction and tumorigenesis. Mechanistic investigations revealed that obesity reversibly silenced guanylin expression through calorie-dependent induction of endoplasmic reticulum stress and the unfolded protein response in intestinal epithelial cells. In transgenic mice, enforcing specific expression of guanylin in intestinal epithelial cells restored GUCY2C signaling, eliminating intestinal tumors associated with a high calorie diet. Our findings show how caloric suppression of the guanylin-GUCY2C signaling axis links obesity to negation of a universal tumor suppressor pathway in colorectal cancer, suggesting an opportunity to prevent colorectal cancer in obese patients through hormone replacement with the FDA-approved oral GUCY2C ligand linaclotide.
Introduction
The precise molecular mechanisms by which obesity influences neoplastic transformation, including colorectal cancer, continue to be one of the most perplexing and provocative questions in cancer research. In that context, how obesity influences canonical signaling pathways underlying tumorigenesis remains incompletely defined. Guanylyl cyclase C (GUCY2C), expressed selectively in intestinal epithelial cells, is the receptor for diarrheagenic bacterial heat-stable enterotoxins (STs) and the gut paracrine hormones, guanylin in colon and uroguanylin in small intestine (1). This paracrine axis comprises a tumor suppressing circuit whose dysregulation universally characterizes colorectal carcinogenesis across species (2, 3). Indeed, guanylin is one of the most commonly lost gene products in colorectal tumorigenesis and its loss is one of the earliest events in intestinal transformation (2, 4, 5). Loss of guanylin silences GUCY2C producing intestinal epithelial dysfunction disrupting homeostatic mechanisms organizing the crypt-villus axis including proliferation, DNA damage sensing and repair, and metabolic programming which contributes to tumorigenesis (6-8). Here, we demonstrate that diet-induced obesity suppresses guanylin expression and silences GUCY2C through calorie-dependent ER stress, contributing to tumorigenesis.
Materials and Methods
Animal models
C57BL/6 mice were purchased from NCI, while Balb/c (Stock Number 000651) and ob/ob (B6.Cg-Lepob/J; Stock Number 000632) mice were purchased from JAX. Mice were acquired at 4 wks of age, acclimated for 2 wks, and fed Lean diet (LabDiet 5010; 3.0 kcal/g, 12.7% from fat and 58.5% from carbohydrate), HF diet (TestDiet 58Y1; 5.1 kcal/g, 61.6% from fat and 20.3% from carbohydrate) or HC diet (TestDiet 58Y2; 3.8 kcal/g, 10.2% from fat and 71.8% from carbohydrate) as indicated (Supplemental Table 1). Preliminary studies revealed that 4-6 weeks of a high fat diet was required to induce guanylin suppression. Gucy2c−/− mice, generated by inserting a neomycin resistance gene into the first exon, were bred, maintained, genotyped, and functionally characterized as described (7). Sibling Gucy2c+/+ and Gucy2c−/− mice from five different Gucy2c+/− breeding pairs [(generation 16 by backcrossing to the C57BL/6J mice obtained from JAX (Stock Number 000664)] were used to generate mice for the experiments. ROSA-STOPflox-Guca2a mice were generated by standard procedures in the Thomas Jefferson University transgenic mouse facility as described (9). Expression of the GUCY2C ligand, guanylin (GUCA2A), is regulated by the ROSA26 promoter followed by a STOP codon flanked by two loxP sites upstream of full length Guca2a in ROSA-STOPflox-Guca2a mice (Fig. 4A). Removal of the STOP codon by Cre recombinase activates constitutive transcription of Guca2a driven by the ROSA26 promoter (Fig. 4A). The murine villin promoter targets stable and homogeneous expression of transgenes in small and large intestine along the crypt-villus axis, in differentiated enterocytes, as well as in the immature, undifferentiated cells of the crypt. Villin-Cre-ERT2 mice were obtained from S. Robine (Institut Curie, Paris, France). Villin-CreERT2 mice express Cre recombinase in intestinal epithelial cells. Intraperitoneal injection of tamoxifen (20 mg/Kg BW) for 5 consecutive days activates CreERT2 to mediate genetic recombination. Villin-CreERT2 mice were crossed with ROSA-STOPflox-Guca2a mice to generate hemizygous ROSA-STOPflox-Guca2a-vil-Cre-ERT2 mice. Villin-CreERT2 mice were paired with hemizygous ROSA-STOPflox-Guca2a-vil-Cre-ERT2 mice to produce ROSA-STOPflox-Guca2a-vil-Cre-ERT2 mice and corresponding littermate controls lacking the ROSA-STOPflox-Guca2a transgene. Both Villin-CreERT2 and ROSA-STOPflox-Guca2a mice were on the C57BL/6 background. Xbp1ΔIEC (Villin-Cre+-Xbp1fl/fl) mice were generated by breeding Xbp1fl/fl and Villin-Cre mice. Xbp1fl/fl mice were generated by targeting loxP sites to introns flanking exon 2 and backcrossed >8 generations onto C57BL6 mice (10). Xbp1fl/fl mice served as genotype controls (11). Mice were housed in light-cycled and climate-controlled barrier animal facilities at Thomas Jefferson University (C57BL/6J, Balb/c, ob/ob, Gucy2c−/−, ROSA-STOPflox-Guca2a-vil-Cre-ERT2, ROSA-STOPflox-Guca2a) and Harvard Medical School (Xbp1ΔIEC, Xbp1fl/fl). All experiments were performed in compliance with the Thomas Jefferson University and Harvard University Animal Care and Use Guidelines and approved animal protocols.
