Abstract
Microdialysis sampling probes were implanted into the subcutaneous space on the dorsal side of male Sprague Dawley rats to locally deliver dexamethasone-21-phosphate (Dex) with the aim of altering in vivo macrophage polarization. Macrophage polarization is of significant interest in the field of biomaterials since wound healing macrophages are a possible means to extend implant life as well as improve tissue remodeling to an implant. Quantitative analysis of CCL2 in collected dialysates, gene expression and immunohistochemistry performed on the tissue surrounding the microdialysis implant were used to evaluate if Dex polarized macrophages. Dex infusion down regulated IL-6 and CCL2 gene expression and decreased CCL2 concentrations in dialysates collected at the implant site. Dex appeared to have no significant effect on the gene regulation of CD163, a commonly used M2c macrophage surface marker; Arg2; and iNOS2. However, Dex infusion was effective at increasing the number of CD 163+ cells surrounding the implanted microdialysis probe. This work demonstrates the use of microdialysis sampling to deliver agents such as Dex to alter macrophage polarization in vivo while allowing the ability to collect cytokines in the surrounding microenvironment.
1. Introduction
Macrophages play key roles in both innate and adaptive immunity. Their importance to the field of biomaterials has been recognized for decades [1, 2]. Macrophages are known to play opposing roles such as inflammatory vs anti-inflammatory and tissue destruction vs tissue remodeling. The macrophage role is driven by micro-environmental chemical signals present within the extracellular matrix. These cues result in different macrophage polarization states [2–4], which comprise a continuum of macrophage phenotypes. In the case of bacterial infections, pro-inflammatory cytokines, such as Interferon-gamma (IFN-γ) and Tumor Necrosis Factor-alpha(TNF-α), are released inducing an M1-type macrophage. This macrophage phenotype is responsible for a Th1 type response resulting in a pro-inflammatory, phagocytic response that effectively clears pathogens. M1 macrophages are further characterized as producing high levels of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-12 and TNF-α [5–7].
At the other end of the polarization continuum are the M2 macrophages, which are subdivided into three classifications: M2a,b,c [6]. The M2a macrophages are induced by IL-4 and/or IL-13. This polarization state is thought to promote a Th2 type response, formation of foreign body giant cells (FBGC), as well as clearance of parasites [6]. M2a macrophages are characterized as producing collagen type VI, fibronectin, Transforming Growth Factor Beta (TGF-β) and Transforming Growth Factor Beta Induced (TGFβI) [8]. M2b macrophages are induced by FC gamma receptors and toll like receptor activation. M2b macrophages are characterized as producing high levels of the anti-inflammatory cytokine, IL-10, but also high levels of inflammatory cytokines such as IL-1β, IL-6 and TNF-α [2]. M2c macrophages are induced by IL-10 and glucocorticoid steroids and are characterized as producing high levels of IL-10 and low levels of inflammatory cytokines such as IL-1β, IL-6 and IL-12 [5]. M2c macrophages are thought to be anti-inflammatory, pro-wound healing, and pro-tissue remodeling cells.
A recent publication by Murray et. al. provides suggestions for a nomenclature change for macrophage polarization states [9]. These authors suggest that instead of using the M1, M2 nomenclature that the polarizing agent be used to identify the macrophage such as M(LPS) for LPS induced macrophages as a replacement for M1 or M(GC) for macrophages induced by glucocorticoids instead of M2c [9]. In accord with these newly suggested guidelines, this manuscript will use the new nomenclature for descriptions of specific subtypes of macrophages elicited in this study.
Chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte chemoattractant protein-1, is a 13kDa monomeric chemokine [10]. Chemokines are responsible for the recruitment of leukocytes such as monocytes, neutrophils, and lymphocytes, to a wound via chemokine gradients. CCL2 is known to be one of the primary chemokines responsible for the migration and infiltration of monocytes to a wound site [11]. CCL2 has been implicated in biomaterials contexts for attracting monocytes to the site of an implant [12]. Once at the wound site, monocytes differentiate to macrophages. Depending on the cytokine signals present at the wound site, these macrophages will then polarize to either a predominantly M1 or M2 state.
Glucocorticoid steroids are a class of steroids that bind to the glucocorticoid receptor which regulates inflammation and have been used extensively to treat inflammatory conditions [13, 14]. Dexamethasone, a synthetic glucocorticoid steroid, has been shown to regulate pro-inflammatory cytokines in two ways: 1) the glucocorticoid receptor interacts directly with glucocorticoid response elements in the promoter region of genes, thereby inhibiting gene expression [15, 16], 2) by interfering with transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells(NF-κB) and Activator Protein 1 (AP-1) [17–19]. Glucocorticoids have been shown to reduce the transcription of several genes including CCL2, IL-6, and TNF-α and inducible Nitric Oxide Synthase (iNOS) [20] as well as reducing protein levels of CCL2 in plasma and granuloma tissue [13, 21, 22], IL-6 in plasma and sponge exudate [23, 24], and TNF-α in sponge exudate [24]. In human monocytes, dexamethasone has been shown to induce a specific anti-inflammatory subtype marked by the up-regulation of many anti-inflammatory genes and the down regulation of pro-inflammatory genes [25].
Dexamethasone has been widely used in the field of biomaterials to reduce inflammatory responses to implanted materials [26, 27]. There are numerous examples of the use of dexamethasone to reduce inflammation in a biomaterials context [14, 28–31]. However, the endpoint analyses in these studies are frequently histological analysis of H&E and Masson’s Trichrome stains.
Microdialysis sampling is a widely-used, minimally-invasive, diffusion-based in vivo collection technique in which a probe, with a defined molecular weight cut-off (MWCO) semi-permeable membrane, is surgically implanted allowing in situ collection of many different solutes [32]. An isotonic fluid with a pH and ionic strength matching the extracellular space (ECS) is perfused through the membrane lumen in contact with the extracellular fluid allowing free solute exchange and collection of solutes. Since asolute concentration gradient exits between the perfusion fluid and the ECS, solutes diffuse into the membrane lumen and the resulting dialysate is collected and quantified [33]. This allows microdialysis to be used to simultaneously collect analytes from the ECS as well as deliver modulators to the ECS provided they are smaller than the MWCO of the membrane [34]. Dexamethasone-21-phosphate is a water-soluble derivative of the hydrophobic dexamethasone that is converted to dexamethasone in vivo by esterases [35].
Macrophage polarization in the context of biomaterials has been identified as an important process to consider when developing materials that can integrate with the host and provide improved outcomes [36]. Much effort has been focused on reducing the FBR to implanted sensors. Thus, having means to control this outcome via macrophage polarization that provides acceptable outcomes would be highly beneficial to many biomaterials applications. While there has been significant work on macrophage polarization in vitro, there are relatively few in vivo reports. Knowing that M2c macrophages are pro-wound healing and pro-tissue remodeling, it has been postulated that if modulators can be used to shift macrophages to a predominately M2c state, the longevity of the implant would be expected to increase.
In this work, microdialysis sampling probes have been used to locally deliver dexamethasone-21-phosphate at an implant site to alter the cytokine environment toward polarizing macrophages to their M(GC) state. To assess if cytokine responses were altered from the dexamethasone treatment, we quantified CCL2 protein levels in dialysates and performed gene expression studies combined with immunohistochemistry of the tissue surrounding the microdialysis probe implant.
