Abstract
Diabetes is a complex disease affecting 29.1 million (9.3%) of US citizens[1]. Diabetes is a chronic illness that needs continual medical care and ongoing patient self-management, education, and support[2]. There is no cure for diabetes, requiring patients to conduct frequent self-monitoring of blood glucose and dosing of insulin in many cases. Evidence has shown that patients are more adherent to their diabetes management plan when they incorporate personal lifestyle choices[3]. To address the challenge of empowering patients to better manage their diabetes, we have developed a novel mobile application prototype, iDECIDE, that refines rapid-acting insulin dose calculations by incorporating two important patient variables in addition to carbohydrates consumed that are not currently a part of standard insulin dose calculation algorithms: exercise and alcohol intake[4], [5]. A retrospective analysis for the calibration and evaluation of iDECIDE is underway by comparing recommendations made by the application against insulin dosing recommendations made by insulin pumps.
Keywords: Diabetes mellitus, insulin dosing, clinical decision support systems, mobile application, disease self-management
Introduction
Patient-centered care is defined as health care that respects patients’ wants, needs and preferences, and supports patient desires to make decisions and participate in their own care[6]. Too often patients must adapt to pre-existing protocols and guidelines, rather than receiving services designed to focus on their individual needs and preferences[6]. Patient-centered decision support that translates evidence-based care into health care practice in ways that account for individual preferences and goals is needed.
Many patients with chronic conditions such as diabetes can benefit greatly from self-management[7]. Self-monitoring of blood glucose can be empowering for patients with diabetes, but tracking such data can be overwhelming[8]. Additionally, even patients well trained in diabetes self-management often fail to meet personal glycemic goals. Despite ongoing research to identify patient preferences, track treatments, and integrate patient data to provide personalized options, significant advances in the design and deployment of patient-centered decision aids are still to be made[9], [10].
Type 1 diabetes (T1D) is a chronic disease in which a person’s pancreas does not produce insulin, a hormone required to regulate carbohydrate and fat metabolism in the body. Type 2 diabetes (T2D) results from a relative insulin deficit and can be due to a diminished insulin effect or insufficient production to maintain normal blood glucose levels. T2D patients may need insulin injections, oral medications, non-insulin injectable medications, or various combinations of these to control hyperglycemia. Patients with T1D must manage their disease by using insulin injections deliverable through syringes, insulin pens, or insulin pumps. Contemporary insulin pumps utilize a rapid acting insulin analog and deliver continuous basal insulin. Additionally, insulin pumps have bolus calculators that calculate the units of insulin needed based on settings, food intake and active insulin time. Such bolus calculators, which are designed to cover mealtime glucose excursions, do not take into account patient preferences such as alcohol intake and exercise. Evidence shows that these personal preferences can have a significant short-term impact on glucose levels, which in turn affects insulin dosing[4], [5]. Our hypothesis is that by incorporating current evidence regarding the impact of exercise and alcohol intake on insulin dosage, we can further improve postprandial glucose levels for adult individuals with diabetes, thereby empowering them to make informed, evidence-based self-management decisions. We have designed and seek to evaluate a novel, evidence-based decision support tool, iDECIDE, which customizes and refines rapid-acting insulin dosing calculations by incorporating individual preferences for exercise and alcohol. The target population of iDECIDE are adult diabetes patients with T1D or T2D.
Why employ iDECIDE when there are hundreds of mobile applications that allow users to track carbohydrate intake, exercise, medication and insulin dosage? Most of the available mobile applications are not evidence-based[11], while iDECIDE is based on the most current medical evidence.
Methods
A literature search that included diabetes pathophysiology, treatment and management options was conducted. We identified insulin dosage calculations based on glycemic levels, carbohydrate intake, exercise, and alcohol consumption.
Next, we reviewed the literature on smartphone apps for diabetes self-management and apps for healthy eating, physical activity, and personal health and wellness [11], [12]. Based on the review there is a proliferation of apps that are not evidence-based or do not align with well-established behavior change theories.
Following our literature review, we met with an endocrinologist and diabetes care team to further understand diabetes and to discuss current clinical challenges that patients with diabetes encounter. We participated in a guided simulation training session with a diabetes nurse educator at the Mayo Clinic Arizona Simulation Center that included hands-on experience with insulin pumps and continuous glucose monitors. The training excluded review of existing smartphone apps for diabetes management, fitness or nutrition. Based on the trainings we created three prototypical patient cases to reflect the daily regimens and personal preferences encountered on a daily basis by diabetes patients. We learned that diabetes is not a “one size fits all” disease and that personal management requires special consideration for each patient.