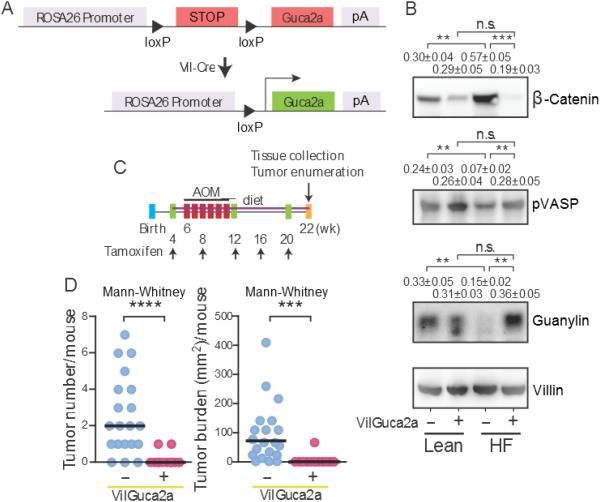
Figure 4. Transgenic guanylin expression prevents calorie-induced tumorigenesis.
(A) Construct for transgenic guanylin expression in intestine. Transgenic guanylin expression (B) rescued GUCY2C signaling reducing epithelial dysfunction induced by a HF diet and (C-D) almost completely eliminated tumorigenesis induced by a HF diet and AOM in colons of VilGuca2a+, but not control VilGuca2a-, mice. Immunoblot results represent the mean ± SEM of 5 mice. VilGuca2a (−), C57BL/6 mice expressing vil-Cre-ERT2 (guanylin wild type); VilGuca2a (+), C57BL/6 mice expressing vil-Cre-ERT2 and carrying the guanylin transgene.
Genotyping
Gucy2c genotype was confirmed by PCR with primers: forward: 5'-AGGTCATGACGTCACTGCTGGGCC-3'; reverse: 5'-TGTCCAGTCCTTCCTCCACAG-3'; neomycin: 5'-GGTGGGCTCTATGGCTTC-3' (7). ROSA-STOPflox-Guca2a genotype was confirmed by PCR with primers: forward: 5'-CCGCCGTTGTTGTTATTGTAG-3'; reverse: 5'-GTTGTGGTG ATAGGTGGCAAG-3'. Villin-Cre-ERT2 genotype was confirmed by PCR with primers: forward: 5'-GAAAATGCTTCTGTCCGTTTG-3'; reverse: 5'-ATTGCTGTCACTTGGTCGTG-3' (9).
Colorectal tumorigenesis model
For Gucy2c+/+ (Lean, HF) and Gucy2c−/− (Lean) mice, azoxymethane (AOM 8 mg/kg; Sigma, St. Louis, MO) was administrated to mice (6 wks old) through intraperitoneal injections weekly for 6 wks. Tumors were enumerated and their sizes were quantified 8 wks after the last AOM dose under a dissecting stereomicroscope by blinded analysis. Tumor burden per animal was calculated as the sum of the area (diameter2) of individual tumors (7). For the ROSA-STOPflox-Guca2a-vil-Cre-ERT2 model and corresponding controls (Fig. 4c), mice were on HF diet starting at 4 wks of age. Tamoxifen (20 mg/kg IP) was administered every 4 wks to enforce guanylin expression starting at 4 wks until tumor enumeration. Six doses of AOM (10 mg/kg) weekly were administrated starting at 5 wks of age. Tumors were enumerated and their sizes quantified at 22 wks of age (6, 7). For the Gucy2c+/+ (Lean, HC) mice, AOM (12 mg/kg) was administrated to mice (6 wks old) weekly for 6 wks. Tumors were enumerated and their sizes quantified 12 wks after the last AOM dose. The doses of AOM in different diet models were established in pilot studies to ensure at least ~50% tumor incidence in experimental cohorts.