2. Materials and Methods
2.1 Chemicals
The following chemicals were used in this study: Anti-CD68 Antibody (Santa Cruz Biotechnology, Inc., Dallas, TX); Anti-CD163 Antibody (Santa Cruz Biotechnology, Inc., Dallas, TX); Apex™ Antibody Labeling Kits (Alexa Fluor 488 and Alexa Fluor 647) (Life Technologies, Carlsbad, CA); BD OptEIA™ Rat MCP-1 ELISA Set (BD Biosciences, San Jose, CA); bovine serum albumin (BSA) (Rockland Immunochemicals, Gilbertsville, PA); chloroform (MP Biomedicals LLC, Solon, OH); dexamethasone-21-phosphate disodium salt (Dex) (Sigma Aldrich, St Louis, MO); Dextran-500 (Sigma Aldrich, St Louis, MO); ethylene oxide (Anderson Sterilizers, Inc, Haw River, NC); Halt Protease Inhibitor (Pierce, Rockford, IL); Hoechst 34580 (Sigma Aldrich, St Louis, MO); HPLC grade water (Fisher Scientific, Waltham, MA); isoflurane (Abbott Laboratories, North Chicago, IL); Optimal Cutting Temperature solution (Sakura® Finetek, Torrance, CA); povidone-iodine (Professional Disposables International Inc, Orange burge, NY); Proteinase K (Qiagen, Venlo, Limburg); RNAlater (Life Technologies, Carlsbad, CA); Trizol (Life Technologies, Carlsbad, CA); Taqman® Gene Expression Assays (IL-6, CCL2, TGFβ-1, TNF-α, CD163, Arg2, iNOS2, and Taf9b) (Life Technologies, Carlsbad, CA) and Vetbond™ (3M, St Paul, MN). Ringer’s solution contained 147mM NaCl, 4.6mM KCl, 2.3 mM CaCl2, pH 7.4 and was prepared in HPLC-grade water. All other chemicals were reagent-grade or better.
2.2 Microdialysis Procedure
All microdialysis sampling was performed using CMA 20 microdialysis probes which consist of a 10 mm long 100 kDa molecular weight cut-off (MWCO) membrane (Harvard Apparatus, Holliston, MA). Prior to implantation all probes were ethylene oxide sterilized (Anderson Sterlizers, Inc, Haw River, NC). The probes were infused using a BAS Bee microdialysis pump (Bioanalytical Systems Inc, West Lafayette, IN) using 1 mL sterile syringes (BD Biosciences, San Jose, CA) with a 23g blunt tip (VWR International, Radnor, PA). Perfusion fluids were autoclaved and filter sterilized prior to use. Post microdialysis probe implantation, animals were placed in a CMA 120 freely moving collection system (CMA Microdialysis, Solna, Sweden).1
2.3 Surgical Procedure
For all experiments, male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN) in a weight range of 325–360g were used. Prior to surgical procedures all animals were kept in a temperature controlled environment at 72°F. Animals were allowed access to food and water ad libitum. All experiments were approved by the University of Arkansas Institutional Animal Care and Usage Committee (IACUC) and conformed to the NIH standards for the ethical treatment of animals.
Animals were anesthetized in an induction chamber using 5% isoflurane in 0.8 L/min O2. The dorsal side was then shaved and the animals were maintained on a nose cone using 3% isoflurane in 0.5 L/min O2. Body temperature was maintained using a CMA 150 temperature controller (CMA Microdialysis, Solna, Sweden). Aseptic technique was used for surgical procedures and all tools were autoclaved prior to use. Two microdialysis probes (Harvard Apparatus, Holliston, MA) were then implanted in the subcutaneous space on the dorsal side. Probes were placed on either side of the spine with ~1 in of space between the probes. Implantation was performed by first making a '⊥' shaped incision at the posterior end of the animal and a '−' shaped incision at the anterior end of the animal, near the nape of the neck. A sterile straw was then passed subcutaneously from the posterior to anterior incision. The inlet and outlet tubes of the microdialysis probe were then passed through the straw from the posterior to anterior end. A needle was then used to place the introducer at the posterior incision. The needle was then removed leaving behind the introducer. The microdialysis probe was then placed in the introducer and the inlet and outlet lines were pulled tight. The straw was then pulled out of the subcutaneous space from the anterior incision and the introducer was removed. The posterior incision was then closed using Vetbond™. Post collection, the animal was anesthetized and the inlet and outlet tubing was placed in a subcutaneous pocket created at the anterior incision site which was then closed using sterile Reflex wound clips (Fine Science Tools, Foster City, CA) and animals were returned to housing. On collection days where no surgery was needed, animals were anesthetized, surgical staples were removed, the incision was swabbed with alcohol, and lines were removed.
2.4 Collection Procedure
Two probes were implanted into the dorsal subcutaneous space, in each rat (n=8) with one probe serving as the control and the other as the treatment. The control perfusion fluid consisted of Ringer's + 0.1% BSA + 4% Dextran-500 while the treatment perfusion fluid contained an added 20 µg/mL Dex. Dextran-500 was used as an osmotic agent to prevent fluid loss through the high molecular weight cut off microdialysis membrane [37] and bovine serum albumin (BSA) was used to reduce non-specific binding to the microdialysis plastic materials [38, 39]. Prior to implantation, control and treatment probes were flushed with Ringer's or Ringer's + 20 µg/mL Dex, respectively. Post probe implantation, animals were moved to a CMA 120 freely moving animal system. An initial flush was then performed using the perfusion fluids which started at 3 µL/min and was reduced to 1.0 µL/min in 0.5 µL/min increments over a 25 min period. Infusions were then performed in 1 hour increments for 6 hours. All collection vials contained Halt Protease Inhibitor and were immediately placed in ice once collection was complete. A final flush was performed following the 6th hour of collection for 30 mins at 3 µL/min using Ringer's or Ringer's + 20 µg/mL Dex for the control and treatment, respectively. Following the flush, the animal was anesthetized and the lines were placed in a subcutaneous pocket at the anterior incision. The animal was then returned to housing. This procedure was performed every day for 3 days. At the end of the 3rd day the animal was euthanized and the probes and tissue surrounding the probes were harvested as previously stated [40]. In some cases, the tissue surrounding the probe (~1–2 mm) was removed from the probe and stored in RNAlater for PCR analysis. Alternatively for image analysis, tissue with the implanted probe was placed in optimal cutting temperature (OCT) solution and flash frozen using liquid nitrogen for immunohistochemical analysis.
2.5 qRT-PCR Procedure
Tissue was harvested from around the membrane portion of the microdialysis probe and placed in RNAlater and stored on ice. Once all tissue was harvested, it was stored at 4°C until RNA was extracted. RNA was extracted using the Trizol method and was purified using an RNeasy Mini kit (Qiagen, Venlo, Limburg) per the manufacturer's instructions. Prior to conversion of RNA to cDNA, the integrity of the RNA was confirmed by gel electrophoresis looking at the 18s and 28s band ratios. RNA was then converted to cDNA using a high capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, CA), per the manufacturer's instructions. Taqman gene expression assays (Life Technologies, Carlsbad, CA) were then used to determine the relative gene expression ratios via a 7500 Real Time PCR Instrument (Life Technologies, Carlsbad, CA). Data were analyzed using REST Gene Quantification Software and Transcription initiation factor TFIID subunit 9B (Taf9b) was used as a control gene.
2.6 Immunohistochemical Staining Procedure
Primary antibodies specific for Cluster of Differentiation 68 (CD68) and Cluster of Differentiation 163 (CD163) (Santa Cruz Biotechnology, Dallas, TX) were used to stain macrophages and M(GC) macrophages, respectively. CD68 was conjugated with Alexa Fluor 488 and CD163 was conjugated with Alexa Fluor 647 using APEX Antibody Labeling Kits (Life Technologies, Carlsbad, CA). Tissue sections were cut at a thickness of ~5µm via a Leica CM3050 S cryostat (Leica Microsystems, Wetzlar, Germany) and mounted on microscope slides. Tissue was then fixed in methanol at −20°C for 20 mins. Tissue sections were then encircled with a hydrophobic pen and incubated in blocking solution for 30 mins at room temperature in a humidified chamber. Once blocked, slides were washed 4 times for 5 mins per wash in phosphate buffered saline. Wash solution was then wicked away using a Kimwipe from the tissue and tissue was incubated with CD68 (1:125) and CD163 (1:50) antibodies overnight at 4°C in a humidified chamber. Slides were then washed 4 times for 5 mins per wash and wash solution was wicked away from the tissue sections. A nuclear counterstain (Hoescht 34580) was then applied to the tissue sections and allowed to incubate in the dark for 12 minutes. Slides were then washed 3 times for 5 mins per wash and wash solution was wicked away from the tissue sections. VECTASHIELD® (Vector Laboratories, Burlingame, CA) was then applied and cover slips were sealed. Images were then visualized using a Leica TCS SP5 II confocal microscope (Leica Microsystems, Wetzlar, Germany). Manual cell counts were performed on cells staining positive for CD68 as well as cells staining positive for both CD68 and CD163. From this, the percentage of cells staining positive for CD68 and CD163 was determined.