We also reviewed existing insulin pump technologies commercially available in the US. State of the art insulin pumps compute mealtime insulin doses based on proprietary formulas that are approved by regulatory entities like the US Federal Food and Drug Administration (FDA). While alcohol intake and exercise can have an impact on blood glucose levels, no insulin pump takes into consideration alcohol and exercise to compute the insulin needed to correct for a meal. While insulin pumps provide bolus wizards to compute pre-meal insulin boluses, diabetes patients can manually compute pre-meal insulin bolus using an equation from Colin et al. (Equation 1) which takes into consideration important factors, except alcohol and exercise, for choosing the correct insulin dose[13].
Equation 1 for Standard Insulin Dosing
In Equation 1, the variable U represents units of insulin. The first fraction in the equation, “carbs/ICR”, calculates the relationship between the grams of carbohydrates (carbs) intended to be consumed covered by one (1) unit of insulin (ICR). ICR is calculated as 450/TDD, where Total Daily Dose of insulin (TDD) = body weight (lbs) × 0.23. The second fraction in the equation calculates the difference between the actual blood glucose level (cBG) and the target blood glucose level (tBG) and divides this difference by the Correction Factor (CF). The correction factor, also called insulin sensitivity factor (ISF) is defined as how much one (1) unit of rapid acting insulin lowers an individual’s blood glucose over the course of 2–4 hours during a fasting or pre-meal state. These correction doses can account for approximately 9% of the TDD by compensating for the deficits in basal rates or carbohydrate boluses. CF is calculated as (1700mg/dl)/TDD. The final segment of the equation subtracts the Insulin On Board (IOB) i.e. the theoretical amount of insulin remaining in the body after the last bolus dose.
The ADA states that regular physical activity is important for maintaining health and fitness for those diagnosed with diabetes. People with diabetes are advised to participate in at least 150 minutes of moderate-intensity physical activity per week. Regular exercise has been shown to improve blood glucose control, reduce cardiovascular risk factors, contribute to weight loss and improve well-being[14]. Evidence suggests that most forms of low-to-moderate intensity physical activity result in an increase of insulin sensitivity, which produces a drop in blood glucose levels. When glucose levels drop to abnormally low levels it is called hypoglycemia. Hypoglycemia can be averted by reducing the bolus insulin, increasing food intake, or a combination of both [5]. The evidence recommends ingestion of carbohydrates (e.g. snacks) before exercising to avoid hypoglycemic events.
Alcoholic beverages present an even more complex insulin dosing challenge. Depending on the specific content of the drink, alcoholic beverages can be a carbohydrate source and/or result in delayed hypoglycemia. It is difficult for patients to factor alcoholic drinks into their insulin dosing calculations. Also, they frequently are not aware that more than 2 alcoholic drinks can increase the probability of hypoglycemia a few hours after alcohol consumption[15].
We therefore propose a new insulin dosing equation (patent pending) that accounts for the intensity and duration of physical exercise as well as the alcohol load and related carbohydrates from alcoholic beverages. We have added to the standard equation (Equation 1) parameters to account for patient preferences for exercise and alcohol consumption. As we noted previously, insulin pump calcuators do not consider exercise when calculating insulin dosage, neither do they factor in the effects of alcohol on insulin sensitivity. iDECIDE incorporates these factors to suggest the dosage of rapid acting insulin and sets an alarm to recommend glucose level monitoring in certain circumstances related to alcohol consumption.
Results
Several prototyping platforms such as WireframeSketcher, POP and Proto.io™ were compared. Proto.io™ emerged as the best choice due to its drag-and-drop intuitive interface for building interfaces. Figure 1 depicts screenshots of the resulting iDECIDE prototype built with Proto.io™.
Figure 1.

Screenshots of the iDECIDE: a) the patient inputs 150 mg/dl as current blood glucose and that no carbs will be consumed, b) he also inputs that he will be performing 30 minutes of medium intensity exercise; then c) iDECIDE summarizes the input data, the parameters set up by the patient’s endocrinologist (e.g. target glucose), and the computed active insulin or IOB; finally d) iDECIDE generates recommendations (take 0 U of insulin and consume a snack with 10 grams of carbohydrates) and a breakdown of how the suggested insulin dosage was computed (0 U= 0 U to cover carbs + 1 U for correction factor −0.75 U of active insulin – 0.25 U for planned exercise).
