Abstract
Many of the chronic illnesses that plague modern society derive in large part from poor food choices. Thus, it is not surprising that the Dietary Guidelines for Americans, aimed at the population ≥2 y of age, recommends limiting consumption of salt, fat, and simple sugars, all of which have sensory properties that we humans find particularly palatable, and increasing the variety and contribution of fruits and vegetables in the diet, to promote health and prevent disease. Similar recommendations may soon be targeted at even younger Americans: the B-24 Project, led by the US Department of Health and Human Services and the USDA, is currently evaluating evidence to include infants and children from birth to 2 y of age in the dietary guidelines. This article reviews the underinvestigated behavioral phenomena surrounding how to introduce vegetables and fruits into infants’ diets, for which there is much medical lore but, to our knowledge, little evidence-based research. Because the chemical senses are the major determinants of whether young children will accept a food (e.g., they eat only what they like), these senses take on even greater importance in understanding the bases for food choices in children. We focus on early life, in contrast with many other studies that attempt to modify food habits in older children and thus may miss sensitive periods that modulate long-term acceptance. Our review also takes into consideration ontogeny and sources of individual differences in taste perception, in particular, the role of genetic variation in bitter taste perception.
Keywords: breastfeeding, eating behavior, infant feeding, fruits, vegetables, diet, infants, taste, repeated exposure, variety
Introduction
Many of the chronic illnesses that plague modern society derive in large part from poor food choices. To promote health and prevent disease through diet and nutrition, the most recent Dietary Guidelines for Americans (1) and the US Department of Health and Human Services’ newest 10-y national objective, Healthy People 2020 (2), recommend, in part, limiting consumption of salt, fat, and simple sugars, all of which have sensory properties that we humans find particularly palatable, and increasing the variety and contribution of fruits and vegetables in the diets of the population ≥2 y of age [Nutrition and Weight Status Goals 14 and 15 (2)]. Similar recommendations may soon be targeted at even younger Americans: the B-24 Project, led by the Department of Health and Human Services Office of Disease Prevention and Health Promotion and the USDA’s Center for Nutrition Program and Policy, is currently evaluating the evidence base to support including infants and children from birth to 2 y of age in the Dietary Guidelines for Americans (3).
Why the focus on fruits and vegetables? Not only are they important sources of a wide range of vital micronutrients that are underconsumed by many, but evidence suggests that increased consumption of these foods can reduce the risks of a number of chronic diseases, including cardiovascular diseases and certain cancers (4). Increasing fruits and vegetables in the diet can also aid in weight management for children and adults (5–9), especially when these foods are substituted for more energy-dense foods in the diet (10, 11). However, despite the recommendations of health authorities worldwide, people and even young children eat too much salt, fat, and simple sugars and too few fruits and vegetables (12), although fruits continue to be more accepted by children than green vegetables (12–15).
The 2002 and 2008 Feeding Infants and Toddlers Studies (16, 17), designed to update knowledge on the feeding patterns of American children, revealed that 1 in 3 infants between the ages of 6 and 8.9 mo and 1 in 5 infants between the ages of 9 and 11.9 mo did not consume any fruit or vegetable on a given day. By 1 y of age, the top fruits and vegetables were not baby food but rather fresh or canned, indicating that children who eat these foods are making the appropriate transition to fruits and vegetables at the table (16). However, not 1 of the top 5 vegetables eaten was a dark green vegetable (12, 13, 16). Instead, young children were more likely consuming potatoes, sweet- and salty-tasting snacks, and sweetened beverages (12, 13), a dietary pattern similar to that observed in older children (18–21).
For older children, fruits are more accepted than vegetables, and some progress has been made in recent years (14): total fruit intake among children increased between 2003–2004 and 2009–2010 because of increases in whole fruit consumption (i.e., all fruit excluding juice). Over this time period, fruit juice intake (i.e., 100% juice, foods, and other beverages) also decreased. However, total vegetable intake did not change during this period. It is important to note that no sociodemographic group met the Healthy People 2020 target consumption for vegetables, and only children between the ages of 2 and 5 y met the target for fruits.
In this article, we review the ontogeny of the chemical senses, one of the major determinants of whether young children will initially accept or reject a food, and how children learn to like a food more. Because young children eat only what they like (22), these senses take on even greater importance in understanding the bases for food acceptance during childhood. We also review the underinvestigated behavioral phenomena surrounding how to introduce vegetables and fruits into infants’ diets, for which there is much medical lore (23, 24) but, to our knowledge, little evidence-based research (23, 25). Our focus is on early life, in contrast with many other studies that attempt to modify food habits in older children and thus may miss sensitive periods that modulate long-term acceptance.
Current Status of Knowledge
The biology of flavor and chemical sensing.