Isotype controls were stained in the same manner as the experimental tissue sections. Briefly, Alexa Fluor (AF) 488 and Alexa Fluor 647 were both conjugated to mouse IgG1, kappa monoclonal [MOPC-21] isotype control (Abcam Cambridge, England) using the respective Apex Antibody Labeling Kits. The isotype control is a monoclonal antibody with an unknown specificity which has been tested on fixed rat tissue. Sections were cut to ~5 µm sections and fixed in methanol for 20 mins at −20°C. Sections were then circled with a hydrophobic pen and blocked for 30 mins in a humidity chamber. Blocking solution was then washed away and sections were incubated overnight at 4°C with isotype controls for both AF 488 and AF 647. Slides were then washed, sections covered in VECTASHIELD®, coverslips placed, and sealed.
2.7 Histological Analyses
Tissue was embedded in OCT solution and stored at −80°C. Tissue sections were cut at a thickness of ~5µm and mounted on microscope slides. Tissue was fixed in 10% neutral buffered formalin. Tissue sections were stained using standard protocol for hematoxylin and eosin (H&E) and Masson's Trichrome. Sections were imaged using a Zeiss Axioskop II plus microscope (Carl Zeiss Inc., Thornwood, NY) with Cannon EOS Digital Software for Rebel T2i camera.
2.8 CCL2 Quantification in Microdialysis Dialysates
Dialysates were collected from both the control and treatment probes once per hour over a 6 hour period plus the initial 25 min flush. CCL2 concentrations in the dialysates were determined using a BD OptEIA™ rat MCP-1 ELISA set (BD Biosciences, San Jose, CA) per the manufacturer's protocol with the exception that 60 µL of sample were loaded and the remaining reagents were adjusted accordingly. Briefly, a 96 well plate was blocked for 1 hour, standards of known concentration along with samples were then loaded and incubated for 2 hours, samples were incubated with enzyme working solution for 1 hour, TMB substrate solution for 30 mins in the dark, and then the reaction was stopped by adding Stop Solution. The absorbance was then read at 450 nm with a 570 nm reference on a Tecan Infinite M200 (Tecan, Maennedorf, Switzerland).
2.9 Statistical Analysis
Kruskal-Wallis ANOVA was performed with a Bonferroni post-hoc test to determine significance, with p≤0.05 being considered statistically significant. A two sample t-test was used to determine if there was a significant difference in the percent of M (GC) cells in treatment vs control tissue. Origin 8.6 statistical software was used.
3. Results
3.1 qRT-PCR
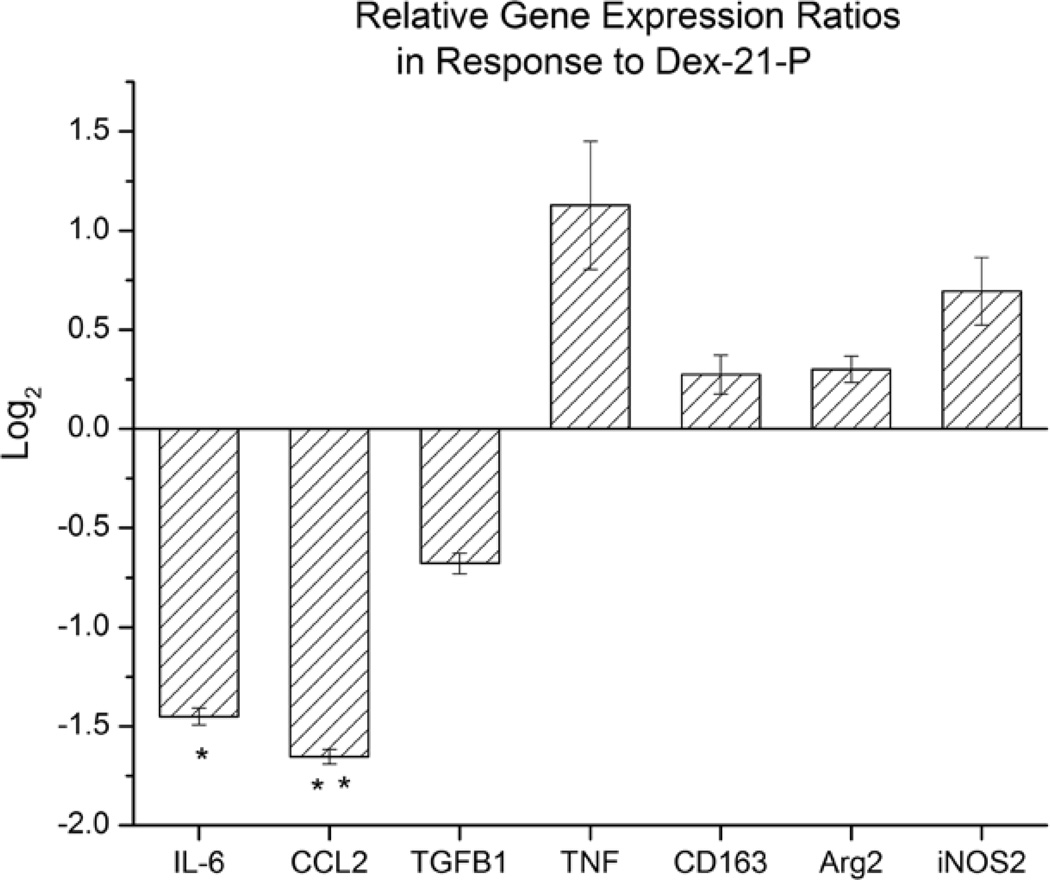
Transcription levels of seven different genes (Arginase (Arg2), CCL2, CD163, IL-6, iNOS2, TGF-β, and TNF-α) were analyzed using qRT-PCR from the extracted tissue in which the microdialysis probe was implanted (Figure 1). CCL2, IL-6, TGF-β, and TNF-α were chosen as markers to determine if Dex had any effect on the cytokine environment which would be expected in the FBR. Both IL-6 and CCL2 transcript levels were found to be significantly reduced, 2.7 fold (p≤0.01) and 3.4 fold (p≤0.001) respectively, in response to the Dex treatment as compared to controls. The transcription levels between TNF-α and TGF-β showed no significant difference between Dex treatments and controls. CD163, Arg2, andiNOS2 were chosen to investigate the effects of Dex on known differential markers for macrophage polarization state at the implant site. Transcript levels of CD163, Arg2, or iNOS2 were unchanged in response to Dex treatment as compared to controls.
Figure 1.
