Abstract
Our understanding of the impact of poor hepatic choline/phosphatidylcholine availability in promoting the steatosis characteristic of human nonalcoholic fatty liver disease (NAFLD) has recently advanced and possibly relates to phosphatidylcholine/phosphatidylethanolamine concentrations in various, membranes as well as cholesterol dysregulation. A role for choline/phosphatidylcholine availability in the progression of NAFLD to liver injury and serious hepatic consequences in some individuals requires further elucidation. There are many reasons for poor choline/phosphatidylcholine availability in the liver, including low intake, estrogen status, and genetic polymorphisms affecting, in particular, the pathway for hepatic de novo phosphatidylcholine synthesis. In addition to free choline, phosphatidylcholine has been identified as a substrate for trimethylamine production by certain intestinal bacteria, thereby reducing host choline bioavailability and providing an additional link to the increased risk of cardiovascular disease faced by those with NAFLD. Thus human choline requirements are highly individualized and biomarkers of choline status derived from metabolomics studies are required to predict those at risk of NAFLD induced by choline deficiency and to provide a basis for human intervention trials.
Keywords: choline, liver diseases, microbiome, NAFLD, nonalcoholic steatosis, phosphatidylcholine
Introduction
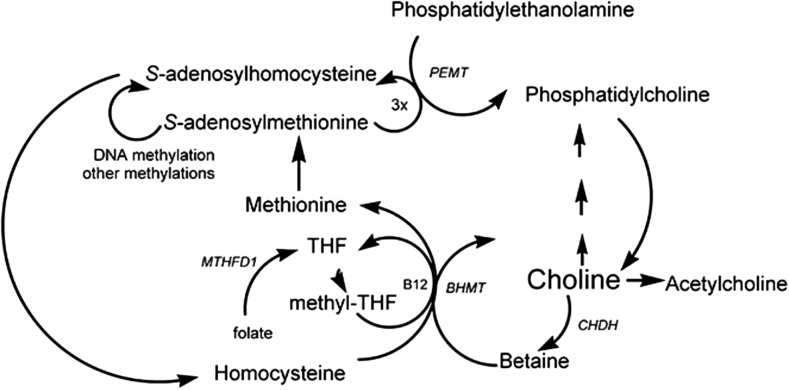
Choline is a nutrient obtained through both dietary intake and endogenous synthesis. In chemical terms it is a methyl-rich quaternary amine, present in free or esterified forms in all mammalian tissues. Choline is used for the synthesis of the neurotransmitter acetylcholine (1), and is also involved in methyl-group metabolism, particularly in the liver, because it is a major dietary source of methyl groups via its irreversible oxidation to betaine and the subsequent synthesis of S-adenosylmethionine (2) (Figure 1). The other sources of methyl groups are dietary methionine and betaine, and de novo synthesis via the one-carbon folate pool (Figure 1).
FIGURE 1.
Choline metabolism, including the BHMT gene for betaine–homocysteine S-methyltransferase, the CHDH gene for choline dehydrogenase, the MTHFD1 gene for methylenetetrahydrofolate dehydrogenase 1, and the PEMT gene for phosphatidylethanolamine N-methyltransferase. B12, vitamin B-12; BHMT, betaine-homocysteine S-methyltransferase; CHDH, choline dehydrogenase; MTHFD1, methylenetetrahydrofolate dehydrogenase 1; PEMT, phosphatidylethanolamine N-methyltransferase; THF, tetrahydrofolate. Reproduced with permission from reference 2.
The liver is probably the major site of choline metabolism, where it is found primarily as phosphatidylcholine (1). Phosphatidylcholine and other choline-containing phospholipids, lysophosphatidycholine, sphingomyelin, and choline plasmalogen, are components of plasma and organelle membranes (1, 2). The choline moiety provides these phospholipids with a larger and more cylindrical head group than the smaller and more conical shapes of other membrane phospholipids (1). Phosphatidylcholine is also required for the assembly/secretion of lipoproteins (2) and for solubilizing cholesterol in bile (3). It undergoes enterohepatic circulation, with estimates of the amount of human biliary phosphatidylcholine ranging from 5 mmol (∼4 g) (4) to 10–20 g/d (5), thus possibly making a greater contribution to phosphatidylcholine entering the small intestinal lumen than dietary phosphatidylcholine (Table 1), the most abundant form of choline in the diet (7).
TABLE 1.
Free choline, choline as PC, total choline, and betaine concentrations of some common food sources1
| NDB no.2 | Food description | Free choline, mg/100 g | PC, mg choline moiety/100 g | Total choline, mg choline moiety/100 g | Betaine, mg/100 g |
| 05013 | Chicken, broilers and fryers, meat only, roasted | 5.7 | 54.0 | 79.0 | 5.7 |
| 01009 | Cheese, cheddar | 1.6 | 7.4 | 17.0 | 0.7 |
| 01077 | Milk, whole, 3.25% milk fat | 3.7 | 1.9 | 16.0 | 0.6 |
| 01129 | Egg, whole, hard-boiled | 0.7 | 210.0 | 230.0 | 0.6 |
| 05661 | Chicken liver | 69.0 | 210.0 | 330.0 | 21.0 |
| 08001 | Kellogg’s All-Bran, original | 26.0 | 18.0 | 49.0 | 360.0 |
| 98013 | Spinach, frozen, whole leaf, microwaved | 2.2 | 24.0 | 28.0 | 130.0 |
| 98019 | Beans, baked, canned, plain, or vegetarian, heated | 14.0 | 11.0 | 28.0 | 0.1 |
| 10068 | Pork, fresh, top loin (chops) separable lean only, broiled | 1.1 | 57.0 | 78.0 | 3.0 |
| 11084 | Beets, canned, drained solids | 0.3 | 5.4 | 7.5 | 260.0 |
| 11136 | Cauliflower, boiled, drained | 25.0 | 12.0 | 39.0 | 0.1 |
| 12061 | Almonds | 9.4 | 40.0 | 52.0 | 0.5 |
| 12036 | Seeds, sunflower, kernels, dried | 18.0 | 29.0 | 55.0 | 35.0 |
| 15260 | Fish, salmon, pink, canned, drained solids with bone | 4.3 | 52.0 | 88.0 | 9.0 |
| 18075 | Bread, whole wheat, commercially prepared | 18.0 | 3.3 | 27.0 | 38.0 |
| 23040 | Steak, separable lean and fat trimmed to 0” fat, grilled | 0.7 | 91.0 | 110.0 | 13.0 |
From the USDA Database for the Choline Content of Common Foods, Release 2 (6). Total choline refers to the sum of free choline, glycerophosphocholine, phosphocholine, phosphatidylcholine, and sphingomyelin. NDB, Nutrient Database; PC, phosphatidylcholine.