Flavor, a powerful determinant of human ingestive behavior, is a product of several sensory systems, most notably those of taste and smell (odors perceived retronasally). The anatomically independent flavor senses are well developed at birth and continue to change throughout childhood and adolescence, serving as gatekeepers by controlling one of the most important decisions an animal makes: whether to eat something or reject it [see Forestell and Mennella (26) for review].
Experimental research conducted during the past century has repeatedly revealed that children live in different “taste” worlds than do adults. Early in life they have sensory systems that detect and prefer the once rare calorie- and mineral-rich foods that taste sweet or salty while rejecting the potentially toxic ones that taste bitter. However, there are age-related changes in taste perception and preference such that the rewarding properties of sweet and salty and the aversive properties of bitter are more pronounced during childhood and adolescence, changing to resemble the adult pattern around midadolescence (27–30). The following sections focus on age-related changes in sweet, salty, and bitter tastes because, to our knowledge, little research has focused on such changes in the other basic tastes of sour [but see Liem and Mennella (31, 32) and Liem and de Graaf (33)] and umami.
That the ability to detect sweet tastes is functioning and interacting with systems controlling effect is evident early in life (34, 35), presumably because it attracts children to the predominant taste quality of mother’s milk and it is our biological signal of calories (34, 36). Within hours after birth, newborns differentiate varying degrees of sweetness and consume more of a sugar solution than water (37). When a sweet solution is placed in the oral cavity, infants relax the face and sometimes smile (38). Both cross-sectional and longitudinal studies have revealed that preferences for sweets remain heightened throughout childhood (28) and early adolescence, declining to adult levels during midadolescence (28, 39). The taste of sugars is not just liked by children; tasting something sweet can also blunt expressions of pain (40, 41). The more children like sweets, the better its analgesic properties.
Similar age-related changes have also been observed for salty taste. Although the ability to detect salt does not emerge until infancy, children prefer more concentrated salt solutions and saltier foods than do adults. Preferences for salt and sweet are not only elevated during childhood but also are related to each other (42), as well as to how much the child is growing: children who are growing, as evidenced by higher levels of a biomarker for bone growth, prefer sweeter and saltier solutions (42, 43).
As much as children like sweet and salty flavors, they initially reject all that tastes bitter. Shortly after birth, babies reject bitter-tasting substances, as evidenced by decreased intake and pronounced facial expressions (44, 45). In other words, they can detect and respond accordingly to bitter tastes. Of all the taste qualities, bitter taste perception is the most varied across individuals (46). We posit that the individual differences in acceptance of vegetables may arise from genetic differences among infants, although this body of research has focused on how bitter taste perception of vegetables relates to variation in taste receptor genotype in adults (47) or how vegetable intake relates to individual differences in bitter taste perception among older children (48), but not among infants.
There has been major progress toward identifying the initial events in taste recognition, and new light has been shed on the molecular mechanisms underlying why children and adults live in different sensory worlds and why some children may be more accepting of some bitter tastes than are other children (49, 50). For example, because of genotype differences, some individuals are more sensitive to the bitter taste of some vegetables (47, 51) and are thus less likely to eat these foods (47, 52, 53). Genetically based differences in receptors for bitter taste are particularly well known for the related bitter compounds phenylthiocarbamide and 6-n-propylthiouracil (PROP); some people can detect these at low concentrations, but others need much higher concentrations or cannot detect at all (54, 55).
Because people perceive taste-active chemicals differently, they also perceive the tastes of foods differently. Although many bitter receptor genes have alleles that affect bitter perception (56), their role in food preferences has not been fully explored. Perhaps most is known about the taste receptor, type 2, member 38 (TAS2R38) gene, which is related to the ability to taste PROP and phenylthiocarbamide, codes for 1 member of the family of 25 taste receptors that respond to bitter stimuli (55). Some vegetables contain phenylthiocarbamide-like glucosinolates (e.g., turnips and broccoli) (47); the different TAS2R38 alleles correlate with differences in perceptions of bitter intensity of these vegetables (47, 51).
We investigated whether age modified the genotype-phenotype relations in the TAS2R38 gene and bitter taste sensitivity of PROP (27, 57). Like the age-related changes in sweet and salt preference, children become less sensitive to some bitter tastes with age. Not only does PROP sensitivity vary with age but children heterozygous for a TAS2R38 variant perceived a bitter taste at lower PROP concentrations than did adults who were heterozygous (27, 57), with intermediate thresholds for heterozygous adolescents (27). In other words, childhood may be a time of heightened bitter sensitivity for many children, and such sensitivity may affect food acceptance. Because bitter sensitivity has been shown to be related to taste acceptance of certain vegetables in both children [those less sensitive to bitter flavors are more accepting or perceive less bitterness than those who are more bitter sensitive (53, 58–60)] and adults (47, 51, 61, 62), research is currently under way in our laboratory to determine whether variations in the TAS2R38 genotype contribute to individual differences in infants’ initial liking of cruciferous vegetables, as well as the number of repeated exposures needed to develop a liking for these foods during infancy.