Relative gene expression ratios from excised tissue in response to dexamethasone-21-phosphate (20 µg/mL) infused as compared to controls (n=3). Error bars represent the SEM with *p≤0.01 **p≤0.001
3.2 CCL2 Quantification
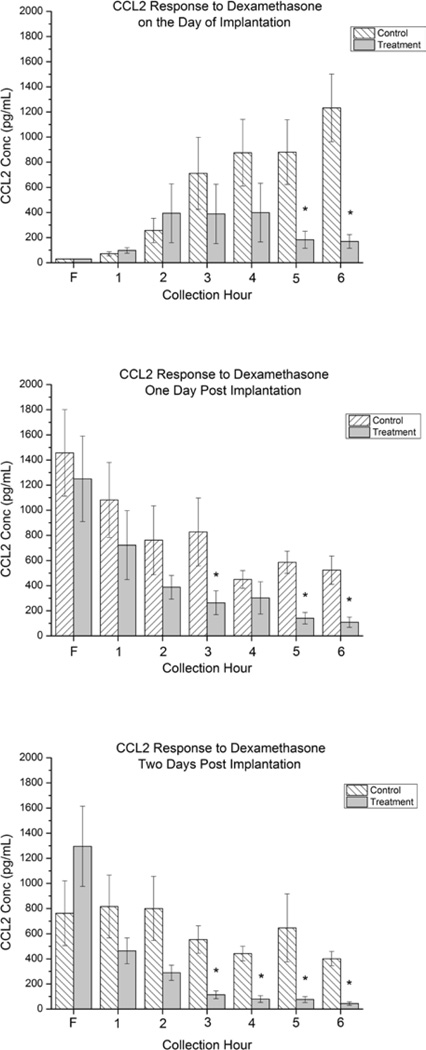
Figure 2 shows the CCL2 concentrations in dialysates from control and treatment probes. On the day of implantation (Fig 2a) CCL2 concentrations from the control probes steadily rose over the 6 hour collection period ranging from ~100 pg/mL in the first hour of collection to ~1300 pg/mL by the 6th hour. In dialysates collected from the Dex-treatment probes, CCL2 concentrations were found to be ~100 pg/mL during the 1st hour, and peaked during hours 2 through 4 to a level of approximately 400 pg/mL. During hours 5 and 6, CCL2 concentrations decreased to ~200 pg/mL. There was no significant difference in CCL2 levels found in control and treatment dialysates in the flush or hours 1 through 4. In hours 5 and 6, CCL2 levels in the treatment were found to be significantly lower (~200 pg/mL) than in control (~ 1200 pg/mL).
Figure 2.
Bar graphs showing the concentration of CCL2 collected from the control and treatment probes perfused at 1 µL/min for each hour on the day of implantation (Top, 2a), one day post implantation (Middle, 2b), and 2 days post implantation (Bottom, 2c). Error bars represent the SEM with *p≤0.05 for n=8 (Top) and n=6 (Middle, Bottom) animals. F represents the initial flush period.
One day post implantation (Fig 2b), CCL2 concentrations collected from control probes ranged from 1100 pg/mL to 600 pg/mL during the collection period (1 to 6 hrs), decreasing from 1–3 and stabilizing over hours 4–6 but showing no statistical difference over the collection period. CCL2 concentrations collected from treatment probes decreased from ~750 to ~300 pg/mL during hours 1 through 3, remained constant in hour 4 and decreased in both hours 5 and 6 to a level similar to the first hour of collection on the day of implantation. No significant difference was seen between CCL2 concentrations collected from control and treatment probes during the flush or hours 1,2, and 4, but CCL2 concentrations were found to be significantly lower in the treatment probes as compared to the control probesin hours 3,5, and 6.