Human tissues
Human distal colonic mucosal specimens were obtained from patients undergoing surgery under a protocol approved by the Thomas Jefferson University Institutional Review Board (control no. 01.0823).
Cell culture and lentivirus infection
Caco2 C2BBE1 cells were obtained from ATCC (CRL-2101), maintained and extended in Dulbecco’s Modified Eagle’s Medium (DMEM, Cellgro, Catalog No. 10-013), supplemented with 0.01 mg/ml human transferrin, and 10% fetal bovine serum (FBS). HEK293T cells (ATCC, Catalog No. ACS-4500) were maintained in DMEM with 10% fetal bovine serum. For PERK inhibitor experiments, C2BBE1 cells were incubated in DMEM supplemented with 0.01 mg/ml human transferrin without FBS. Cells were pre-treated with 10 μg/ml PERK inhibitor I (GSK2606414, EMD Millipore, Catalog No. 506190) for 30 min before induction of ER stress by 2.5 μg/ml tunicamycin (Sigma-Aldrich, Catalog No. T7765) for 24 h. For ER stress experiments, cells were treated with various concentration of tunicamycin, or 500 nM thapsigargin (Sigma-Aldrich, Catalog No. T9033), for 24 h and 48 h before analysis. For siRNA experiments, HEK293 cells were transfected with Stealth siRNA (Life Technologies) siPERK (HSS190343) or Negative Control siRNA (Catalog Number 12935-300) using Lipofectamine RNAimax Transfection Reagent (Life Technologies). The next day, cells were transfected with 0.5 μg of a bi-directional CMV plasmid designed to co-express guanylin protein and a zsgreen fluorescence marker using FUGENE HD Transfection Reagent (Promega). After 3 h, cells were incubated in media containing 1 μg/mL tunicamycin or DMSO (control) for 24 h and then analyzed.
Tissue preparation
Mucosal layers from mouse distal colons were frozen immediately in liquid nitrogen and stored at −80°C until protein or RNA analysis. For immunostaining, specimens were fixed overnight in 4% formaldehyde at 4°C, dehydrated through a series of graded acetone and ethanol washes, and embedded in paraffin (6, 7). Paraffin sections (5 μm) were mounted (5 sections/slide/mouse) for immunohistochemistry or immunofluorescence staining (7).
RNA analysis
Total RNA was isolated by RNeasy Mini Kit (Qiagen, Catalog No. 74104) as directed by the manufacturer's instructions. Two-step RT-PCR was performed using TaqMan® reverse transcription reagents (Life Technologies, Catalog No. N8080234) and TaqMan® universal master mix (Life Technologies, Catalog No. 4440038) to perform quantitative RT-PCR using GUCA2A primer/probe for TaqMan® Gene Expression Assays (Mm00433863_m1, Hs 00157859_m1) in an ABI 7000 Sequence Detection System (Applied Biosystems)(7, 12). Relative expression was calculated using the 2−ΔΔCT method utilizing villin1 (Life Technologies, Mm00494146_m1, Hs00200229_m1) as the internal control (9).
Immunostaining and immunofluorescence
Ki-67 served as a marker of proliferation, and immunohistochemistry was performed as described (7). Tissue specimens were deparaffinized and rehydrated, followed by heat-induced epitope retrieval (10 mmol/L citrate buffer, pH 6.0). After quenching with 3% H2O2 in TBS-T (Tris-buffered saline with 0.5% Tween 20) and blocking with 5% milk in TBS-T, proliferative cells were detected by overnight incubation with anti-Ki67 antibody (Dako, Clone TEC-3, Code No. M7249), followed by biotin-labeled secondary antibody and Histostain IHC detection kit for visualization (Life technology). Ki-67-positive cells were quantified by blinded-analysis from 5 to 15 crypt-villus units/segment/mouse. Crypt number was estimated from complete crypts per transverse cross-section in 5 sections per mouse. Results reflect means ± SEM of at least 5 animals in each group. For guanylin detection, heat-induced antigen retrieval was done in 10 mmol/L citrate buffer pH 8.5, blocked in 5% milk in TBS-T supplemented with 3% donkey serum for 1 h at RT and followed by overnight incubation with rabbit anti-guanylin antibody at 4°C (gift from Dr. Michael Goy, University of North Carolina, NC). Specimens were mounted in ProLong® Gold antifade reagent with DAPI after incubation with fluorescence labeled secondary antibody (Alexa Flour, Life Technologies) for visualization. Fluorescence was visualized using a Zeiss LSM 510 Meta Confocal Laser Scanning Microscope. Intestine specimens from Xbp1ΔIEC and control Xbp1fl/fl mice were obtained from Drs. F.M. Tomczak and R.S. Blumberg (11) and paraffin sections prepared as described.