It is interesting to note, from a culinary perspective, that the 2 tastes that are most preferred (i.e., salty and sweet) can block or mask bitter taste for children and adults (63, 64). For sodium salts, the efficacy of blocking bitter tastes depends on both the age of the subject and the chemical nature of the blocker and bitter agent, and its site of action appears to be at the receptor level in the periphery. Although there was variation among individuals and among bitter agents in the ability of sucrose to suppress bitterness, for some people and for some compounds sucrose is an unequivocally effective masker of bitter taste, and for some bitter agents it worked better than sodium salts (64, 65). A clinical study of school-age children showed that the addition of dilute solutions of the nonnutritive sweetener aspartame to the vegetables decreased children’s perception of the bitterness and increased their liking of the vegetables (66) [see also Sharafi et al. (65)].
In summary, our basic biology does not predispose children to favor the recommended low-sugar, low-sodium, vegetable-rich diets and makes them especially vulnerable to our current food environment high in salt and refined sugars. Thus, the struggle parents have in modifying their children’s diets to reduce added sugars and salt appears to have a strong biological basis. However, as discussed in the following sections, the senses that underlie flavor perception are “plastic” and can be modified by early experience, suggesting new, research-based strategies to improve children’s diets.
Learning about foods: amniotic fluid and mother’s milk.
Although innate taste likes and dislikes may create an obstacle to healthy eating by children, early experiences can teach children (like other mammalian young) what foods are safe and part of the culinary traditions of their family. During the past few decades, our research program and others have systematically studied the transfer of dietary volatiles to amniotic fluid and human milk to determine its effects on the behavior of breastfed infants [see Mennella (67) for review]. This research has revealed that a wide variety of flavors either ingested (e.g., fruits, vegetables, alcohol, and spices) or inhaled (e.g., tobacco) by the mother are transmitted to her amniotic fluid and/or milk (67–76). In general, the intensity of the flavor in milk increased within hours after consumption. That amniotic fluid and breast milk share flavor profiles with foods eaten by the mother suggests that breast milk “bridges” experiences with flavors in utero and those in solid foods (77). These variations in flavor from mother to mother and from feeding to feeding suggest that breastfeeding, unlike formula feeding, provides the infant with the potential for a rich source of sensory variety. The types and intensity of flavors experienced may be unique for each infant and characteristic of the culinary traditions of the family. These are the foods their mothers eat (78, 79) and will be the foods that their mothers will feed them as they grow. This is the first way, but not the only way, we learn about flavors of the foods of the family, culture, and environment.
During the first year of life, infants make the important transition from an all-milk diet to one containing solid foods; thus, like other mammals, developmental processes must ensure that they learn both what and how to eat (67, 80). To determine how pre- and postnatal flavor experiences affect liking of flavors at weaning in humans, to our knowledge, we conducted the first randomized clinical trial in which we assigned pregnant women who planned to breastfeed their infants to groups that differed in whether and when they drank carrot juice (81). One group of women was randomly assigned to drink carrot juice for several days per week during the last trimester of pregnancy; another group was randomly assigned to drink carrot juice for a similar time period during the first 3 mo of lactation, whereas the control group drank water and avoided carrots and carrot juice during both pregnancy and lactation. When mothers weaned their infants to solid foods, we tested their acceptance of plain cereal on 1 d and carrot-flavored cereal on another day. Infants who experienced the flavor of carrots in either amniotic fluid or mother’s milk responded more favorably (e.g., ate more and made fewer faces of distaste) to carrot-flavored cereal than did nonexposed control infants.
Learning about foods through dietary transmitted flavors in amniotic fluid and mother’s milk may be a fundamental feature of mammalian dietary learning (67). Such experiences early in life may cause a variety of neurologic and physiologic changes that influence later behaviors, and there is some evidence that dietary learning is more pronounced during early life (82). When mothers ate fruits or vegetables, infants experienced the flavors in amniotic fluid and then mother’s milk, which, in turn, increased the palatability of these foods by their infants. Animal models show that early experiences with retronasally perceived odors (flavors) in milk result in enhanced detection of these learned odors that in nature would facilitate selection of foods that mothers find palatable. Not only does breastfeeding confer an initial advantage to babies in their acceptance of fruits and vegetables when these foods are part of the maternal diet, but this continuity in flavor helps the infant transition to solid foods.