Two days post implantation (Fig 2c), CCL2 concentrations from control probes ranged from ~750 pg/mL to ~400 pg/mL and seemed to remain stable over the collection period showing no statistically significant difference. CCL2 concentrations from treatment probes were ~450 pg/mL during the 1st hour of collection and steadily decreased during the 6 hour collection period. During hours 3 through 6, CCL2 concentrations in dialysates collected from treatment probes were significantly lower than CCL2 concentrations in dialysates collected from control probes. Furthermore, by the 6th hour of collection, two days post implantation, Dex reduced CCL2 concentrations from the treatment probes to the level that was seen during the initial flush on the day of implantation.
3.3 Immunohistochemistry
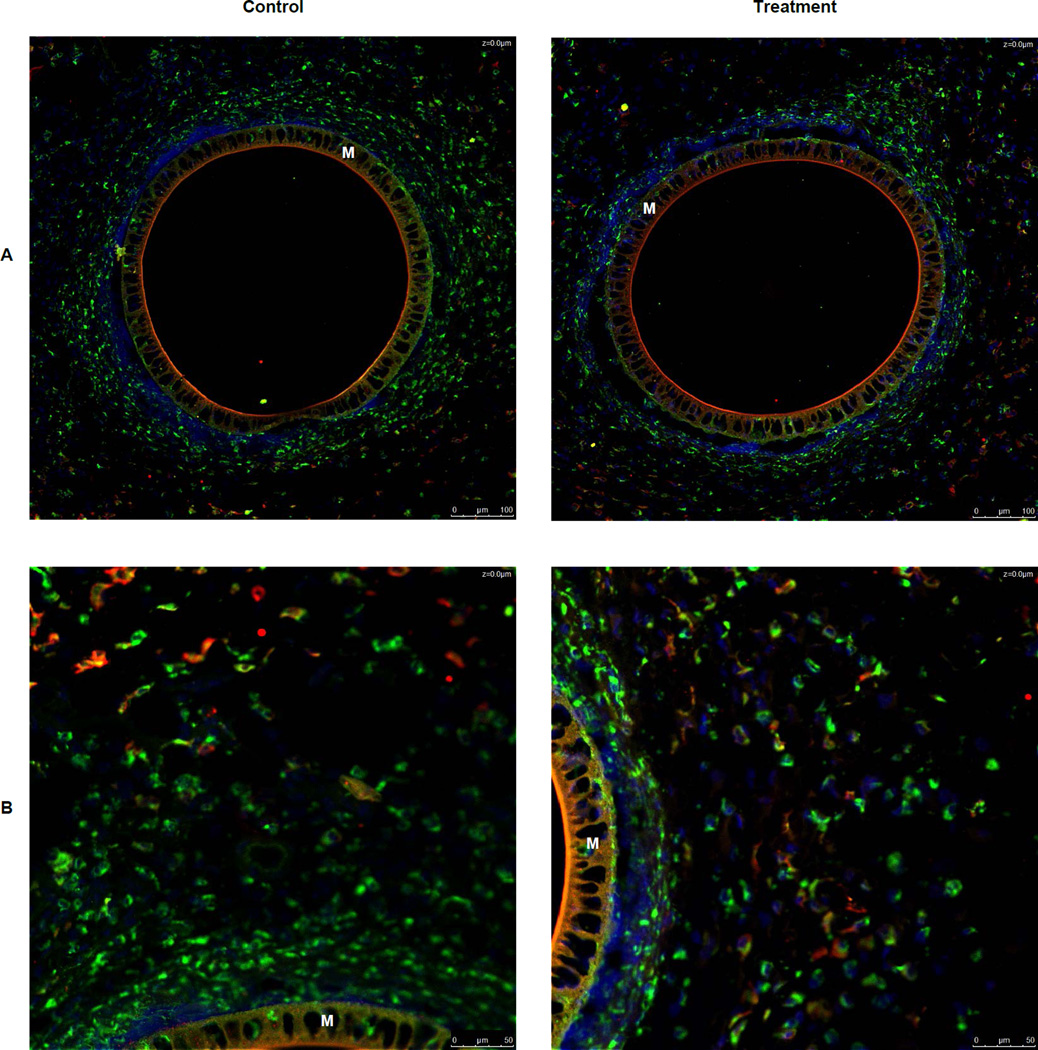
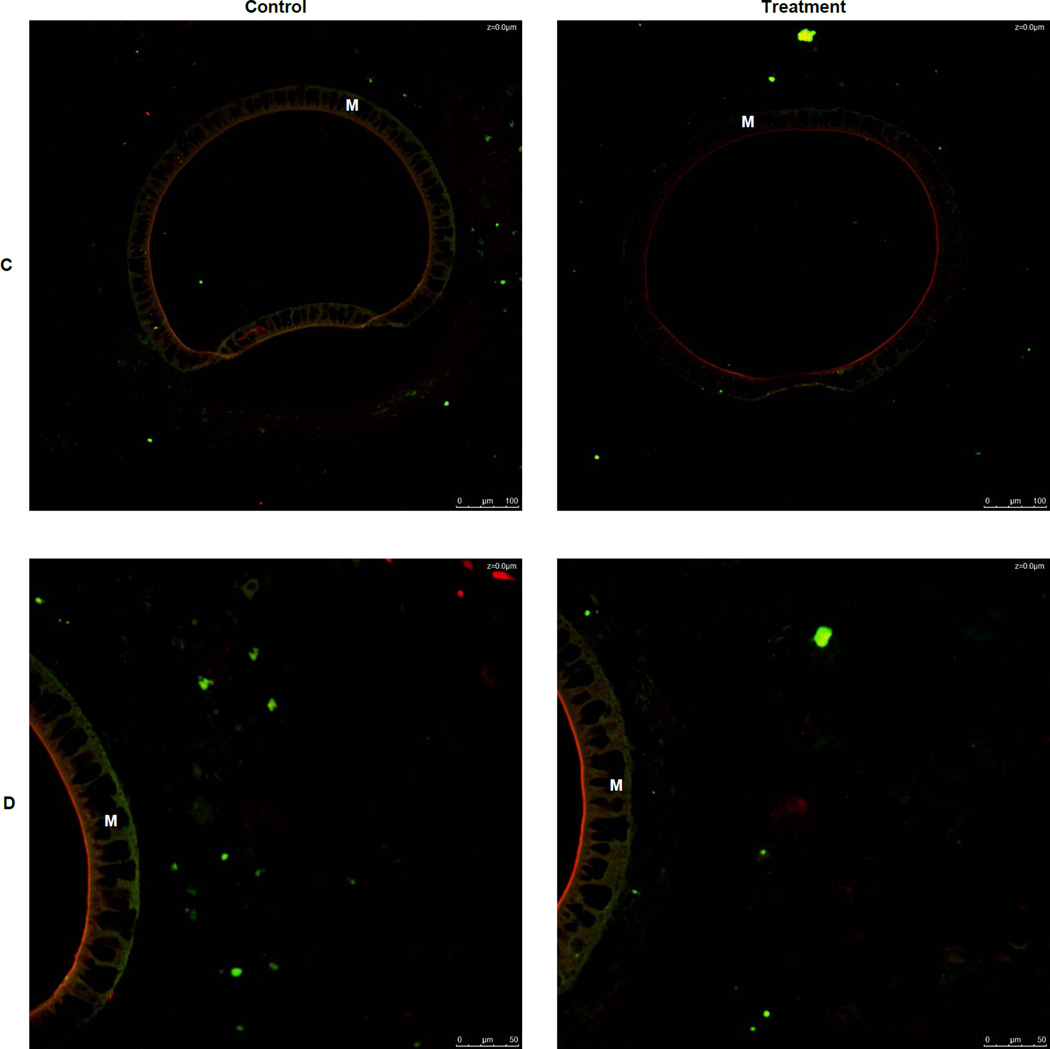
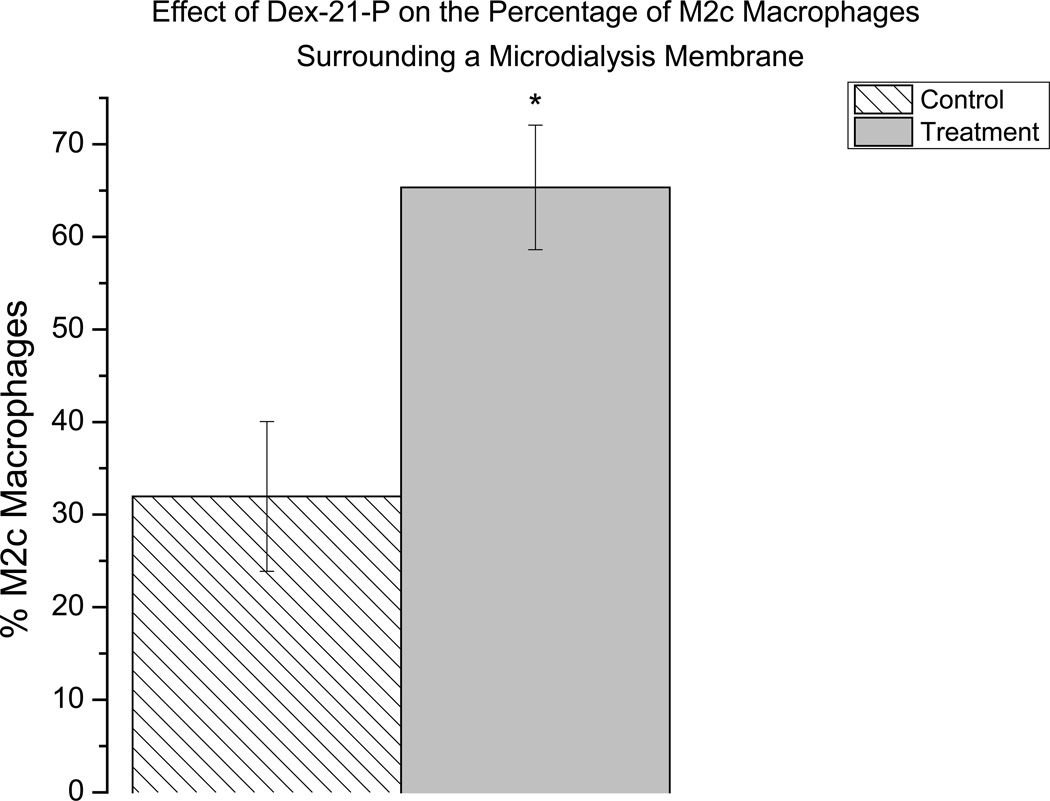
Immunohistochemistry was used to identify macrophages in the tissue surrounding an explanted microdialysis probe using a CD68 antibody. To determine if Dex caused macrophages to convert to the M(GC) polarization state and the formerly called M2c state, a CD163 antibody was used to immunohistochemically stain these types of macrophages. At lower magnifications (20×) there appeared to be no CD 163+ stained cells immediately surrounding the microdialysis probe (Figure 3A). At the lower magnification, pockets of CD 163+ cells were seen more distal (350–500 µm) to the microdialysis probe in both control and treatment tissue with these cells appearing to be more distal to the control probe (Figure 3A). However, at higher magnification (40×) it was found that CD 163+ cells are present in both control and treatment tissue immediately surrounding the microdialysis probe (Figure 3B). Further, in the higher magnification images, it seen that there is a greater amount of CD163+ cells surrounding the treatment probe than the control (Figure 3B). The percentage CD163+cells found in the 40× images were calculated for both treatment and control. The percentage of CD163+ cells found was significantly greater in the treatment tissue than in the control tissue (Figure 4).
Figure 3.
Immunohistochemical staining for CD 68 (green), CD 163 (red), and nuclei (blue) in tissue surrounding a microdialysis probe. Overlapping colors represent M2c macrophages. A) Images of both control and treatment tissue surrounding a microdialysis probe membrane. 20× Magnification B) Images of both control and treatment tissue surrounding a microdialysis probe membrane. 40× Magnification C) Images of isotype controls at 20× Magnification D) Images of isotype controls at 40× Magnification M indicates the microdialysis probe membrane Scale bars in lower right of each image represent 100 µm
Figure 4.