To exemplify the use of iDECIDE, Figure 1 demonstrates a T2D patient using the app to decide if insulin should be taken before starting a 30 minute, medium-intensity bike ride. Based on the exercise plan and his current blood glucose level of 150 mg/dl, (cBG=150) iDECIDE recommends no insulin and suggests consuption of an additional 10 g of carbohydrates before starting the exercise to achieve a target glucose level of 130 mg/dl (tBG=130) and to avoid hypoglycemia. iDECIDE is using the ICR=10 and CF=20, based on input from the patient’s endocrinologist. The IOB=0.75 because the previous insulin dose was 1.25 units from 2 hours prior[16]. To account for exercise (Ex), 0.25 is subtracted off given the short duration (30 minutes) to be completed[17]. The suggested carbohydrates (10 g) were derived from the evidence regarding the patients weight of 150 pounds and the choice of performing 30 minutes of moderate exercise[5].
Figure 2 exemplifies another use case scneario showing how iDECIDE can set up an alarm if the patient chooses to consume more than 2 alcoholic drinks. The alarm is to remind the patient to monitor blood glucose levels to help avoid hypoglycemic events.
Figure 2.
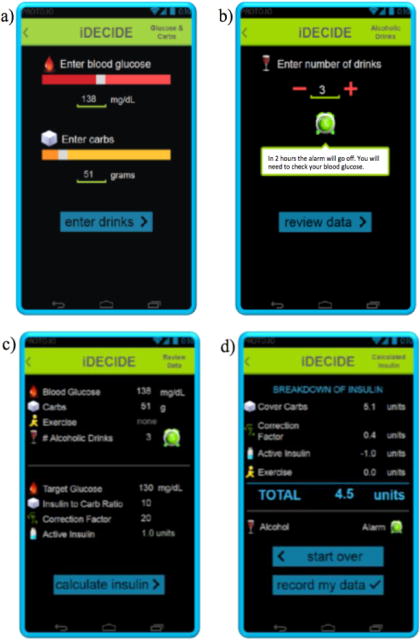
Screenshots of the iDECIDE prototype: a) the patient inputs 138 mg/dl as current blood glucose and that 51 grams of carbs will be consumed, b) he also inputs that he will be drinking 3 alcoholic drinks, to what iDECIDE reacts by setting an alarm to remind checking blood glucose levels to avoid hypoglycemia; then c) iDECIDE summarizes the input data, the parameters set up by the patient’s endocrinologist, the computed IOB and indicates that it has setup un alarm; finally d) iDECIDE generates recommendations (take 4.5 U of insulin and check the blood glucose levels in 2 hours when the alarm rings) and a breakdown of how the suggested insulin dosage was computed.
Both Figures 1 and 2 assume that the user enters information immediately before eating, drinking or exercise in order to compute the insulin bolus. It is not uncommon for diabetes patients to input data after they eat or drink to account for last-minute changes.
We incorporated feedback on this mobile application and on the iDECIDE evidenced-based insulin dosing equation from domain experts in clinical decision support systems and usability, as well as fellow biomedical informatics graduate students. The iDECIDE prototype displayed in Figures 1 and 2 resulted from these recommendations. Then, we deployed the resulting improved interfaces and functionalities as an Android app and we performed a usability study. We secured IRB approval from Arizona State University to recruit 5 students to participate in the study. Participants were given 7 tasks to complete after a 5 minute period of self-guided exploration of the tool. Afterwards they were given a usability survey to complete. A total of 7 usability issues were identified. The exploratory task resulted in the most issues, 5.8, with the final two tasks resulting in no reportable issues. This may suggest that users were able to learn to use the system over time.
The class diagram in Figure 3 shows the main classes (domain concepts) and relationships used for designing iDECIDE. When possible, the domain knowledge of iDECIDE was mapped into terminologies and thesaurus like the Current Procedural Terminology (CPT), the SNOMED Clinical Terms, the National Cancer Institute thesaurus (NCI) and RXNORM. For instance, the concept currentBG was mapped to the NCI with the code C0392201.The Diabetes Patient using iDECIDE takes daily multiple measurements of blood glucose (currentBG), which is a type of Endocrine Finding. We are also modeling that, for example, a patient can use iDECIDE to set up clinical goals (hasDesiredState) related to Target Glucose, Target Carbs, Target Exercise, and Target Alcohol. For instance, one goal could be to have no more than 2 alcoholic drinks per day during weekends. Every time the patient interacts with iDECIDE he is requested to input his daily preferences (hasPreferences) on Carbs Plan, Alcohol Plan, Exercise Plan and Insulin Plan. For example, the patient plans to have 3 alcoholic drinks and a dinner, which account for 51 grams of carbs. Based on the input, iDECIDE triggers recommendation messages to remind the patient that his goal was to consume less than 3 drinks, and can also remind the patient the ADA guidelines on alcohol consumption. The patient can decide to follow iDECIDE’s recommendations (makesRecommendations) or can decide to stick to the original plan, committing to a plan (hasCommitedTo). Patients with chronic illnesses, such as diabetes, frequently encounter Obstacles when trying to achieve goals or follow treatment recommendations; they are more likely to be successful if back up plans are identified in advance (hasBackUpState) and suggested to the patient when obstacles are encountered.