This pattern makes evolutionary sense because the foods that a mother eats when she is pregnant and nursing are the flavors associated with nutritious foods or, at the very least, with foods the mother has access to and, hence, the foods to which the child will have the earliest exposure. Because food habits established during infancy track into later childhood and adolescence (79, 83), early experiences with nutritious foods and flavor variety should maximize the chance that, as infants grow, they will enjoy a more healthy diet because they like the taste. Because mothers typically feed children foods that are part of their own diet and culture, the breastfed infant continues to learn the flavors of the foods they will be offered. Sensory experiences with food flavors in mother’s milk in children whose mothers eat a varied diet may explain why children who were breastfed tend to be less picky and more willing to try new foods during childhood (84).
Learning about foods: repeated exposure and dietary variety.
Learning about foods and flavors continues after weaning because experiences can modify liking of fruits and vegetables (25). As summarized in Table 1, through basic research we have discovered that regardless of whether infants are breastfed, formula fed, or both, once they are weaned to solid foods acceptance of fruits and vegetables can be facilitated by different types of early dietary experiences (85–92). The vast majority of this research uses a within-subject design and evaluates the infant’s acceptance by a variety of measures, including intake, facial reactivity, and maternal perceptions, using an infant-led feeding paradigm both immediately before and after the exposure period, which typically lasts 8–10 d [see Forestell and Mennella (86, 90) for review of methodologies).
TABLE 1.
Summary of research findings from experimental research on how repeated exposure and exposure to variety affect acceptance of fruits and vegetables during infancy
| Type of exposure1 | Exposure, d | Target complementary food2 | Result | Reference |
| Repeated exposure | 8 | Pears | Increased acceptance of pears; no effect on acceptance of novel vegetable (green beans) | Mennella et al. (85) |
| Repeated exposure | 8 | Peaches | Increased acceptance of peaches | Forestell and Mennella (86) |
| Repeated exposure | 9–10 | Carrots | Increased acceptance of carrots | Gerrish and Mennella (87); Caton et al. (88) |
| Repeated exposure | 8 | Green beans | Increased acceptance of green beans | Sullivan and Birch (89); |
| Mennella et al. (85); | ||||
| Forestell and Mennella (86, 90) | ||||
| Repeated exposure | 10 | Artichoke | Increased acceptance of artichoke | Remy et al. (91); Caton et al. (88) |
| Repeated exposure | 10 | Apples3 | Increased acceptance of more complex texture forms of the target food | Lundy et al. (92) |
| Repeated exposure (instructions)4 | 6 | Green beans, peas, squash, and carrots | Increased acceptance of green beans, peas, and squash but not carrots | Paul et al. (93) |
| Between-meal variety | 8 | Peaches, prunes, and apples | Increased acceptance of novel fruit (pears); no effect on acceptance of novel vegetable (green beans) | Mennella et al. (85) |
| Between-meal variety | 9 | Peas, potatoes, and squash | Increased acceptance of novel vegetable (carrots) | Gerrish and Mennella (87) |
| Between-meal variety | 8 | Squash, spinach, and carrots | Increased acceptance of carrots and spinach; increased acceptance of novel vegetable (green beans) | Mennella et al. (85) |
| Between- and within-meal variety | 8 | Squash/peas, carrot/peas, squash/spinach | Increased acceptance of carrots and spinach; increased acceptance of novel vegetable (green beans) | Mennella et al. (85) |
| Repeated exposure | 12–35 | Carrots, green beans, spinach, broccoli | Increased acceptance of vegetables; carrots were liked more than green beans | Hetherington et al. (94) |
Repeated exposure: infant was fed the same food during daily target meals throughout the experimental exposure period. Between-meal variety: infant was fed one food during the target meal that was different from the one experienced during the previous day’s target meal. Within-meal variety: infant was fed two different foods (e.g., green and orange vegetables) during the target meal that were different from those experienced during the previous day’s target meal.
All foods were in pureed form, unless otherwise indicated.
Apples were presented as pureed, diced, and/or lumpy textures.
Unlike other studies, one group of mothers was randomly assigned to receive instructions to repeatedly expose their infants to vegetables.
One type of experience entails repeated exposure to a particular vegetable or fruit for 8–10 d (85–87, 89) or longer (94). Like children (95), infants ate more of the fruit or vegetable to which they were repeatedly exposed. Merely looking at the food does not appear to be sufficient; children have to experience the flavor and taste of the fruit or vegetable to learn to like it (96, 97). Through use of the Facial Action Coding System (98), which measures the contraction of individual facial muscles that change the appearance of the face and is an indicator of liking or disliking (99), we found that facial expressions became less negative after repeated dietary experience with a particular food, and in some cases mothers judged that their infants enjoyed the foods more after the exposure (86, 89, 90).