Immunoblot analyses
Protein was extracted and homogenized in M-PER reagent (Thermo Fisher Scientific, Catalog No. 78501) supplemented with protease and phosphatase inhibitors (Roche Applied Science, No. 05892970001 and 04906837001), and then subjected to SDS-PAGE and immunoblotting using antibodies as follows: actin (Cell Signaling, 4967S), Bip (Cell Signaling, 3177S), β-catenin (Cell Signaling, 8480S), CHOP (Cell Signaling, 2895S), cyclin D1 (Cell Signaling, 2978S), eIF2α (Cell Signaling, 9722S), GUCA2A (LSBio, LS-C3244, LS-C166741), hexokinase II (Cell Signaling, 2106S), Phospho-AKT (Cell Signaling, 9271S), Phospho-eIF2α (Cell Signaling, 3597S), γ-H2AX (Cell Signaling, 2577S), Phospho-VASP (Cell Signaling, 3114S), villlin-1 (Cell Signaling, 2369S). Secondary antibodies were from Santa Cruz Biotechnology. Staining intensity of specific bands quantified by densitometry (Kodak) was normalized to that for villin-1. Immunocomplexes were detected by SuperSignal West Dura Substrate (Thermo Fisher Scientific, No. 37071). Average relative intensity reflects the mean of 5~15 individual animals per cohort or ≥3 independent experiments with cells.
Growth curves and food intake
Five mice were housed together, each mouse was weighed weekly, and at least 20 mice from each cohort were followed. In the ob/ob restricted diet experiment, 3 mice were housed together and 6 mice from each cohort were weighed. For food intake, mice were separated into individual cages with wire-mesh floors, and given a pre-weighed amount of chow each day. All mice were given ad libitum access to water for the duration of the experiment. Food consumption was measured daily for 7 d to establish average food intake.
Statistical analyses
Minimum cohort sizes were computed using a power of 80% and a significance level of 0.05 (1-tailed test) employing a priori predictions of effect size and variance established by preliminary studies or literature review. Operators were blinded to sample identities for analyses. Comparisons between two groups at single time points were analyzed by Student’s t test, or by the Mann-Whitney test for measures not satisfying normality assumptions, and comparisons between >2 groups employed One-way ANOVA. All statistical tests were calculated using GraphPad Prism. Analyses represent mean ± SEM of n=5, unless otherwise indicated, and * p<0.05, ** p<0.01, *** p<0.001, ****, p<0.0001. The association of hormone level with body mass index (BMI) in patients was completed using a linear mixed model incorporating hetero-skedastic variances aligned with BMI category. A global test of mean differences across BMI levels was calculated, and differences in least squares means were used to assess differences for BMI groups in relation to lean individuals.