Figure 3.
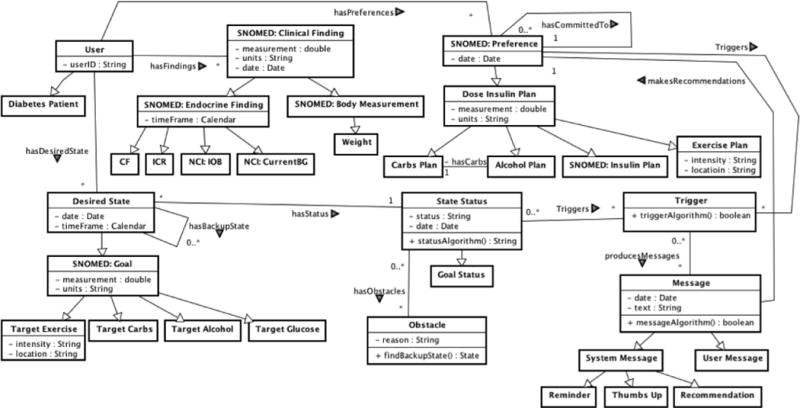
Class diagram depicting iDECIDE’s main classes and relationships
Also, we are incorporating patient Specific, Measurable, Attainable, Realistic and Timely (SMART) goals related to diabetes management, fitness and nutrition to attempt to further empower patients to achieve a healthier lifestyle. For instance, “walk more” is too general as a goal. Instead, “I will walk three times a week for 20 minutes” can be measured, is action oriented, can be chosen based on clinician assessment of the patient’s clinical state and self-motivation to change behavior, and has a time frame. Patients can understand SMART goals, and the achievement of SMART goals can be assessed and tracked by decision support systems. Therefore, we are currently working on decision mechanisms to provide suggestions to help patients achieve their chosen goals. For instance, in the example case-scenario described above, the patient has a fitness SMART goal of daily lunchtime exercise for 30 minutes at medium intensity. An obstacle arises for the patient: rain. The model incorporates a back-up plan for inclement weather, and suggests an exercise at home (e.g. a 30 minute WiiFit activity) that will achieve his goal. The proposed decision mechanism is inspired by the goal-based clinical decision support planning framework proposed and implemented by Grando, et al. [18], [19] to detect and recover from deviations to standard clinical care plans. In order to specify and reason on SMART goals we have built an ontology using the Ontology Web Language (OWL) using the Protégé tool. Figure 4 depicts a screenshot of the Protégé tool, demonstrating how we model a SMART goal for exercising and the encountered obstacle. The resulting ontology will support the decision rules that recommend behavioral changes, such as a specific, pre-identified home exercise option to use when there is inclement weather. Furthermore, using the ontology’s goal achievement status (full, partial and none) the achievement status will be automatically determined and tracked. In our example above, the suggested back-up plan, WiiFit, is considered equivalent to the initial outdoor plan, so our patient achieves the prescribed exercise goal.
Figure 4.
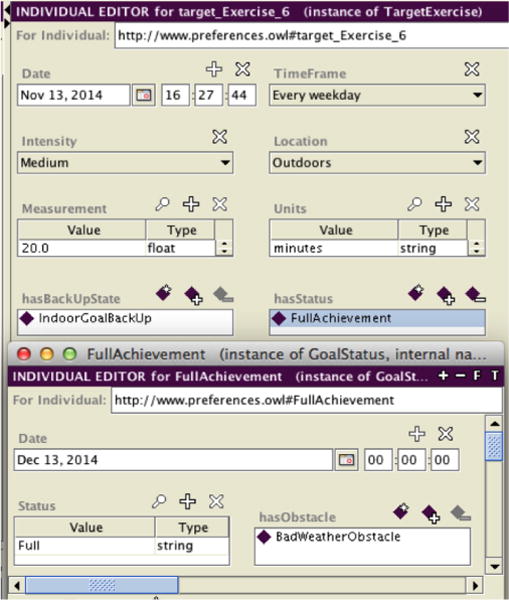
Screenshot from Protégé,, displaying the use case scenario of a patient who chooses the goal of walking outdoors every weekday for 30 minutes with medium intensity. In case of inclement weather, the back-up plan is to exercise at home. The status of the goal achievement (full, partial, none) can be monitored and tracked.