A 5-digit numerical code used in the USDA Nutrient Database for Standard Reference.
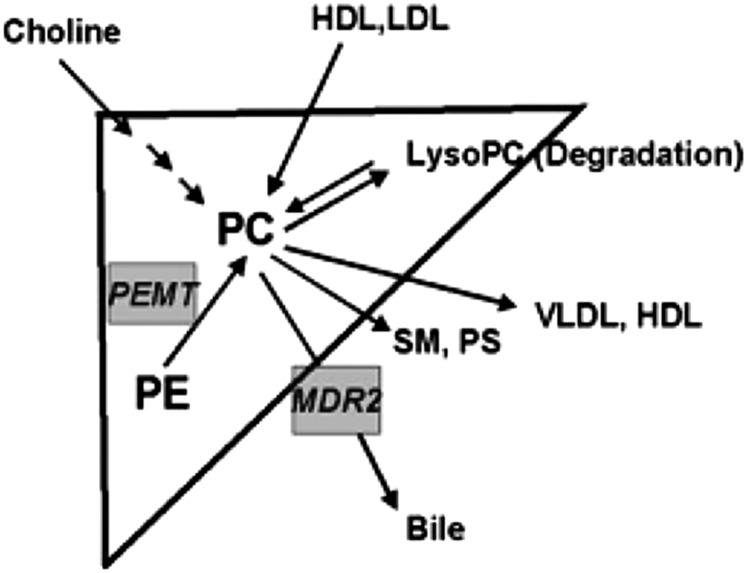
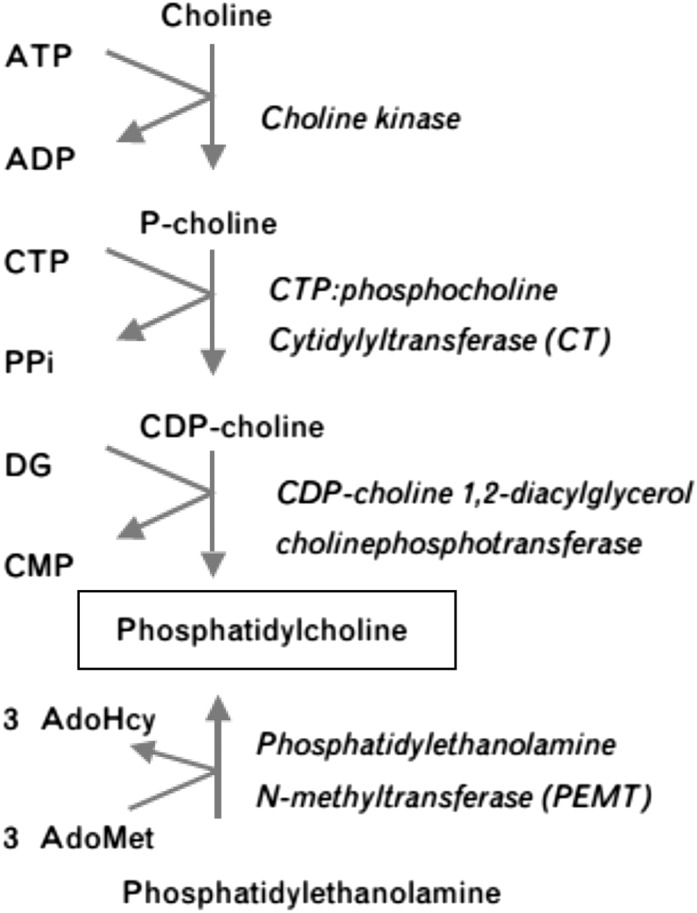
Hepatic homeostasis of phosphatidylcholine is achieved by balancing phosphatidylcholine/choline/betaine uptake (Table 2) and anabolism with phosphatidylcholine catabolism and secretion (12) (Figure 2). Studies of phosphatidylcholine anabolism in mice led to the estimation that 70% of the hepatic synthesis of phosphatidylcholine is derived from the CDP-choline pathway, requiring dietary choline (Figure 3), and 30% from the phosphatidylethanolamine N-methyltransferase (PEMT)7 pathway (1). The PEMT pathway becomes increasingly important for the maintenance of phosphatidylcholine supply within the liver when choline intake is insufficient, but it requires the involvement of S-adenosylmethionine (Figure 1). Regulation of S-adenosylmethionine availability is crucial to the capacity of the PEMT pathway to maintain the supply of phosphatidylcholine in the absence of adequate dietary choline.
TABLE 2.