Results
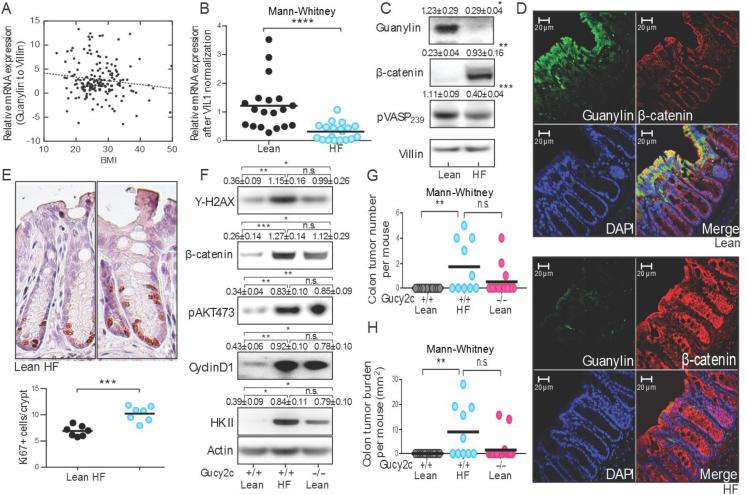
There is an inverse relationship between guanylin mRNA expression in normal colonic epithelium and body mass index (BMI) in humans (p<0.001), and morbidly obese patients (BMI≥35 kg/m2) exhibit an 80% decrease in guanylin mRNA expression compared to lean individuals (Fig. 1A). Loss of guanylin mRNA expression in obesity recapitulated guanylin loss in colon tumors compared to matched normal adjacent tissues in patients (12). Similarly, a high fat (HF) diet reduced guanylin mRNA expression in normal colon epithelia in mice (Fig. 1B), associated with reduced guanylin protein expression (Figs. 1C-D), in proximal and distal colon (Supplemental Fig. 1), recapitulating guanylin protein loss in colon tumors in patients (12). Loss of guanylin expression in obesity silenced GUCY2C, without changing its expression (Supplemental Fig. 2), eliminating canonical cyclic GMP-dependent phosphorylation of the vasodilator-stimulated phosphoprotein (VASP) at serine 239 and increasing the expression of β-catenin (Fig. 1C). Beyond β-catenin, silencing GUCY2C produced characteristic intestinal epithelial dysfunction (6, 7, 13) increasing DNA damage (γ-H2AX); activating drivers of proliferation (AKT phosphorylation); accelerating the cell cycle (cyclin D1) increasing the proliferating crypt compartment (Ki67); and reprogramming metabolism by increasing the glycolytic machinery (hexokinase (HK) II; Figs. 1E-F). Epithelial dysfunction produced by guanylin suppression and functional silencing of GUCY2C in obesity recapitulated that induced by genetic elimination of GUCY2C expression (Fig. 1F). Moreover, in an established model of colon cancer in obesity (14), tumorigenesis induced by a HF diet associated with guanylin suppression mimicked that produced by genetic silencing of GUCY2C expression (Fig. 1G-H).
Figure 1. Suppression of the guanylin-GUCY2C axis with epithelial dysfunction and tumorigenesis in obesity in C57BL/6 mice.
(A) Guanylin mRNA in human colon inversely correlates with BMI. (B) Guanylin mRNA in colons from mice on high fat (HF) or Lean diets. (C) Loss of guanylin in mice on HF diet silences GUCY2C, increasing epithelial dysfunction. (D) Guanylin (green), β-catenin (red) and (E) Ki67 in colons from mice on Lean and HF diets. (F) Epithelial dysfunction was compared in GUCY2C wild type (+/+) and deficient (−/−) mice on Lean and HF diets. (G) Tumor number and (H) burden in GUCY2C (+/+) and (−/−) mice on Lean or HF diet receiving AOM. Immunoblot results represent the mean ± SEM of 5 mice.
The established paradigm suggests that tumorigenesis associated with diet-induced obesity reflects reprogramming of the endocrine, adipokine, and inflammatory milieu (14-16). However, a high carbohydrate (HC) diet which increased caloric intake ~40% (Supplemental Fig. 1A) without producing weight gain (Supplemental Fig. 1B) reduced colon expression of guanylin (Fig. 2A-B), induced epithelial dysfunction (Fig. 2B), and amplified tumorigenesis (Fig. 2C) that recapitulated the effects of a HF diet. Also, there is a phenotypic polymorphism in C57BL/6 mice in which about 20% consume excess calories (Supplemental Fig. 2A) but resist diet-induced obesity (Supplemental Fig. 2B)(17). These mice, which remain lean on a HF diet, nevertheless lose guanylin mRNA (Supplemental Fig. 2C) and protein expression associated with epithelial dysfunction marked by increased β-catenin that recapitulates those effects produced in obese mice (Fig. 2D). Similarly, while Balb/c mice are genetically resistant to obesogenic diets and remain lean (Supplemental Fig. 3A-B)(18), they lose guanylin expression on a HF diet (Supplemental Fig. 3C). Further, ob/ob mice have a genetic defect in leptin expression producing obesity even on a calorie-restricted Lean diet (Supplemental Fig. 4A-B)(19). However, even in the context of obesity (Supplemental Fig. 4b) consumption of a diet restricted to normal caloric intake did not reduce guanylin expression or increase β-catenin in ob/ob mice (Supplemental Fig. 4C; Fig. 2E). Finally, obese wild type mice on a HF diet recover colonic guanylin expression, signaling, and β-catenin levels after 4 wks on a Lean diet (Fig. 2F), although they remain persistently obese (Supplemental Fig. 5). Taken together, these observations demonstrate that guanylin suppression, GUCY2C silencing and epithelial dysfunction underlying intestinal tumorigenesis reflect the quantity of ingested calories, rather than the type of calories, or the endocrine, adipokine, and inflammatory milieu associated with obesity.