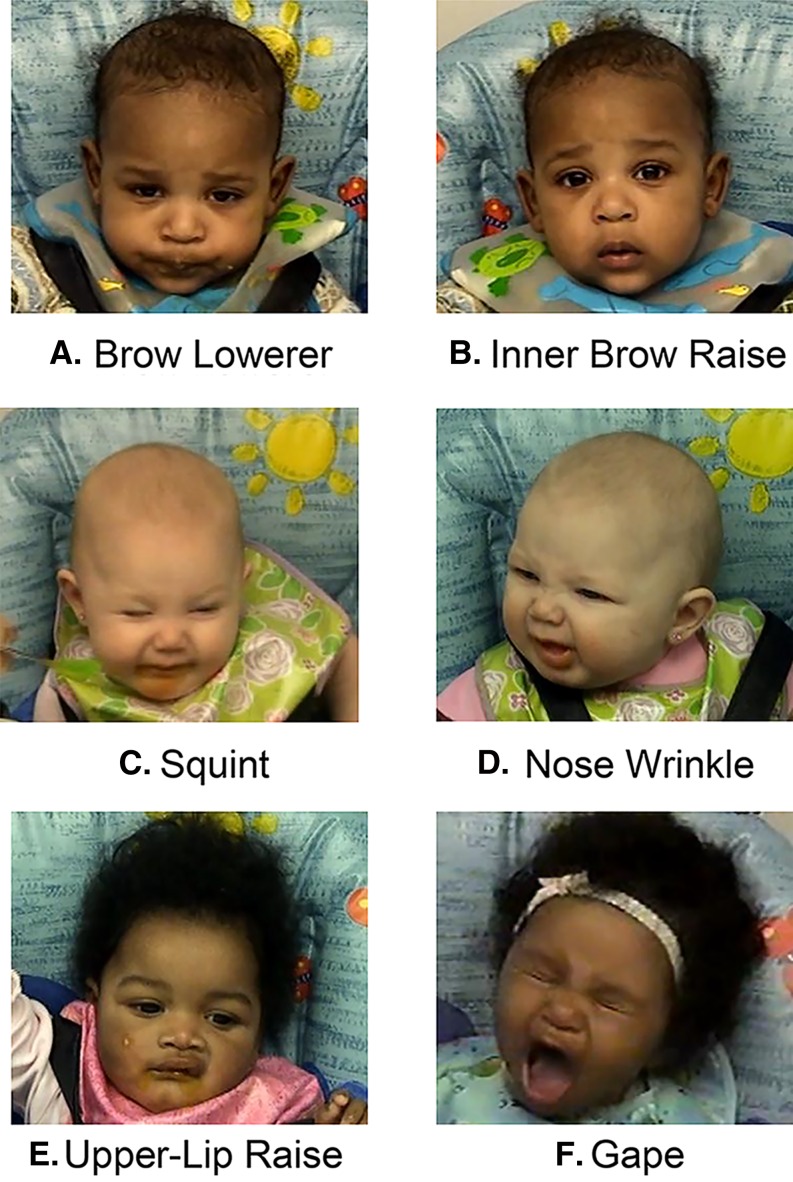
As shown in Figure 1, eating fruits and vegetables activates distinct, stereotyped motor behaviors of the orofacial region, which provide insights into the hedonic response to the taste of the food (e.g., liking). There were individual differences in the display of these facial expressions while eating that, in some cases, predicted the rate at which infants ate a particular food (86). For example, repeated exposure to green beans and/or peaches modified intake but only those who experienced peaches after green beans appeared to like the taste of the green beans more after the 8-d exposure. We hypothesize that the sweet taste of peaches masked some of the bitterness of the green beans, increasing its palatability and liking (64). When vegetables are fed alone (without the masking of the fruit), it takes longer (>8 d) for the face to change than changes in intake (86, 89). In other words, measures of liking and of intake are related but are governed by separate neural substrates (100) and, consequently, do not always change in tandem (100, 101). This suggests that mothers should be encouraged to focus on their infants’ willingness to eat a food, not just the facial expressions made during feeding (86, 102). They should also be made aware that with repeated dietary exposure, it may take longer to observe changes in facial expressions than changes in intake [see Paul et al. (93)].
FIGURE 1.
Feeding fruits and vegetables to weaning infants activates distinct, stereotyped motor behaviors of the orofacial region: brow lowerer (A), inner brow raise (B), squint (C), nose wrinkle (D), upper lip raise (E), and gape (F). Reproduced with permission from Pediatrics, Vol 120, pages 1247–54, Copyright © 2007 by the AAP (86).