Discussion
Future plans include providing reminders, encouragement messages and alternatives to help patients achieve their SMART goals. For our previous example, iDECIDE could remind at lunchtime the patient to exercise with the personalized message, “Time for your lunchtime break exercise. It feels good to be in shape!” The patient can choose to answer the reminder selecting from a set of predefined options including “I cannot exercise today, the weather is bad.” Based on the patient’s feedback and back up plans iDECIDE can provide suggestions, I see … do you feel like trying some WiiFit tonight instead? I can send you a reminder if you want.”
We are recruiting twenty Arizona Mayo Clinic adult patients with T1D who currently use Medtronic™ insulin pumps to begin a retrospective calibration of the evidenced-based formula used by iDECIDE. We are limiting this study to users of Medtronic™ pumps to streamline the data analysis. Participants will be asked to keep records for one month, including alcohol intake and performed exercise (see Tables 1 and 2). The study was approved by the Mayo Clinic IRB.
Table 1.
My Diabetic Diary: Tracking alcohol intake
| DATE (m/d/y) | TIME (Hour: Min) | TYPE OF DRINK (Beer, wine, etc) | # OF DRINKS | MEASURE (small glass, pint, can, etc) | Did You Input Drink’s Carbs Into Insulin Pump? | |
|---|---|---|---|---|---|---|
| __/_/_ | __:__ | YES carbs: | NO | |||
Table 2.
My Diabetic Diary: Tracking exercise performed
| DATE (m/d/y) | TIME (Hour: Min) | INTENSITTY – check one | DURATION (minutes) | ||
|---|---|---|---|---|---|
| __/_/_ | __: __ | LIGHT | MODERATE | VIGOROUS | |
Initially we tried to reuse existing retrospective data repositories generated from insulin pumps and patients’ diabetes dairies, but the available repositories lacked information on alcohol consumption or they could not be shared due to human subject protection constraints. Following the completion of the study, all participants will provide the data generated during the study period by their insulin pumps and their daily records on alcohol intake and performed exercise. We will input the provided data into iDECIDE. As part of the projected retrospective calibration, domain experts will compare the insulin recommendations generated by iDECIDE against those generated by insulin pumps. We will consider that a recommendation from iDECIDE is as good as the one from the insulin pump when the recommendations are close in range and the postprandial glucose target is achieved. We will say that a recommendation from iDECIDE is better than the one from the insulin pump if iDECIDE recommends a higher (lower) dose and the postprandial reading is higher (lower) than target.
We are adopting a user-centered design approach for iDECIDE. Numerous otherwise well-conceived applications that target patients and health consumers have failed to achieve their desired effect because they have not involved users in the development process. Usability issues identified from the completed usability study will be considered to make appropriate design changes. We also plan to conduct another usability study with diabetes patients at the Arizona Mayo Clinic to further improve the interfaces and functionalities of the mobile app.
iDECIDE does not communicate with continuous glucose monitors or insulin pumps via wireless or Bluetooth technologies because insulin pump and glucose reader manufacturers do not share the application programming interfaces (APIs) that could facilitate such interactions. iDECIDE currently requires patients to manually input first their glucose reading, meal carbohydrates, alcohol intake and exercise. iDECIDE recommends an insulin dosage to be injected using an insulin pump or syringe. The current study utilizes patients on insulin pumps as a model to test and refine the iDECIDE methodology. Insulin pumps utilize only rapid acting insulin. Future refinement of the system to account for differences in insulin pharmacokinetics will be needed. There are situations where the actions of diabetes patients digress from what was previously entered into the pump’s bolus wizard. In these situations there is no technology to account for such behavior. Most patients who use insulin pumps are fairly disciplined and adhere to an established routine, in such cases iDECIDE would be a useful tool.