Overview of steps involved in delivery of the choline moiety to the liver1
| Choline moiety | Metabolism in SI lumen | Uptake and metabolism in SI enterocytes | Transfer in circulation and delivery to liver |
| Dietary and biliary PC | Conversion to LPC by phospholipase A2 (8) | Uptake from micelles, reacylation of LPC to PC, incorporation into chylomicrons, or further hydrolysis of LPC (4) | Some transfer of chylomicron–PC to HDLs via phospholipid transfer protein (9) |
| Uptake of chylomicron remnants | |||
| Dietary unesterified choline | Uptake of choline | Portal vein | |
| Dietary betaine | Uptake of betaine | Portal vein | |
| HDL PC | Derived from chylomicrons, VLDLs or extrahepatic tissues (reverse PC transport) (10). Uptake by scavenger receptor B1 and other mechanisms (10). Preferentially channeled into bile secretion (11) | ||
| LDL PC | Taken up via LDL receptors and scavenger receptor B1 (9) |
LPC, lysophosphatidylcholine; PC, phosphatidylcholine; SI, small intestine.
FIGURE 2.
Uptake, anabolism, catabolism, and secretion of PC in the liver. LysoPC, lysophosphatidylcholine; MDR2, multiple drug–resistant protein 2; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PEMT, phosphatidylethanolamine N-methyltransferase; PS, phosphatidylserine; SM, sphingomyelin. Reproduced with permission from reference 12.
FIGURE 3.
Two pathways generate phosphatidylcholine in the liver: the choline pathway and the phosphatidylethanolamine N-methylase pathway. AdoHcy, adenosylhomocysteine; AdoMet, S-adenosylmethionine; DG, diacylglycerol; PPi, pyrophosphate. Reproduced with permission from reference 9.
Initially, choline was not considered a dietary essential because of the existence of the PEMT pathway for de novo synthesis of phosphatidylcholine (Figure 2) and then release of choline from phosphatidylcholine by phospholipase D where required. However, the presence of a pathway for endogenous synthesis of any nutrient does not guarantee that requirements can be met through this pathway alone (13). Choline was recognized as a required nutrient by the US Institute of Medicine in 1998 (13), and guidelines regarding the daily adequate intake for various age-groups and a tolerable upper intake limit were established; however, they have not been revised since. It is now recognized that human dietary requirements for choline vary, with the 2 primary reasons being estrogen status and the presence of polymorphisms in key genes of choline and folate metabolism (14, 15). In addition, phosphatidylcholine (or possibly lysophospatidylcholine) is now recognized as a substrate for intestinal bacterial metabolism, adding a third factor to the complexity of quantifying choline requirements. It also must be remembered that preformed betaine, found in whole-grain foods and some vegetables, including beetroot and spinach (Table 1), can lower the amount of dietary choline required, because it reduces the need to convert choline to betaine (Figure 1).
The link between choline deficiency and accumulation of hepatic lipid has been recognized for over 50 y (16), leading to the establishment of choline-deficient diets to induce models of nonalcoholic fatty liver disease (NAFLD) in animals. In humans, NAFLD is the most common liver condition worldwide, affecting up to 30% of Western and 17% of Eastern populations (17). NAFLD is an umbrella term for a histologic spectrum of disease ranging from hepatic steatosis to nonalcoholic steatohepatitis (NASH), an inflammatory phenotype with hepatocyte injury, with or without fibrosis, which can progress to cirrhosis with complications of liver failure and hepatocellular carcinoma (17). The current treatment for NAFLD is based on reducing body fat through caloric restriction and physical activity (18). Little is known about the role of choline in the potential prevention or treatment of NAFLD. Disturbances in host or bacterial choline metabolism may have a substantial impact on the genesis of NAFLD, the development of liver injury, and the association between NAFLD and cardiovascular disease, which remains the leading cause of death in these patients. Given the restricted dietary options for treating NAFLD, this review will focus on recent understanding of the provision and functions of choline in the liver, including the role of the gut microbiota in determining bioavailability and the impact of polymorphisms of selected genes of choline metabolism. These factors may explain why some individuals who are not meeting their personal choline requirements can encounter detrimental changes in hepatic lipid homeostasis.
Current Status of Knowledge
Choline/phosphatidylcholine in the liver
Within hepatocytes, choline may be oxidized to betaine in mitochondria and then supply a methyl group (Figure 1), or enter the CDP–choline pathway (Figure 3) for phosphatidylcholine synthesis. As well as synthesis, phosphatidylcholine is supplied by reverse phosphatidylcholine transport (Table 2); 50% of the hepatic phosphatidylcholine in mice was shown by van der Veen et al. (10) to be derived from the circulation. Hepatic phosphatidylcholine is used to form the monolayers of lipid droplets, VLDL and HDL, but this phosphatidylcholine is not obtained from a single source. Phosphatidylcholine, the main phospholipid of the lipid droplet monolayer (19), is synthesized at the growing lipid droplet surface via targeted activation of the rate-limiting enzyme CTP:phosphocholine cytidyltransferase in the CDP–choline pathway (Figure 3) (19). Similarly, the CDP–choline pathway appears to be optimal for the phosphatidylcholine in the endoplasmic reticulum and Golgi that is released to apoA1 during the genesis of HDL (9), but the PEMT pathway is essential for VLDL synthesis and secretion (20). Some of the phosphatidylcholine in mixed micelles in bile is generated by the PEMT pathway (4), with the reverse transport of HDL–phosphatidylcholine accounting for 38% of rat biliary phosphatidylcholine (11). Thus reverse transport of both HDL– and LDL–phosphatidylcholine returns phosphatidylcholine that originated from the liver, as well as phosphatidylcholine from the periphery.