Figure 2. Guanylin expression is reversibly suppressed by ingested calories.
(A) High carbohydrate (HC) and HF diets suppressed guanylin mRNA in colon. (B) HC diet reduced guanylin and GUCY2C signaling, increasing epithelial dysfunction. (C) Mice fed a HC, compared to a Lean, diet are more sensitive to AOM-induced colon tumorigenesis. (D) HF diet reduced guanylin and GUCY2C signaling, increasing epithelial dysfunction, in mice resistant to diet-induced obesity (HF-R). (E) ob/ob on a calorie-restricted diet (10 Kcal/d per mouse) maintained guanylin expression and GUCY2C signaling, without epithelial dysfunction. (F) Diet effects on guanylin expression, GUCY2C signaling, and epithelial dysfunction were reversed by switching mice on a HF diet for 20 wks to a Lean diet for 4 wks (HF-Lean). Immunoblot results represent the mean ± SEM of 5 mice.
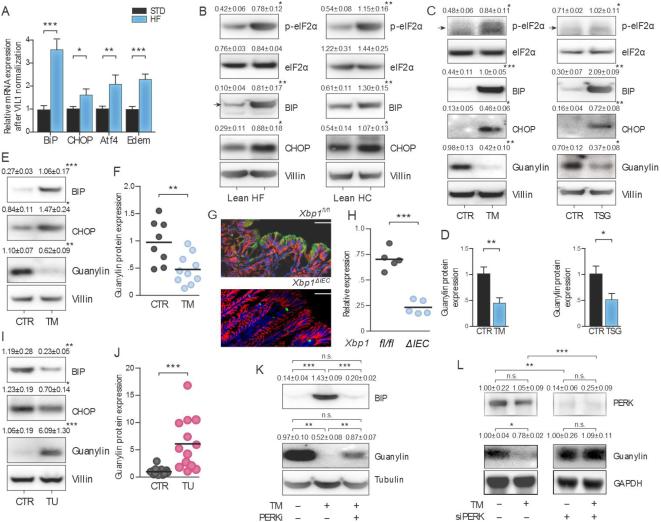
Ingesting excess calories induces endoplasmic reticulum (ER) stress associated with an unfolded protein response in extra-intestinal tissues (20, 21). Here, HF or HC diets induced colon ER stress in mice, increasing canonical mRNA and protein markers of the unfolded protein response (Fig. 3A-B)(21, 22). In that context, ER stress pharmacologically induced by tunicamycin or thapsigargin eliminated guanylin expression in Caco2 human colon cancer cells in vitro (Fig. 3C-D). Similarly, intraperitoneal (IP) tunicamycin induced ER stress associated with loss of guanylin expression in mouse colon (Fig. 3E-F). Further, ER stress genetically induced by eliminating expression of Xbp1 (10, 11), a key transcription factor mediating the unfolded protein response (21, 22), almost completely abolished colon guanylin expression (Fig. 3G-H). Conversely, blocking ER stress in mice on a HF diet by oral supplementation with the chemical chaperone taurodeoxycholic acid (TUDCA)(23) reconstituted colonic guanylin expression (Fig. 3I-J). Similarly, blocking the unfolded protein response by pharmacologic (Fig. 3K) or genetic (Fig. 3L) inhibition of PERK, an essential sensor of ER stress (22), restored guanylin expression suppressed by tunicamycin in vitro. These observations highlight the role of ER stress and the associated unfolded protein response induced by ingested calories as one mechanism suppressing the expression of guanylin in obesity.
Figure 3. ER stress mediates guanylin suppression by ingested calories.