Graphical representation of the percent of CD 163+ (M2c) cells found in both control and treatment tissue. Error bars represent the SEM, n=5 where n is equal to the number of measurements *p≤0.05
3.4 Histological Analyses
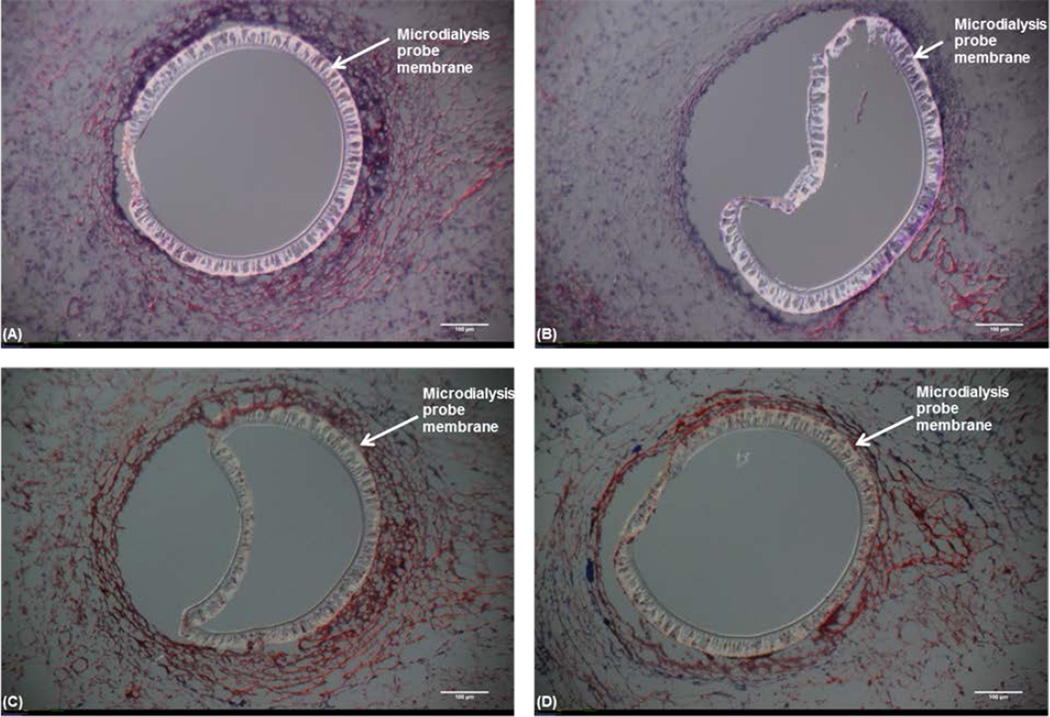
Histology (H&E and Masson’s Trichrome) was performed to determine the effects of Dex on the cellular density and collagen amounts surrounding the probe (Figure 5). The Dex-treated tissue was more fragile than control tissue resulting in unusable treatment sections. The Dex-treated tissue fragility may be a result of reduced cellular density. Tissues with Dex treatment are often difficult to process since the tissue surrounding the implant is not well integrated. In the cases where sections were obtained, it is clear the Dex treatment has reduced the number of infiltrating cells.
Figure 5.
Hematoxylin and Eosin (H&E) and Masson’s Trichrome staining of the tissue surrounding the microdialysis probe implanted into the subcutaneous space.Top: (H&E) stained tissue (nuclei – blue, eosinophilic structures - red, basophilic structures – purple and erythrocytes – bright red) (A) Control microdialysis probe. (B) Treatment microdialysis probe (20 µg/mL Dex). Bottom: Masson's Trichrome stained tissue (nuclei – dark brown/black, cytoplasm -light red/pink and collagen – blue). (C) Control microdialysis probe. (D) Treatment microdialysis probe (20 µg/mL Dex). Images are 10× magnification with 100 µm scale bar and are representative of tissue where tissue sections were obtainable.
4. Discussion
The implantation of biomaterials is known to lead to the FBR and eventual fibrotic encapsulation. While the encapsulation of some biomaterials is not problematic, other biomaterials suffer from loss of reliable function due to encapsulation [41]. In the case of degradable biomaterials, the formation of scar tissue at the implant site as opposed to wound resolution is considered problematic. Until recently, these tissue outcomes of biomaterial implantation were viewed as detrimental, yet unavoidable.
Macrophage polarization has been described as playing a potential key role in the outcome of biomaterial implantation [36]. Controlling macrophage polarization states at an implant site may lead to improved tissue remodeling and reduced fibrosis and has been the topic of several recent review articles [42–44]. However, the available literature describing macrophage polarization as an observed outcome in biomaterials studies are limited. Most of the macrophage polarization literature is derived from the field of tumor biology. One recent work has used genetic engineering of IL-10 to different types of macrophages in cell culture to promote macrophage polarization [45]. The same group has used genetic engineering techniques in tissue to alter leukocyte infiltration in vivo [46].
Use of microdialysis sampling procedures allows both the localized delivery of modulators to the tissue space and a concomitant collection of bioactive solutes that may be affected by the modulator. This characteristic makes microdialysis sampling a useful model technique in biomaterials science for investigating responses to locally-delivered modulators and their effects on the local tissue biochemistry. Solutes collected by the microdialysis probe reflect the localized chemical milieu surrounding the implanted microdialysis probe. Combining bioactive chemical content analysis with tissue analysis that includes judicious choices regarding gene transcription coupled with immunohistochemical staining of defined macrophage surface markers provides a comprehensive approach for elucidating the molecular mechanisms of macrophage polarization in vivo.
Dexamethasone was used in this study for several reasons. First, glucocorticoids are known to dampen inflammation and Dex has been shown previously to reduce fibrosis at an implant site and has been widely applied in biomaterials studies [26, 47]. Additionally, Dex is known to induce macrophages to an M(GC) state which was our desired goal in these experimental studies. Dex likely reduces fibrosis at implants due in part to Dex's ability to decrease pro-inflammatory cytokines at both the gene and protein level [48, 49]. It is interesting to note that most of the work with biomaterials and Dex for in vivo studies have relied on histological outcomes and have not elucidated alterations in molecular mechanisms in vivo. To our knowledge, this work is the first that aims to gain a more complete molecular snapshot of how modulators affect the FBR in vivo.
Dexamethasone delivery from the microdialysis probe caused significant decreases in both IL-6 and CCL2 at the gene transcript level. Since both of these cytokines are considered to be inflammatory, it is expected that Dex treatment would cause significant decreases in their overall transcript levels. While PCR analysis has been used to elucidate gene transcript changes in the presence of implanted biomaterials in an in vivo setting [50], it has not been used in vivo in the context of using modulators to affect macrophage polarization. The changes in transcription levels with Dex are in accordance with previous studies which have shown Dex treatment to be able to reduce transcript levels of CCL2 in rat pancreatitis models [17] as well as in an inflammatory state induced by potassium permanganate [21]. Dex has been shown to reduce IL-6 transcript levels in a rat arthritis model [51].
While Dex significantly decreased CCL2 and IL-6 gene transcripts, it did not significantly change the gene transcripts for another inflammatory cytokine, tumor necrosis factor-α (TNF-α). While this was unexpected, it is not novel in that TNF-α levels have been shown to be constitutively expressed in the spleen, liver, and small bowel of rats even when treated with Dex [52]. Further, TNF-α transcript levels have been shown to rise immediately following Dex treatment in a rat arthritis model [51]. The remaining genes showed no differential expression due to Dex treatment.
In the case of TGF-β1, reports of transcript regulation have been controversial. Dex has been shown to have different effects including no changes in TGF-β1 transcript levels in human T cells collected from asthma patients [53], increases in TGF-β1 transcript levels in human T cell cultures [54] as well as decreases in TGF-β1 transcript levels in rats [55]. The effect Dex has on TGF-β1 may be transient and dose dependent and perhaps the time course and/or dosage was unable to produce a difference in TGF-β1 transcript levels.
The genes for CD163, Arg2, and iNOS2 were chosen for analysis based on reports of differential expression of their protein products in an M(GC) state. While transcript levels of CD163, Arg2, and iNOS2 were unchanged, this does not mean that protein levels also remained unchanged as changes in transcript and protein levels do not always share a correlated response [56, 57]. Since quantitative analysis was not performed on Arg2 or iNOS2 protein concentrations, it is not possible to determine if these protein levels changed in response to the Dex-treatment. However, evidence of increased expression of CD163 was found using immunohistochemical staining. This increase may be due to tissue being harvested and RNA processed after the transcript levels peaked. Alternatively, this may be due to the relative amount of total tissue obtained relative to that which would be nearest to the microdialysis probe releasing Dex.