Phosphatidylcholine synthesis via the PEMT pathway competes for S-adenosylmethionine, which is also used for methylation of DNA, RNA, histones, and other targets, as well as being a precursor to polyamines and glutathione (4, 21). Communication between folate metabolites and the ratio of S-adenosylmethionine to S-adenosylhomocysteine (Figure 1) maintains S-adenosylmethionine homeostasis. S-adenosylmethionine inhibits the synthesis of methyltetrahydrofolate, a source of the methyl group independent of betaine. Moreover, methyltetrahydrofolate inhibits an enzyme that catabolizes S-adenosylmethionine [glycine N-methyltransferase (GNMT)] (22). The main biological role of GNMT, an enzyme found at high concentrations in the liver, is postulated to be to optimize the ratio of S-adenosylmethionine to S-adenosylhomocysteine (21).
Choline/phosphatidylcholine and hepatic steatosis
Hepatic steatosis develops when FA influx, de novo hepatic lipogenesis, or TG synthesis from diacylglycerol exceeds lipid export or oxidation. Alterations in choline and phosphatidylcholine metabolism may have an impact on several pathways, potentially predisposing one to fatty liver. Choline deficiency as a cause of hepatic steatosis is exemplified in the setting of total parenteral nutrition, in which choline replacement led to a reversal of fatty infiltration (23). Furthermore, dietary choline deficiency is routinely used in animal models of NAFLD (24).
Phosphatidylcholine deficiency increases de novo hepatic lipogenesis.
The products of de novo lipogenesis are SFAs rather than unsaturated FAs, and TGs enriched with SFAs have been shown to be increased in subjects with NAFLD compared with those without, supporting the contribution of de novo lipogenesis to hepatic steatosis (25). An elevation in TG synthesis may be secondary to low phosphatidylcholine concentrations (26). The resultant reduction in the phosphatidylcholine-to-phosphatidylethanolamine ratio increases the curvature of membranes, and in the case of the endoplasmic reticulum, leads to activation of sterol regulatory element–binding protein 1c independently of other triggers, e.g., elevated insulin concentrations (26). A reduction in the phosphatidylcholine-to-phosphatidylethanolamine ratio may arise from 1) decreased dietary choline and/or decreased choline bioavailability for the CDP pathway, 2) decreased PEMT activity because of genetic variants, or 3) reduced methylation capacity (Figure 1) (26). Notably, rare functional mutations in the CDP pathway lead to reduced phosphatidylcholine synthesis and severe fatty liver in humans (27), reinforcing the observation that the PEMT pathway cannot compensate fully for the absence of the CDP pathway.
Phosphatidylcholine as a substrate for TG synthesis in the liver.
Reverse HDL–phosphatidylcholine transport has been shown to provide a substrate for TG synthesis in mice (10). Phosphatidylcholine is hydrolyzed into diacylglycerol and phosphocholine, with diacylglycerol subsequently undergoing acylation to TG. Mice have high HDL concentrations, and HDL–phosphatidylcholine contributes to one-half of their liver phosphatidylcholine content. In turn, two-thirds of hepatic TG is derived from hepatic phosphatidylcholine, underlying its important contribution to hepatic steatosis (10, 21). It is not known what contribution to hepatic TG synthesis reverse HDL–phosphatidylcholine makes in humans; however, the rare functional mutations in the CDP–choline pathway described by Payne et al. (27) resulted in fatty liver but very low HDL concentrations. Interestingly, the phosphatidylcholine that becomes associated with apoA1 before secretion is optimally derived from the CDP–choline pathway (9), thus potentially explaining the reduced HDL concentrations in these individuals.
Hepatic lipid storage.
In hepatocytes, a critical function of lipid droplets is to regulate the intracellular concentrations of both unesterified FAs and cholesterol, these being cytotoxic at increased concentrations (28). Phosphatidylcholine in the lipid droplet monolayer acts as a surfactant to prevent the coalescing of lipid droplets that would yield larger lipid droplets less likely to undergo lipolysis (19). Proteins that orchestrate lipid storage and utilization reside in this monolayer. Townsend et al. (29) demonstrated that the reduced phosphatidylcholine content of NIH 3T3 fibroblasts results in increased association of intracellular perilipin 2 with the lipid droplets and altered binding of as-yet unidentified proteins. Perilipin 2 is a negative regulator of VLDL secretion in hepatocytes, and phosphatidylcholine deficiency results in the accumulation of larger lipid droplets (30); thus, investigation of the impact of reduced phosphatidylcholine content of hepatocytes on perilipin 2 and other proteins associated with VLDL secretion may be informative.
Hepatic TG export.
In the nonfatty liver there is a homeostatic balance between TG synthesis and excretion. Given that phosphatidylcholine is required for VLDL synthesis/secretion, reduced phosphatidylcholine would be expected to account for at least some of the TG accumulation in the liver seen in NAFLD (20). The PEMT pathway is essential for VLDL synthesis and secretion (20), although the exact mechanism for this has not been defined (9). Several aspects of VLDL maturation remain unresolved (30), but the study of GNMT knockout mice by Martínez-Uña et al. (31) led to the suggestion that excess S-adenosylmethionine plays a role in disrupting VLDL assembly.
Biliary cholesterol.
Min et al. (32) demonstrated multiple aberrations of hepatic cholesterol metabolism in subjects with NAFL and NASH, leading to an accumulation of free cholesterol without a corresponding increment in cholesterol esters. These aberrations include decreased LDL receptor expression (and subsequently reduced LDL–phosphatidylcholine reverse transport), increased expression of the gene responsible for the hydrolysis of cholesterol esters, and decreased bile acid synthesis. Excess cholesterol in the bile may result in gallstones, and using a longitudinal stud design, Liu et al. (33) reported NAFLD to be an independent risk factor for gallstone development, but only in women (mostly postmenopausal at baseline). Further understanding of the reasons for this increased risk may shed light on any change in the usual secretion of phosphatidylcholine in bile that reduces the solubility of cholesterol.