(A) High calorie diets induced the transcription (A) and translation (B) of markers of ER stress in colon in C57BL/6 mice. (C-D) ER stress induced by 2.5 μg/ml tunicamycin (TM) or 500 nM thapsigargin (TSG) increased markers of ER stress and suppressed guanylin in Caco2 cells. (E-F) IP administration of 1 mg/kg TM to C57BL/6 mice increased markers of ER stress and suppressed guanylin expression in colon. (G-H) Colons from Xbp1ΔIEC mice exhibited reduced expression of guanylin (green) compared to wild type (Xpbfl/fl) mice [β-catenin (red)]. (I-J) TUDCA (150 mg/kg by oral gavage for 12 d) relieved ER stress and rescued guanylin expression in C57BL/6 mice on a HF diet. (K) Inhibition of PERK by GSK2606414 relieved ER stress and restored guanylin expression in Caco2 cells treated with TM. (L) PERK siRNA restored guanylin expression in HEK 293 cells treated with 1.0 μg/ml TM. In vitro results represent the mean ± SEM of three independent experiments performed in triplicate. In vivo results represent the mean ± SEM of 5 mice unless otherwise indicated.
The present observations suggest that colon tumorigenesis associated with obesity reflects suppression of guanylin expression which silences the GUCY2C tumor suppressor by reversible calorie-dependent induction of ER stress and the unfolded protein response in colon epithelial cells. This hypothesis was directly tested employing a genetic mouse model in which enforced guanylin expression is induced selectively in intestinal epithelial cells (Fig. 4A)(7). Induction of transgenic guanylin expression overcame endogenous guanylin suppression and GUCY2C silencing, reconstituting VASP phosphorylation and normalizing β-catenin levels in mice on a HF diet (Fig. 4B). Importantly, preventing the loss of guanylin expression and maintaining GUCY2C signaling (Fig. 4C) almost completely eliminated intestinal tumorigenesis associated with obesity induced by a HF diet (Fig. 4D).
Discussion
While essential molecular details linking energy balance and cancer in any tissue have remained elusive, the present study establishes a direct link between signaling pathways underlying colon tumorigenesis and mechanisms directly contributing to obesity. In that context, GUCY2C is a tumor suppressing receptor whose silencing through loss of the paracrine hormone guanylin universally contributes to epithelial dysfunction underlying the initiation of sporadic colorectal cancer (1, 2, 4, 6-8, 12, 13). Although loss of guanylin mRNA and protein uniformly characterizes the earliest stages of intestinal tumorigenesis (2, 4, 12), the precise molecular mechanisms modulating hormone expression remain undefined (1). Here, we reveal that one mechanism contributing to guanylin suppression is the induction of ER stress and the unfolded protein response (20-22). While a role for ER stress in intestinal tumorigenesis has been suggested (24), its role in regulating guanylyl cyclase signaling within, or outside, the intestine has not been recognized previously. The prevailing paradigm suggests that intestinal tumorigenesis related to obesity reflects dysregulation of the endocrine, adipokine, and inflammatory milieu characterizing the associated abnormal metabolic state (14-16). Surprisingly, the present study reveals that hormone suppression reflects ER stress induced by hyperphagia and excess ingested calories (20, 21), rather than the abnormal metabolic state produced by obesity. Indeed, ER stress and hormone suppression was induced by hypercaloric nutrition in the absence of obesity in mice on a high carbohydrate diet, and in obesity-resistant Balb/c (17) and C57BL/6 (18) mice. Conversely, caloric restriction even in the context of obesity, reconstituted guanylin expression in ob/ob (19) and C57BL/6 mice. These studies expand the established mechanistic model, adding calorie-induced ER stress to the constellation of processes contributing to tumorigenesis in obesity.
Mechanistic insights provided here offer unique therapeutic opportunities with immediate potential for clinical translation. Indeed, as highlighted in this study, caloric suppression of guanylin was reversible, even in the context of persistent obesity. Additionally, conditional expression of guanylin by intestinal epithelial cells that could not be suppressed by hyperphagia and ingestion of excess calories almost completely eliminated obesity-related colorectal cancer in mice. The challenges of lifestyle modification notwithstanding, these observations suggest that dietary modification in the form of caloric restriction to reconstitute guanylin expression and GUCY2C signaling may be an effective strategy to prevent colon cancer in obesity. Further, although guanylin is suppressed in obesity, the GUCY2C tumor suppressor is persistently expressed in apical membranes of intestinal epithelial cells, directly accessible to the lumenal compartment. These considerations suggest that colon cancer in obesity could be prevented by oral ligand supplementation, to replace hormone eliminated by calories and ER stress and maintain the GUCY2C tumor suppressor signaling axis. It is noteworthy that linaclotide, an oral GUCY2C ligand, received FDA approval for the treatment of constipation-type irritable bowel syndrome (25). Moreover, linaclotide recently entered clinical development through the NCI Division of Chemoprevention for the oral prophylaxis of colon cancer (ClinicalTrials.gov Identifier: NCT01950403). Beyond the colorectum, there is an established epidemiological association between obesity and cancer in many tissues (26). In that context, recent studies revealed an essential role for the GUCY2C signaling axis in maintaining the intestinal epithelial barrier, preventing systemic genotoxic insult associated with extra-intestinal tumorigenesis (9). It is tempting to speculate that caloric suppression of guanylin expression, silencing GUCY2C, disrupts the intestinal epithelial barrier, producing systemic genotoxic stress contributing to extra-intestinal tumorigenesis in obesity which also could be prevented by oral hormone supplementation.