A second type of dietary experience involves experiencing a variety of flavors rather than any one specific food or flavor. Infants who were repeatedly exposed to different vegetables on alternate days not only ate more of the vegetables to which they were exposed but also ate more of the novel vegetables than did infants repeatedly exposed to only one vegetable (87). That is, they also learn through repeated exposure to a variety of such foods that vary in both flavor and texture. This, in turn, promotes willingness to eat not only the introduced foods but also other, novel foods. Exposing infants to multiple sensory contrasts (between- and within-meal flavor variety) provided more opportunities to develop flavor preferences, perhaps based on postingestive reinforcing effects of nutritious foods.
Further study revealed a wide range of individual differences in vegetable acceptance by infants (90) relating to their 1) exposure—those who had been eating vegetables over the previous weeks displayed fewer facial expressions of distaste, and 2) temperament—those who were more approaching ate more of the green beans for a longer time and showed fewer facial expressions of distaste. Mothers were sensitive to certain facial behaviors expressed during feeding, which affected their judgments of their infants’ liking of the food.
Such findings are consistent with research in older children (103, 104). Although vegetables in particular are most cited by children as being a food they do not like (105), children can continue to learn to like these foods, but it is a more difficult task as they grow older (106, 107), perhaps because they refuse to taste these foods and therefore cannot learn to like them. We cannot underestimate the role of the caregivers because they feed their children what they themselves are eating [see also Worobey et al. (108), Birch et al. (109), Savage et al. (110), and Hart et al. (111)]. One strategy is to have caregivers try eating more fruits and vegetables because children whose mothers were instructed to do so tried more vegetables (112). In addition, the more fruits and vegetables eaten by the mother, the more likely their child followed their lead (108). Also, in a childcare setting, providing a variety of vegetables and fruits may not only lead to increased consumption of these foods by children but also increase the sensory experiences needed to learn to like these foods (113).
Conclusions
The period from birth to 24 mo of age is a time of rapid development, including feeding behavior. Emerging evidence suggests that individual patterns of food preferences and eating behaviors emerge and differ depending on the foods offered and the contexts of feeding during this early period of dietary transition. For many children, by the time they reach 2 y of age, they have essentially completed the transition to “table foods” and are consuming diets similar to those of other family members.
Research has shown that as children make this transition, early experiences with nutritious foods and flavor variety may maximize the likelihood that they will choose a healthier diet because they like the tastes and variety of the foods it contains. These foods need to be part of the family’s diet and food environment so that once the preference develops, the child continues to be exposed to the actual food to maintain the preference, learning to like more complicated flavors and textures (114). Ultimately, the goal is to gradually familiarize children to a varied diet that meets nutritional needs for growth and development and provides them with opportunities to learn to like and prefer a variety of healthy foods. The task ahead is challenging and there is not going to be just one solution to the problem, especially for those children who do not have their first taste of vegetables until they enter school (115).
Although there are inborn responses to the basic tastes, and some individuals may be more sensitive to some tastes because of genotype, the development of these chemical senses has inherent plasticity that interacts with early life experiences to ensure a child is not genetically restricted to a narrow range of foodstuffs [see Mennella (67) and Mennella and Beauchamp (116) for review]. Beginning very early in life, sensory experiences can shape and modify flavor and food preferences (25, 117). Such functional plasticity, one of the main characteristics of the brain, highlights the ability to change behavior based on experience. In other words, our biology is not necessarily our destiny. However, because consumption of vegetables (and some fruits) is so low among children and their family members, many children are deprived of the sensory experiences as well as parental and peer modeling and the type of food environments needed to learn to like these foods. In animal models, early taste deprivation remodels the central nervous system (118), whereas experience with bitterness during early life enhances liking of bitter taste in adulthood (119). We acknowledge that although food neophobia in the older child is in part a heritable trait (120, 121), we posit that the rejection of certain types of foods, in particular bitter-tasting vegetables, in the infant also may be caused in part by heritability of bitter taste sensitivity (not neophobia) and in the child, a combination of heritable taste sensitivity as well as nongenetic (environmental) factors such as maternal feeding practices and lacking food environments (122, 123).
Because there is no consistent, evidence-based guidance for caregivers who are feeding infants and toddlers [the current Dietary Guidelines are not intended to apply to Americans <2 y of age (1)], research is needed to develop evidenced-based strategies that take into account individual differences in initial acceptance of fruits and vegetables, to understand how infants and toddlers develop the food preferences and the self-regulatory processes necessary to promote healthy growth, particularly in today’s food environment. We need to know whether there are optimum times during the life span when experience promotes greater liking of these and other healthy foods and, conversely, when deprivation of such foods has the greatest consequences on diet and health for generations to come.