CCL2 is a chemokine known to recruit monocytes to a site of inflammation. Dex reduces CCL2 concentrations in rats during different inflammatory states in both plasma and whole tissue [13, 17, 21]. On the day of implantation, a steady increase in CCL2 concentration was quantified in the dialysate of the control probe during the collection period. This was expected as CCL2 is needed at the wound site to recruit macrophages. On one and two days post implantation, no statistically significant difference was seen in CCL2 concentrations over the 6 hour collection period, possibly in result of CCL2 reaching homeostasis. Interestingly, in the dialysate from the treatment probe, an increase in CCL2 concentration is seen between the first and second hour followed by a decrease in the fifth and sixth hours of collection on the day of probe implantation. This suggests that while the Dex may begin exerting anti-inflammatory effects as early as two hours post administration, as indicated by the leveling off of CCL2 concentrations in the treatment dialysates, the anti-inflammatory effects do not peak until later time points as indicated by suppression of CCL2 amounts quantified. On the following two collection days a general trend of suppression of CCL2 in treatment dialysates was seen in response to Dex, with the CCL2 concentration by hour 6 two days post implantation being similar to the concentrations initially detected after microdialysis probe implantation. Interestingly, CCL2 concentrations were found to be much higher in the initial hours of collection as compared to the final hours of collection the previous day. In fact, CCL2 concentrations in the initial flush each day showed no difference between control and treatment dialysates even though Dex was allowed to remain in the probe overnight and freely diffuse out into the ECS from the treatment probe. It is not entirely clear why this has been observed. The lack of difference in the initial flush may suggest that a certain continuous dose has to be applied. Alternatively, from an area-under-the-curve approach, it is clear the overall amount of CCL2 is significantly lower surrounding the probe. This could be a combination of diffusion/mass transport changes combined with CCL2 generation that lead to these differences. If CCL2 is being produced or generated locally by macrophages in the control implant, then its concentration will not be drained from the tissue space through the continuous sampling (removal) process with the microdialysis sampling probe.
Glucocorticoids, such as Dex, have been reported to shift macrophages to a predominantly M2c (M(GC)) state [5, 6]. In a recent study it was shown that dexamethasone was able to shift human macrophages to an M2c (M(GC)) state resulting in increased CD163 expression and increased clearance of early apoptotic cells, in vitro [58]. The desire to shift macrophages to an M2c (M(GC)) state at an implant site is based on the hypothesis that by doing so the implant will better integrate into the surrounding tissue resulting in reduced fibrosis, reduced failure rates, and improved healing. Badylak's group has shown that a predominantly M2c macrophage response to a scaffold implantation results in improved healing and integration of the scaffold [59, 60]. It is important to note at this point that while the Badylak group's findings are important, they differ from this work in that 1) they investigated degradable biomaterials whereas our study utilizes a non-degradable biomaterial, 2) the Badylak group looked at the polarization state of macrophages as a predictor of outcome in response to different biomaterials whereas we focus on using a modulator to shift the polarization state of macrophages at an implant site. However, the same factors can be used as a predictor of outcome. What we found was that two distinct populations of macrophages were found surrounding the implant at lower magnification (20×). This gave the appearance that tissue proximal to the implant was predominantly CD68+ CD163− macrophages, while tissue more distal from the probe was predominantly CD68+CD163+ macrophages, representing the expected conversion for M(GC) macrophages. This finding is in concert with a previous report that showed areas of predominantly M2 macrophages at the periphery of an implant, though these cells were stained with the pan M2 marker (CD206) and not CD 163, which is an M2c specific marker [61]. However, upon further investigation, we found that at higher magnification CD 163+ macrophages were seen in areas proximal to the probe. Further, we found that there were more CD 163+ macrophages surrounding the treatment probe as compared to the control probe. We also found that the tissue surrounding the Dex probes contained a significantly higher percentage of CD 163+ macrophages. This suggests that Dex is able to shift macrophages to a pro-wound healing state in vivo.
Previous studies have used different biomaterials to release Dex in an effort to reduce fibrosis using histological analyses as an endpoint [14, 28–31]. Our own histological findings are similar to this previous work since Dex is able to reduce the cellular density surrounding the biomaterial. However, it should be noted that previous studies have a wide range (5.3 µg – 7 mg) of different amounts of Dex being delivered to the biomaterial implant site. These are much higher doses than used in this study with the amount of delivered Dex being ~3.6 µg per six hour collection period giving a total of ~10.8 µg of Dex being delivered over the three days. It is important to note that these numbers do not take into account the Dex which was left in the probes at the conclusion of each sampling period and allowed to freely diffuse out. Interestingly, even though less Dex was delivered in this study, our findings are consistent with previous findings which showed Dex to result in a decrease in the amount of infiltrating cells [14, 28–31]. This may be due in part to the fact that our technique does not suffer from the initial high bursts of drug that is commonly associated with controlled release biomaterials.
5. Conclusions
Implanted microdialysis sampling probes were used to locally deliver dexamethasone-21-phosphate to the subcutaneous space of rats. This treatment altered the localized concentration of the chemokine, CCL2, and damped gene transcription for CCL2 and IL-6. While no changes in gene transcription were observed for the macrophage CD163 protein, this marker was identified in immunohistochemical analyses. While different materials have been used successfully in vivo to polarize macrophages to a pro-wound healing M2c (CD 163+) state, the use of modulators to shift macrophage polarization states has been focused primarily on in vitro studies. The ability to bridge the gap between successful in vitro modulators and in vivo implants could result in improved tissue remodeling outcomes to a host of biomaterials. This study is the first to show that Dex can be used to shift macrophages to a CD 163+ state at a non-degradable implant site. While this study has some limitations with respect to a limited number of markers and investigation as to the long-term effects on tissue remodeling, it shows great promise for the ability to use modulators to shift macrophage polarization states in vivo resulting in improved tissue remodeling.