Supplementary Material
Acknowledgments
Supported by grants from NIH (CA75123, CA95026, CA146033, CA56036, CA170533, DK088199), the Harvard Digestive Diseases Center (HDDC) DK034854 (R.S.B.), the PA Department of Health (SAP #4100059197, SAP #4100051723), and Targeted Diagnostic and Therapeutics, Inc. The PA Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. G.W.K. received the ASPET Graduate Award for Integrative Research in Pharmacology and the PhRMA Foundation Predoctoral Fellowship Award in Pharmacology. J.E.L. and F.C.-G. were supported by NIH T32 GM08562. J.E.L. received the ASCPT Young Investigator Award. F. C.-G. received the PhRMA Foundation Postdoctoral Fellowship Award in Clinical Pharmacology. S.A.W. is the Samuel MV Hamilton Professor of Thomas Jefferson University.
Footnotes
Conflict of Interest: SAW is the Chair of the Data Safety Monitoring Board for the Chart-1 Trial™ sponsored by Cardio3 Biosciences, and the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
Author Contributions:
JEL-Data acquisition, analysis and manuscript preparation.
FC-G, EB, GWK, AA, BS, JR-Data acquisition.
AES-Data analysis and manuscript preparation.
TZ, TMH-Analysis of guanylin expression and body mass index in patients.
MT, RSB-Analysis of guanylin expression in Xbp1fl/fl and XbpΔIEC mice.
SAW-Concept development, data analysis, manuscript preparation, funding.
References
- 1.Pitari GM, Li P, Lin JE, Zuzga D, Gibbons AV, Snook AE, et al. The paracrine hormone hypothesis of colorectal cancer. Clin Pharmacol Ther. 2007;82:441–7. doi: 10.1038/sj.clpt.6100325. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–72. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 3.Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, et al. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochemical & Biophysical Research Communications. 2000;273:225–30. doi: 10.1006/bbrc.2000.2917. [DOI] [PubMed] [Google Scholar]
- 4.Notterman DA, Alon U, Sierk AJ, Levine AJ. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer research. 2001;61:3124–30. [PubMed] [Google Scholar]
- 5.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer research. 2000;60:5151–7. [PubMed] [Google Scholar]
- 6.Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, et al. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138:241–54. doi: 10.1053/j.gastro.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, et al. Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci U S A. 2003;100:2695–9. doi: 10.1073/pnas.0434905100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PloS one. 2012;7:e31686. doi: 10.1371/journal.pone.0031686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–56. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Bock J, Martinez-Naves E, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–6. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, et al. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2328–37. doi: 10.1158/1055-9965.EPI-14-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons AV, Lin JE, Kim GW, Marszalowicz GP, Li P, Stoecker BA, et al. Intestinal GUCY2C prevents TGF-beta secretion coordinating desmoplasia and hyperproliferation in colorectal cancer. Cancer research. 2013;73:6654–66. doi: 10.1158/0008-5472.CAN-13-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujisawa T, Endo H, Tomimoto A, Sugiyama M, Takahashi H, Saito S, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–8. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–71. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 16.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res. 2007;9:R91. doi: 10.1186/bcr1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearnside JF, Dumas ME, Rothwell AR, Wilder SP, Cloarec O, Toye A, et al. Phylometabonomic patterns of adaptation to high fat diet feeding in inbred mice. PloS one. 2008;3:e1668. doi: 10.1371/journal.pone.0001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107:19320–5. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niederreiter L, Fritz TM, Adolph TE, Krismer AM, Offner FA, Tschurtschenthaler M, et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med. 2013;210:2041–56. doi: 10.1084/jem.20122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2013;365:527–36. doi: 10.1056/NEJMoa1010863. [DOI] [PubMed] [Google Scholar]
- 26.Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2014 doi: 10.1016/S1470-2045(14)71123-4. doi.org/10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.