Conclusion
iDECIDE is a novel mobile application prototype that personalizes insulin dose calculations by incorporating two important patient variables that are not currently a part of standard insulin dose calculation algorithms: exercise and alcohol intake. Unlike the proprietary algorithms currently employed by insulin pump manufacturers to calculate insulin dose recommendations, iDECIDE is based on available clinical evidence that can be reviewed and discussed by the patient with the endocrinologist and care team. Also, iDECIDE will empower patients to improve disease management, fitness and nutrition by incorporating SMART goals. The app will help to track the achievement of SMART goals, but also provide reminders, encouragement messages and alternatives to help patients achieve their goals.
Acknowledgments
This research was supported by iDECIDE: Patient-centered decision support based on device data (1U54HL108460), funded by NIH National Library of Medicine. We would like to thank Jelena Mirkovic, PhD, for her advice on implementation options. We are grateful to Anita Murcko, MD, FACP for her clinical advice and to Marilyn Bailey, RN for providing training on insulin pumps.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation. National Diabetes Statistics Report, 2014. 2014 [Google Scholar]
- 2.American Diabetes Association. Standards of Medical Care in Diabetes—2011. Diabetes Care. 2011 Jan;34(Supplement_1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montori VM, Gafni A, Charles C. A shared treatment decision-making approach between patients with chronic conditions and their clinicians: the case of diabetes. Health Expect. 2006 Mar;9(1):25–36. doi: 10.1111/j.1369-7625.2006.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips PJ, Carapetis M, Stanton C. Alcohol and diabetes. [Online]. Available: http://www.medicinetoday.com.au/2008/june/handout/alcohol-and-diabetes#.VGPZTUtREm8. [Accessed: 12-Nov-2014]
- 5.Scheiner G, Sobel RJ, Smith DE, Pick AJ, Kruger D, King J, Green K. Insulin pump therapy: guidelines for successful outcomes. Diabetes Educ. 2009 Apr;35(Suppl 2):29S–41S. 42S–43S. doi: 10.1177/0145721709333493. quiz 28S. [DOI] [PubMed] [Google Scholar]
- 6.Hurtado MP, Swift EK, Corrigan JM. Envisioning the national health care quality report. National Academies Press; 2001. [PubMed] [Google Scholar]
- 7.Glasgow RE, Funnell MM, Bonomi AE, Davis C, Beckham V, Wagner EH. Self-management aspects of the improving chronic illness care breakthrough series: implementation with diabetes and heart failure teams. Ann Behav Med. 2002;24(2):80–87. doi: 10.1207/S15324796ABM2402_04. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, Entwistle VA, Fiset V, Holmes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner D. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;(3):CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. JAMA. 2008 Jul;300(4):436–438. doi: 10.1001/jama.300.4.436. [DOI] [PubMed] [Google Scholar]
- 10.Quill TE, Holloway RG. Evidence, preferences, recommendations–finding the right balance in patient care. N Engl J Med. 2012 May;366(18):1653–1655. doi: 10.1056/NEJMp1201535. [DOI] [PubMed] [Google Scholar]
- 11.Bastawrous A, Armstrong MJ. Mobile health use in low- and high-income countries: an overview of the peer-reviewed literature. J R Soc Med. 2013 Apr;106(4):130–142. doi: 10.1177/0141076812472620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West JH, Hall PC, Hanson CL, Barnes MD, Giraud-Carrier C, Barrett J. There’s an App for That: Content Analysis of Paid Health and Fitness Apps. Journal of Medical Internet Research. 2012 May;14(3):e72. doi: 10.2196/jmir.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colin IM, Paris I. Glucose Meters with Built-In Automated Bolus Calculator: Gadget or Real Value for Insulin-Treated Diabetic Patients? Diabetes Ther. 2013 Jun;4(1):1–11. doi: 10.1007/s13300-012-0017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Fitness. http://www.diabetes.org/food-and-fitness/fitness/ Accesed: 01/29/2014.
- 15.American Diabetes Association. Alcohol. http://www.diabetes.org/food-and-fitness/food/what-can-i-eat/making-healthy-food-choices/alcohol.html. Accesed: 11/25/2014.
- 16.Walsh J, Roberts R, Bailey T. Guidelines for optimal bolus calculator settings in adults. J Diabetes Sci Technol. 2011 Jan;5(1):129–135. doi: 10.1177/193229681100500118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolderman KM, American Diabetes Association . Putting your patients on the pump. Alexandria, Va.: American Diabetes Association; 2013. [Google Scholar]
- 18.Grando MA, Boxwala A. Division Biomedical Informatics Report. 2012. An ontology-based engine for enacting clinical guidelines in normal and abnormal scenarios. (DBMI-01-15-2012-001). [Google Scholar]