Acknowledgments
All authors read and approved the final manuscript.
References
- 1.USDA and US Department of Health and Human Services. Dietary guidelines for Americans, 2010 [Internet]. 7th ed. Washington (DC): US Government Printing Office; 2010 [cited 2014 Oct 8]. Available from: http://www.health.gov/dietaryguidelines/2010.asp.
- 2.US Department of Health and Human Services. Healthy people, 2020 topics and objectives: nutrition and weight status. Washington (DC): US Department of Health and Human Services; 2014. [cited 2014 Nov 18]. Available from: http://www.healthypeople.gov/2020/topics-objectives/topic/nutrition-and-weight-status/objectives.
- 3.Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“the B-24 Project. ” Am J Clin Nutr 2014;99:663S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knai C, Pomerleau J, Lock K, McKee M. Getting children to eat more fruit and vegetables: a systematic review. Prev Med 2006;42:85–95. [DOI] [PubMed] [Google Scholar]
- 5.Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Prev Chronic Dis 2008;5:A35. [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes LA, Arts IC, Ambergen T, Brants HA, Dagnelie PC, Goldbohm RA, van den Brandt PA, Weijenberg MP. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: a longitudinal analysis from the Netherlands Cohort Study. Am J Clin Nutr 2008;88:1341–52. [DOI] [PubMed] [Google Scholar]
- 7.Carper JL, Orlet Fisher J, Birch LL. Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite 2000;35:121–9. [DOI] [PubMed] [Google Scholar]
- 8.Lakkakula AP, Zanovec M, Silverman L, Murphy E, Tuuri G. Black children with high preferences for fruits and vegetables are at less risk of being at risk of overweight or overweight. J Am Diet Assoc 2008;108:1912–5. [DOI] [PubMed] [Google Scholar]
- 9.Gidding SS, Lichtenstein AH, Faith MS, Karpyn A, Mennella JA, Popkin B, Rowe J, Van Horn L, Whitsel L. Implementing American Heart Association pediatric and adult nutrition guidelines: a scientific statement from the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular Disease in the Young, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council for High Blood Pressure Research. Circulation 2009;119:1161–75. [DOI] [PubMed] [Google Scholar]
- 10.Epstein LH, Paluch RA, Beecher MD, Roemmich JN. Increasing healthy eating vs. reducing high energy-dense foods to treat pediatric obesity. Obesity (Silver Spring) 2008;16:318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser KA, Brown AW, Bohan Brown MM, Shikany JM, Mattes RD, Allison DB. Increased fruit and vegetable intake has no discernible effect on weight loss: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MK, Pac S, Devaney B, Jankowski L. Feeding infants and toddlers study: what foods are infants and toddlers eating? J Am Diet Assoc 2004;104:s22–30. [DOI] [PubMed] [Google Scholar]
- 13.Mennella JA, Ziegler P, Briefel R, Novak T. Feeding Infants and Toddlers Study: the types of foods fed to Hispanic infants and toddlers. J Am Diet Assoc 2006;106:S96–106. [DOI] [PubMed] [Google Scholar]
- 14.Kim SA, Moore LV, Galuska D, Wright AP, Harris D, Grummer-Strawn LM, Merlo CL, Nihiser AJ, Rhodes DG. Vital signs: fruit and vegetable intake among children—United States, 2003–2010. MMWR Morb Mortal Wkly Rep 2014;63:671–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz MB, Henderson KE, Read M, Danna N, Ickovics JR. doi: 10.1089/chi.2015.0019. New school meal regulations increase fruit consumption and do not increase total plate waste. Child Obes 2015 Mar 3 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc 2010;110:S38–51. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler P, Hanson C, Ponza M, Novak T, Hendricks K. Feeding Infants and Toddlers Study: meal and snack intakes of Hispanic and non-Hispanic infants and toddlers. J Am Diet Assoc 2006;106:S107–23. [DOI] [PubMed] [Google Scholar]
- 18.Siega-Riz AM, Carson T, Popkin B. Three squares or mostly snacks–what do teens really eat? A sociodemographic study of meal patterns. J Adolesc Health 1998;22:29–36. [DOI] [PubMed] [Google Scholar]
- 19.Nicklas TA, Demory-Luce D, Yang SJ, Baranowski T, Zakeri I, Berenson G. Children's food consumption patterns have changed over two decades (1973–1994): The Bogalusa heart study. J Am Diet Assoc 2004;104:1127–40. [DOI] [PubMed] [Google Scholar]
- 20.Mannino ML, Lee Y, Mitchell DC, Smiciklas-Wright H, Birch LL. The quality of girls’ diets declines and tracks across middle childhood. Int J Behav Nutr Phys Act 2004;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Affenito SG, Striegel-Moore R, Khoury PR, Barton B, Crawford P, Kronsberg S, Schreiber G, Obarzanek E, Daniels S. Fast-food intake and diet quality in black and white girls: the National Heart, Lung, and Blood Institute Growth and Health Study. Arch Pediatr Adolesc Med 2005;159:626–31. [DOI] [PubMed] [Google Scholar]