Acknowledgements
Funding from NIH EB 014404 is acknowledged for support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CMA Microdialysis is now owned by Harvard Apparatus
References
- 1.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Martinez FO. Regulators of macrophage activation. Eur J Immunol. 2011;41:1531–1534. doi: 10.1002/eji.201141670. [DOI] [PubMed] [Google Scholar]
- 8.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 11.Ashida N, Arai H, Yamasaki M, Kita T. Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem. 2001;276:16555–16560. doi: 10.1074/jbc.M009068200. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes NP, Hunt JA, Williams DF. Macrophage subpopulation differentiation by stimulation with biomaterials. J Biomed Mater Res. 1997;37:481–488. doi: 10.1002/(sici)1097-4636(19971215)37:4<481::aid-jbm6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Chao J, Viets Z, Donham P, Wood JG, Gonzalez NC. Dexamethasone blocks the systemic inflammation of alveolar hypoxia at several sites in the inflammatory cascade. Am J Physiol Heart Circ Physiol. 2012;303:H168–H177. doi: 10.1152/ajpheart.00106.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webber MJ, Matson JB, Tamboli VK, Stupp SI. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012;33:6823–6832. doi: 10.1016/j.biomaterials.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Ma Y-Y, Song X-L, Cai H-Y, Chen J-C, Song L-N, et al. Upregulations of glucocorticoid-induced leucine zipper by hypoxia and glucocorticoid inhibit proinflammatory cytokines under hypoxic conditions in macrophages. J Immunol. 2012;188:222–229. doi: 10.4049/jimmunol.1002958. [DOI] [PubMed] [Google Scholar]
- 16.Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 17.Yubero S, Ramudo L, Manso MA, De Dios I. Mechanisms of dexamethasone-mediated chemokine down-regulation in mild and severe acute pancreatitis. Biochim Biophys Acta - Molecular Basis of Disease. 2009;1792:1205–1211. doi: 10.1016/j.bbadis.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 19.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adcock IM, Caramori G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 2001;79:376–384. doi: 10.1046/j.1440-1711.2001.01025.x. [DOI] [PubMed] [Google Scholar]
- 21.Conti P, Reale M, Feliciani C, Frydas S, Trakatellis M, Placido FC, et al. Augmentation of monocyte chemotactic protein-1 and mRNA transcript in chronic inflammatory states induced by potassium permanganate (KMnO4) in vivo. Immunology. 1997;92:300–306. doi: 10.1046/j.1365-2567.1997.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh C-S, Wang P-W, Lee S-Y, Huang C-C, Chang N-K, Chen C-M, et al. Glucocorticoid pretreatment suppresses chemokine expression and inflammatory cell infiltration in cholestatic rats receiving biliary intervention. J Pediatr Surg. 2006;41:1669–1675. doi: 10.1016/j.jpedsurg.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Ramudo L, Yubero S, Manso MA, Recio JS, Weruaga E, De Dios I. Effect of dexamethasone on peripheral blood leukocyte immune response in bile-pancreatic duct obstruction-induced acute pancreatitis. Steroids. 2010;75:362–367. doi: 10.1016/j.steroids.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Hori Y, Hu D-E, Yasui K, Smither RL, Gresham GA, Fan T-PD. Differential effects of angiostatic steroids and dexamethasone on angiogenesis and cytokine levels in the rat sponge implants. Br J Pharmacol. 1996;118:1584–1591. doi: 10.1111/j.1476-5381.1996.tb15578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrchen J, Steinmüller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–1274. doi: 10.1182/blood-2006-02-001115. [DOI] [PubMed] [Google Scholar]
- 26.Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, de Scheerder I, Desmet W. Dexamethasone-eluting stent: an anti-inflammatory approach to inhibit coronary restenosis. Expert Rev Cardiovasc Ther. 2004;2:653–660. doi: 10.1586/14779072.2.5.653. [DOI] [PubMed] [Google Scholar]
- 28.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81A:858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vacanti NM, Cheng H, Hill PS, Guerreiro JDT, Dang TT, Ma M, et al. Localized Delivery of Dexamethasone from Electrospun Fibers Reduces the Foreign Body Response. Biomacromolecules. 2012;13:3031–3038. doi: 10.1021/bm300520u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norton LW, Park J, Babensee JE. Biomaterial adjuvant effect is attenuated by anti-inflammatory drug delivery or material selection. J Controlled Release. 2010;146:341–348. doi: 10.1016/j.jconrel.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward WK, Hansen JC, Massoud RG, Engle JM, Takeno MM, Hauch KD. Controlled release of dexamethasone from subcutaneously-implanted biosensors in pigs: localized anti-inflammatory benefit without systemic effects. J Biomed Mater Res, Part A. 2010;94A:280–287. doi: 10.1002/jbm.a.32651. [DOI] [PubMed] [Google Scholar]
- 32.Westerink BHC, Cremers TIFH. Handbook of Microdialysis Sampling: Methods, Applications, and Clinical Aspects. Amsterdam: Academic Press; 2007. [Google Scholar]
- 33.Nandi P, Lunte SM. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: A review. Anal Chim Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mou X, Lennartz MR, Loegering DJ, Stenken JA. Modulation of the foreign body reaction for implants in the subcutaneous space: microdialysis probes as localized drug delivery/sampling devices. J Diabetes Sci Technol. 2011;5:619–631. doi: 10.1177/193229681100500316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derendorf H, Rohdewald P, Hochhaus G, Moellmann H. HPLC determination of glucocorticoid alcohols, their phosphates and hydrocortisone in aqueous solutions and biological fluids. J Pharm Biomed Anal. 1986;4:197–206. doi: 10.1016/0731-7085(86)80042-5. [DOI] [PubMed] [Google Scholar]
- 36.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahlin AP, Wetterhall M, Caldwell KD, Larsson A, Bergquist J, Hillered L, et al. Methodological aspects on microdialysis protein sampling and quantification in biological fluids: An in vitro study on human ventricular CSF. Anal Chem. 2010;82:4376–4385. doi: 10.1021/ac1007706. [DOI] [PubMed] [Google Scholar]
- 38.Trickler WJ, Miller DW. Use of osmotic agents in microdialysis studies to improve the recovery of macromolecules. J Pharm Sci. 2003;92:1419–1427. doi: 10.1002/jps.10410. [DOI] [PubMed] [Google Scholar]
- 39.Helmy A, Carpenter Keri LH, Skepper Jeremy N, Kirkpatrick Peter J, Pickard John D, Hutchinson Peter J. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma. 2009;26:549–561. doi: 10.1089/neu.2008.0719. [DOI] [PubMed] [Google Scholar]
- 40.Keeler GD, Durdik JM, Stenken JA. Comparison of microdialysis sampling perfusion fluid components on the foreign body reaction in rat subcutaneous tissue. Eur J Pharm Sci. 2014;57:60–67. doi: 10.1016/j.ejps.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius J Anal Chem. 2000;366:611–621. doi: 10.1007/s002160051556. [DOI] [PubMed] [Google Scholar]
- 42.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta-Molecular Basis of Disease. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the culprits: Macrophages-versatile regulators of wound healing. Adv Wound Care. 2013;2:357–368. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ariel A, Timor O. Hanging in the balance: endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J Pathol. 2013;229:250–263. doi: 10.1002/path.4108. [DOI] [PubMed] [Google Scholar]
- 45.Boehler RM, Kuo R, Shin S, Goodman AG, Pilecki MA, Leonard JN, et al. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol Bioeng. 2014;111:1210–1221. doi: 10.1002/bit.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gower RM, Boehler RM, Azarin SM, Ricci CF, Leonard JN, Shea LD. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials. 2014;35:2024–2031. doi: 10.1016/j.biomaterials.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric "smart" coatings to prevent foreign body response to implantable biosensors. J Controlled Release. 2013;169:341–347. doi: 10.1016/j.jconrel.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 48.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 49.Buttgereit F, Straub RH, Wehling M, Burmester G-R. Glucocorticoids in the treatment of rheumatic diseases: An update on the mechanisms of action. Arthritis Rheum. 2004;50:3408–3417. doi: 10.1002/art.20583. [DOI] [PubMed] [Google Scholar]
- 50.van Putten SM, Ploeger DTA, Popa ER, Bank RA. Macrophage phenotypes in the collagen induced foreign body reaction in rats. Acta Biomater. 2013;9:6502–6510. doi: 10.1016/j.actbio.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 51.Earp JC, DuBois DC, Molano DS, Pyszczynski NA, Almon RR, Jusko WJ. Modeling corticosteroid effects in a rat model of rheumatoid arthritis II: Mechanistic pharmacodynamic model for dexamethasone effects in Lewis rats with collagen-induced arthritis. J Pharmacol Exp Ther. 2008;326:546–554. doi: 10.1124/jpet.108.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ulich TR, Guo KZ, Irwin B, Remick DG, Davatelis GN. Endotoxin-induced cytokine gene expression in vivo. II. Regulation of tumor necrosis factor and interleukin-1 alpha/beta expression and suppression. Am J Pathol. 1990;137:1173–1185. [PMC free article] [PubMed] [Google Scholar]
- 53.Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Rückert B, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114:1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 54.Ayanlar Batuman O, Ferrero AP, Diaz A, Jimenez SA. Regulation of transforming growth factor-beta 1 gene expression by glucocorticoids in normal human T lymphocytes. J Clin Invest. 1991;88:1574–1580. doi: 10.1172/JCI115469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hakim M, Hage W, Lovering RM, Moorman CT, III, Curl LA, De Deyne PG. Dexamethasone and recovery of contractile tension after a muscle injury. Clin Orthop Relat Res. 2005:439. doi: 10.1097/01.blo.0000177716.70404.f9. [DOI] [PubMed] [Google Scholar]
- 56.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 58.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 61.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978–987. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]