Dietary choline and human NAFLD
Studies using estimates of choline intake in free-living human subjects with steatosis are limited. A cross-sectional study of >56,000 middle-aged and older Chinese subjects found an inverse relation between sonographically diagnosed NAFLD and dietary choline in the subgroup of normal-weight women only, suggesting that the influence of choline may depend upon host factors such as sex and adiposity (34). Menopausal status also may have had an influence. A cross-sectional study of Americans (35) did not find any relation between choline intake and degree of steatosis measured by liver biopsy, but postmenopausal women consuming <50% of the adequate intake had more substantial fibrosis. The following sections highlight the impact of estrogen status, as well as genetic and bacterial factors, on the relation between dietary choline and risk of NAFLD.
PEMT activity.
The enzyme PEMT plays a key role in the synthesis of hepatic phosphatidylcholine, as demonstrated by PEMT knockout mice developing hepatic steatosis because of reduced TG excretion (36). Interestingly, the PEMT gene is under estrogenic control (37), which explains why premenopausal women were less likely than postmenopausal women or men to develop hepatic steatosis during a controlled feeding trial that included a phase of depleted choline intake (38). However, some premenopausal women were insensitive to this protective effect, and subsequently this has been demonstrated to be due to 2 single nucleotide polymorphisms that reduce estrogen binding in the promoter region of the PEMT gene (37).
Several studies have investigated the frequency of other PEMT variants in subjects with NAFLD compared with controls. The valine to methionine substitution at residue 175 (V175M) is 30% less active than the wild-type PEMT (36), thus increasing reliance on dietary choline. The frequency of those who are homozygous for this variant is high in Caucasians and even higher in those with fatty liver (39, 40). In contrast with Caucasians, the frequency of V175M variant homozygotes has been reported to be lower in African Americans and Hispanic subjects (one-third) (39, 40) and very low (<1%) in Japanese subjects (40). Even so, V175M was more frequent in Japanese NASH patients than in healthy controls, and associated with a lower BMI in NASH patients (41).
GNMT activity.
GNMT is the most abundant hepatic methyltransferase, and serves to regulate the ratio of S-adenosylmethionine to S-adenosylhomocysteine (22). GNMT is a folate-binding enzyme, and the 5,10-methylene tetrahydrofolate dehydrogenase–1958A gene allele may influence GNMT activity. The carriers of this allele in the choline depletion study (38) were significantly more likely to develop signs of organ dysfunction (42). Interestingly, carriers of this allele developed higher plasma concentrations of S-adenosylhomocysteine, elevated concentrations of which inhibit PEMT, making it harder to synthesize phosphatidylcholine de novo (42).
Overall, progress in understanding the role of changes in GNMT activity in relation to human NAFLD is not as advanced as that for changes in PEMT activity. GNMT knockout mice develop NAFLD, which has established a role for excess as well as reduced S-adenosylmethionine in relation to TG accumulation (21, 31). Downregulation of GNMT has been reported in human fatty liver tissue, and this was associated with an increase in cholesterol accumulation (43), a feature of the contents of lipid droplets in NAFLD (28). Unfortunately GNMT expression was not investigated by Min et al. (32), who reported extensive cholesterol dysregulation in their NAFL and NASH patients. Further research is needed to consolidate the roles of GNMT in relation to both hepatic TG and cholesterol accumulation in humans.
Polymorphisms in other genes related to choline metabolism.
da Costa et al. (15) examined 200 single nucleotide polymorphisms in 10 genes related to choline metabolism in their search for associations with liver (or muscle) dysfunction in the controlled choline feeding trial mentioned above (38). Single nucleotide polymorphisms in choline kinase (CHKA) (Figure 3), solute carrier family 44 member 1 (SLC44A1), also known as choline transporter-like protein 1, and choline/ethanolamine kinase (CHKB) have been implicated in increasing or decreasing susceptibility to hepatic dysfunction on a low-choline diet (15); however, their lower prevalence has meant that further studies with larger cohorts are required to determine their importance in relation to the risk of NAFLD.
Choline and progression from steatosis to steatohepatitis.
Animal models suggest that choline deficiency alone is insufficient to lead to the hepatic inflammation and fibrosis characteristic of NASH, and that other contributing factors are required. A diet deficient in both methionine and choline results in NASH; however, this model lacks the weight gain and insulin resistance seen in human NAFLD. Similar changes are observed with chronic feeding of a choline-deficient l-amino acid–defined diet (34, 44).
A pilot study revealed that hepatic phosphatidylcholine-to-phosphatidylethanolamine ratios were lower in human subjects with NASH than in healthy subjects (8), suggesting that S-adenosylmethionine availability may have been limited. Such a change in membrane composition may allow cytokines or bacterial LPSs to enter hepatic cells and stimulate inflammatory pathways, leading to NASH and fibrosis (12).
In summary, evidence to date suggests cholesterol and TG dysregulation, along with the phosphatidylcholine-to-phosphatidylethanolamine ratio of various hepatic membranes, could be the key to the changes that are seen with choline/phosphatidylcholine deficiency. Progression to NASH may result if changes result in the stimulation of inflammatory pathways.
Gut microbiome.
The human gut microbiome actively metabolizes dietary components, including choline, and thus may alter its bioavailability and potentially predispose one to choline deficiency. The gut microbiome is highly diverse between individuals, which may lead to variation in choline absorption for a given dose of dietary choline and differences in choline metabolite profiles. Screening of 79 bacterial isolates found in the human gastrointestinal tract identified 8 species that are avid choline metabolizers (45). Genetic analyses, however, have demonstrated that anaerobic choline metabolism gene sets are widely distributed among 3 major bacterial phyla existing in the human gut microbiota (Proteobacteria, Firmicutes, and Actinobacteria) (46). The functional impact of these bacteria has been demonstrated by the colonization of germ-free mice with choline-metabolizing bacteria, resulting in reduced choline bioavailability and choline serum concentrations (45).
Dietary choline may in turn affect the gut microbiome. Although wholesale changes in the diversity or abundance of the human gut microbiome have not been demonstrated to occur with alterations in dietary choline, the species Gammaproteobacteria appears to be responsive to choline intake, with deficiency and replenishment leading to changes in its abundance (47). In the choline depletion study (38), replenishment of choline resulted in a reduction in the abundance of Gammaproteobacteria to zero in 13 of 15 subjects, indicating a sensitivity of these bacteria to dietary choline, given predominantly in the form of phosphatidylcholine (47). The class Gammaproteobacteria is known to harbor high concentrations of choline-metabolizing enzymes, and concentrations of gut Gammaproteobacteria subsequently influenced susceptibility toward the development of hepatic steatosis with a choline-deficient diet (46, 47).
Choline salts have long been avoided as a supplemental form because of the accompanying fishy body odor from the bacterial production of trimethylamine (48). The use of lecithin (phosphatidylcholine) as an alternative form of choline to treat Huntington disease and tardive dyskinesia (49) was not associated with malodor, suggesting that bacteria cannot access the choline in phosphatidylcholine to the same degree. Although phosphatidylcholine challenges before and after the use of antibiotics have demonstrated that egg yolk phosphatidylcholine can be metabolized to trimethylamine in the human gut (50), it has not been established how intestinal bacteria access esterified choline. However, Vibrio cholera, a gram-negative human pathogen belonging to the class Gammoproteobacteria, was recently shown to use environmental lipids, including lysophosphocholine (51). This bacterium has an externally oriented lysophospholipase that enables it to release the remaining FAs from lysophosphatidylcholine, which is readily available from both food and biliary phosphatidylcholine by the action of pancreatic phospholipase A2 (Table 2). Other gram-negative bacteria may have similar enzyme activity (50), as well as enzymes to allow the hydrolysis of glycerophosphocholine. Small intestinal bacterial overgrowth is relatively common in those with NAFLD (52), and may lead to the equivalent of a choline-deficient diet (53); in this situation, it seems more feasible that the higher bacterial numbers in the small intestine would be able to access lysophosphatidycholine derived from dietary or biliary phosphatidylcholine before it is taken up by enterocytes.
Animal studies have supported the concept that host genetics predispose one to a choline-utilizing gut microbiota that leads to less choline bioavailability and subsequent increased risk in NAFLD. Feeding a high-fat diet to mice that are genetically predisposed to NAFLD leads to low plasma phosphatidylcholine concentrations. This suggests reduced bioavailability of dietary choline, and increased urinary methylamines indicate increased bacterial metabolism of choline by the gut (54). Interaction between host genetic polymorphisms, dietary choline, and gut bacteria has also been demonstrated in a human study in which a PEMT single nucleotide polymorphism, in association with an abundance of gut Gammaproteobacteria and Erysipelotrichi, predicted the development of fatty liver in subjects who were fed a choline-deficient diet (48).
Bacterial choline metabolism, trimethylamine oxide, and atherosclerosis
Choline, phosphatidylcholine and betaine are among the dietary substrates used for trimethylamine production by intestinal bacteria (55). Trimethylamine in turn undergoes hepatic metabolism to trimethylamine oxide. Several members of the flavin mono-oxygenase family, in particular, hepatic flavin mono-oxygenase-3 (FMO3) (56), play key roles in trimethylamine conversion to trimethylamine oxide. The expression of the FMO3 gene is downregulated by testosterone and upregulated by estrogen, but no differences in circulating trimethylamine oxide concentrations between genders were observed in a clinical study (50), suggesting that these are overwhelmed by variations in dietary precursors to trimethylamine and variation in intestinal microflora (56).
In large-scale cohort studies, elevated plasma trimethylamine oxide concentrations have been associated with both angiographic measures of coronary artery atherosclerotic burden and cardiovascular disease risk (55, 57), possibly through increased cholesterol accumulation by macrophages (56). Thus, bacterial metabolism of choline to trimethylamine may result in reduced choline bioavailability and susceptibility to NAFLD, as well as increased trimethylamine oxide and cardiovascular disease risk, potentially providing a mechanism through which patients with NAFLD are at increased risk of cardiovascular disease. It should be noted that carnitine and possibly other dietary methylamines also contribute to plasma trimethylamine oxide concentrations (58).
Serum trimethylamine oxide has been measured in humans who were fed eggs that had a high phosphatidylcholine content (50, 59). The bioavailability from food sources of phosphatidylcholine and its variability between and within individuals was investigated in a pilot study of 6 subjects (59). The authors estimated that ∼11–15% of dietary total choline (mostly as phosphatidylcholine in egg yolk) in their test meals was converted to trimethylamine oxide in the liver from trimethylamine formed by intestinal bacteria. Biliary phosphatidylcholine would have also contributed to the formation of trimethylamine. It would be interesting to repeat this study in subjects with cystic fibrosis, with their reduced capacity for fat digestion and thus increased phosphatidylcholine concentrations in the intestine, for bacterial metabolism.
Estimating choline requirements
Many reasons for individualized requirements have been identified since the adequate intakes were set in 1998, leading to what now can be seen as an inadequate level for many individuals. Yet, analysis of the NHANES 2003–2004 data (60) suggests that many adults do not meet the adequate intake. It has been suggested that the adequate intake should be increased to cover the elevated needs of some (15); however, Tang et al. (50) have warned that overconsumption of rich sources of phosphatidylcholine may be harmful, at least for some, because of the positive relation between plasma trimethylamine oxide concentrations and cardiovascular disease risk. Again, human polymorphisms may contribute to this particular risk, and more information is required on the bioaccessibility of various phosphatidylcholine food sources and the possibility of manipulating the microbiome (45, 55).
Given all the variables associated with individual choline requirements, a clinical measurement would be useful to establish risk of NAFLD (61). To this end, Sha et al. (61) conducted metabolomic profiling and targeted analyses on the subjects from the choline depletion study (38), and were able to predict who would develop liver dysfunction when consuming a choline-deficient diet. The successful prediction rate was 80% in Caucasians, but decreased when the entire sample was considered, presumably reflecting interethnic group differences in the prevalence of single nucleotide polymorphisms in relevant genes (15, 61). In addition, variable intestinal microbial metabolism of the choline moiety also is likely to have contributed to the differences in response to choline-deficient diets.
Conclusion
The impact of low choline intake is dependent upon estrogen status and genetic polymorphisms involving choline metabolism. The intestinal microbial metabolism of choline, phosphatidylcholine, or betaine contributes another level of variability in those already at low or borderline choline status. It is difficult to see how requirements can be set for this nutrient without incorporating at least some genetic testing, given the variables at play and the possibility that excessive concentrations of phosphatidylcholine may place certain individuals at higher risk of cardiovascular disease. Metabolomic screening offers a more comprehensive way forward by incorporating the risk related to gut bacteria; several levels of requirements based on metabolomic profiles could then be created. Although advances have been made, further intervention trials that account for human and bacterial genetic differences are required to clarify our understanding of how dietary choline intake influences hepatic steatosis and its progression, as well as cardiovascular disease risk.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CHKA, choline kinase; CHKB, choline/ethanolamine kinase; FMO3, flavin mono-oxygenase-3; GNMT, glycine N-methyltransferase; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PEMT, phosphatidylethanolamine N-methyltransferase; SLC44AI, solute carrier family 44 member 1; V175M, valine to methionine substitution at residue 175.
References
- 1.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res 2008;49:1187–94. [DOI] [PubMed] [Google Scholar]
- 2.Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev 2009;67:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jüngst D, Lang T, Huber P, Lange V, Paumgartner G. Effect of phospholipids and bile acids on cholesterol nucleation time and vesicular/micellar cholesterol in gallbladder bile of patients with cholesterol stones. J Lipid Res 1993;34:1457–64. [PubMed] [Google Scholar]
- 4.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr 2006;83:5–10. [DOI] [PubMed] [Google Scholar]
- 5.Northfield TC, Hofmann AF. Biliary lipid output during three meals and an overnight fast. I. Relationship to bile acid pool size and cholesterol saturation of bile in gallstone and control subjects. Gut 1975;16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM, Zeisel SH, da Costa KA, Mar MH. USDA database of the choline content of common foods, Release 2 [Internet]. Maryland: U.S. Department of Agriculture; 2008. [cited 2014 Nov 18]. Available from: https://www.ars.usda.gov/Services/docs.htm?docid=6232.
- 7.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline- containing compounds and betaine in common foods. J Nutr 2003;133:1302–7. [DOI] [PubMed] [Google Scholar]
- 8.Homan R, Hamelehle KL. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line Caco-2. J Lipid Res 1998;39:1197–209. [PubMed] [Google Scholar]
- 9.Vance DE. Role of phosphatidylcholine biosynthesis in the regulation of lipoprotein homeostasis. Curr Opin Lipidol 2008;19:229–34. [DOI] [PubMed] [Google Scholar]
- 10.van der Veen JN, Lingrell S, Vance DE. The membrane lipid phosphatidylcholine is an unexpected source of triacylglycerol in the liver. J Biol Chem 2012;287:23418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portal I, Clerc T, Sbarra V, Portugal H, Pauli AM, Lafont H, Tuchweber B, Yousef I, Chanussot F. Importance of high-density lipoprotein-phosphatidylcholine in secretion of phospholipid and cholesterol in bile. Am J Physiol 1993;264:G1052–6. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 2006;3:321–31. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 14.Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 2012;28:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Costa KA, Corbin KD, Niculescu MD, Galanko JA, Zeisel SH. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J 2014;28:2970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura T, Nakamura S, Karoji N, Aikawa T, Suzuki O, Onodera A, Ono Y. Hepatic function tests in heavy drinkers among workmen. Tohoku J Exp Med 1967;93:219–26. [DOI] [PubMed] [Google Scholar]
- 17.Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci 2011;48:97–113. [DOI] [PubMed] [Google Scholar]
- 18.Finelli C, Tarantino G. Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients? J Gastrointestin Liver Dis 2012;21:293–302. [PubMed] [Google Scholar]
- 19.Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmidt-Supprian M, Vance DE, Mann M, et al. . Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab 2011;14:504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem 2002;277:42358–65. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs RL, van der Veen JN, Vance DE. Finding the balance: The role of S-adenosylmethionine and phosphatidylcholine metabolism in development of nonalcoholic fatty liver disease. Hepatology 2013;58:1207–9. [DOI] [PubMed] [Google Scholar]
- 22.Williams KT, Schalinske KL. New insights into the regulation of methyl group and homocysteine metabolism. J Nutr 2007;137:311–4. [DOI] [PubMed] [Google Scholar]
- 23.Buchman AL, Ament ME, Sohel M, Dubin M, Jenden DJ, Roch M, Pownall H, Farley W, Awal M, Ahn C. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: Proof of a human choline requirement: a placebo-controlled trial. JPEN J Parenter Enteral Nutr 2001;25:260–8. [DOI] [PubMed] [Google Scholar]
- 24.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2011;8:35–44. [DOI] [PubMed] [Google Scholar]
- 25.Westerbacka J, Kotronen A, Fielding BA, Wahren J, Hodson L, Perttila J, Seppanen-Laakso T, Suortti JA, Hultcrantz R. Splanchnic balance of free fatty acids, endocannabinoids, and lipids in subjects with nonalcoholic fatty liver disease. Gastroenterology 2010;139:1961–71.e1. [DOI] [PubMed] [Google Scholar]
- 26.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, et al. . A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 2011;147:840–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne F, Lim K, Girousse A, Brown RJ, Kory N, Robbins A, Xue Y, Sleigh A, Cochran E, Adams C, et al. . Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci 2014;111:8901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki M, Shinohara Y, Ohsaki Y, Fujimoto T. Lipid droplets: Size matters. J Electron Microsc (Tokyo) 2011;60 Supplement 1:S101–16. [DOI] [PubMed] [Google Scholar]
- 29.Townsend E, Hains A, Brown E, Rickertsen C, Kharbabda K, Listenberger L. Decreasing phosphatidylcholine on the surface of the lipid droplet alters protein binding. FASEB J 2015; 29:Supplement 71531 [Google Scholar]
- 30.Li X, Ye J, Zhou L, Gu W, Fisher EA, Li P. Opposing roles of cell death- inducing DFF45-like effector B and perilipin 2 in controlling hepatic VLDL lipidation. J Lipid Res 2012;53:1877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Uña M, Varela-Rey M, Mestre D, Fernandez-Ares L, Fresnedo O, Ferdandz-Ramos D, Gutierrez-de Juan V, Martin-Guerrero I, Garcia-Orad A, Luka Z, et al. . S-adenosylmethionine increases circulating very-low density lipoprotein clearance in non-alcoholic fatty liver disease. J Hepatol 2015;62:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min H-K, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab 2012;15:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Lin H, Zhang C, Wang L, Wu S, Zhang D, Tang F, Xue F, Liu Y. Non-alcoholic fatty liver disease associated with gallstones in females rather than males: a longitudinal cohort study in Chinese urban population. BMC Gastroenterol 2014;14:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D, Shu X-O, Xiang Y-B, Li H, Yang G, Gao Y-T, Zheng W, Zhang X. Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J Nutr 2014;144:2034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerrerio AL, Colvin RM, Schwartz AK, Molleston JP, Murray KF, Diehl A, Mohan P, Schwimmer JB, Lavine JE, Torbenson MS, et al. . Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr 2012;95:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs RL, Zhao Y, Koonen DPY, Sletten T, Su B, Lingrell S, Cao G, Peake DA, Kuo M-S, Proctor SD, et al. . Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem 2010;285:22403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resseguie ME, da Costa K-A, Galanko JA, Patel M, Davis IJ, Zeisel SH. Aberrant estrogen regulation of PEMT results in choline deficiency-associated Liver dysfunction. J Biol Chem 2011;286:1649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu T-S, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr 2007;85:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J, da Costa KA, Fischer LA, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J 2005;19:1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeisel SH. People with fatty liver are more likely to have PEMT rs7946 SNP, yet populations with the mutant allele do not have fatty liver. FASEB J 2006;20:2182. [Google Scholar]
- 41.Dong H, Wang J, Li C, Hirose A, Nozaki Y, Takahashi M, Ono M, Akisawa N, Iwasaki S, Saibara T, et al. . The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population. J Hepatol 2007;46:915–20. [DOI] [PubMed] [Google Scholar]
- 42.Kohlmeier M, da Costa K-A, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci 2005;102:16025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Y-J, Chen T-L, Lee T-S, Wang H-A, Wang C-K, Liao L-Y, Liu R-S, Huang S-F, Arthur Chen Y-M. Glycine N-methyltransferase deficiency affects Niemann-Pick type C2 protein stability and regulates hepatic cholesterol homeostasis. Mol Med 2012;18:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kucera O, Cervinkova Z. Experimental models of non-alcoholic fatty liver disease in rats. World Journal of Gastroenterology 2014;20:8364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 2015;6:e02481–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martínez-del Campo A, Bodea S, Hamer HA, Marks JA, Haiser HJ, Turnbaugh PJ, Balskus EP. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. MBio 2015;6:e00042–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011;140:976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther 1983;225:320–4. [PubMed] [Google Scholar]
- 49.Rosenberg GS, Davis KL. The use of cholinergic precursors in neuropsychiatric diseases. Am J Clin Nutr 1982;36:709–20. [DOI] [PubMed] [Google Scholar]
- 50.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pride AC, Herrera CM, Guan Z, Giles DK, Trent MS. The outer surface lipoprotein VolA mediates utilization of exogenous lipids by Vibrio cholerae. MBio 2013;4:e00305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Masciana R, Forgione A, Gabrieli ML, Perotti G, et al. . Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49:1877–87. [DOI] [PubMed] [Google Scholar]
- 53.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med 2011;62:361–80. [DOI] [PubMed] [Google Scholar]
- 54.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, et al. . Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci 2006;103:12511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, et al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. . Trimethylamine-N-oxide, a Metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. . Intestinal microbiota metabolism of l–carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O’Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am J Clin Nutr 2014;100:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen HH, Batres-Marquez SP, Carriquiry A, Schalinske KL. Choline in the diets of the US population: NHANES, 2003–2004. FASEB J 2007;21:219. [Google Scholar]
- 61.Sha W, da Costa KA, Fischer LM, Milburn MV, Lawton KA, Berger A, Jia W, Zeisel SH. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J 2010;24:2962–75. [DOI] [PMC free article] [PubMed] [Google Scholar]