- 22.Birch LL. Psychological influences on the childhood diet. J Nutr 1998;128:407S–10S. [DOI] [PubMed] [Google Scholar]
- 23.Butte N, Cobb K, Dwyer J, Graney L, Heird W, Rickard K. The start healthy feeding guidelines for infants and toddlers. J Am Diet Assoc 2004;104:442–54. [DOI] [PubMed] [Google Scholar]
- 24.Lucas A. Programming by early nutrition: an experimental approach. J Nutr 1998;128:401S–6S. [DOI] [PubMed] [Google Scholar]
- 25.Mennella JA. Ontogeny of taste preferences: basic biology and implications for health. Am J Clin Nutr 2014;99:704S–11S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forestell CA, Mennella JA. The ontogeny of taste perception and preference throughout childhood. In: Doty RL, editor. Handbook of olfaction and gustation. 3rd ed. Wilmington (DE): Wiley, John & Sons, Inc.; 2015. p. 797–830. [Google Scholar]
- 27.Mennella JA, Pepino MY, Duke FF, Reed DR. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet 2010;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mennella JA, Lukasewycz LD, Griffith JW, Beauchamp GK. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem Senses 2011;36:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desor JA, Maller O, Turner RE. Preference for sweet in humans: infants, children and adults. In: Weiffenback JM, editor. Taste and development: the genesis of sweet preference. Washington (DC): US Government Printing Office; 1977. p. 161–72. [Google Scholar]
- 30.Cowart BJ. Development of taste perception in humans: sensitivity and preference throughout the life span. Psychol Bull 1981;90:43–73. [PubMed] [Google Scholar]
- 31.Liem DG, Mennella JA. Sweet and sour preferences during childhood: role of early experiences. Dev Psychobiol 2002;41:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liem DG, Mennella JA. Heightened sour preferences during childhood. Chem Senses 2003;28:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liem DG, de Graaf C. Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol Behav 2004;83:421–9. [DOI] [PubMed] [Google Scholar]
- 34.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev 2001;25:53–74. [DOI] [PubMed] [Google Scholar]
- 35.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mennella JA. The sweet taste of childhood. In: Firestein S, Beauchamp GK, editors. The senses: a comprehensive reference, Vol. 4. Olfaction and taste. San Diego (CA): Elsevier; 2008. p. 183–8. [Google Scholar]
- 37.Maller O, Turner RE. Taste in acceptance of sugars by human infants. J Comp Physiol Psychol 1973;84:496–501. [DOI] [PubMed] [Google Scholar]
- 38.Steiner JE. Human facial expressions in response to taste and smell stimulation. Adv Child Dev Behav 1979;13:257–95. [DOI] [PubMed] [Google Scholar]
- 39.Desor JA, Beauchamp GK. Longitudinal changes in sweet preferences in humans. Physiol Behav 1987;39:639–41. [DOI] [PubMed] [Google Scholar]
- 40.Mennella JA, Pepino MY, Lehmann-Castor SM, Yourshaw LM. Sweet preferences and analgesia during childhood: effects of family history of alcoholism and depression. Addiction 2010;105:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pepino MY, Mennella JA. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain 2005;119:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mennella JA, Finkbeiner S, Lipchock SV, Hwang LD, Reed DR. Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLoS ONE 2014;9:e92201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coldwell SE, Oswald TK, Reed DR. A marker of growth differs between adolescents with high vs. low sugar preference. Physiol Behav 2009;96:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desor JA, Maller O, Andrews K. Ingestive responses of human newborns to salty, sour, and bitter stimuli. J Comp Physiol Psychol 1975;89:966–70. [DOI] [PubMed] [Google Scholar]
- 45.Kajiura H, Cowart BJ, Beauchamp GK. Early developmental change in bitter taste responses in human infants. Dev Psychobiol 1992;25:375–86. [DOI] [PubMed] [Google Scholar]
- 46.Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medicines: overview of basic research on bitter taste. Clin Ther 2013;35:1225–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandell MA, Breslin PA. Variability in a taste-receptor gene determines whether we taste toxins in food. Curr Biol 2006;16:R792–4. [DOI] [PubMed] [Google Scholar]
- 48.Reed DR, Tanaka T, McDaniel AH. Diverse tastes: genetics of sweet and bitter perception. Physiol Behav 2006;88:215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 2010;35:157–70. [DOI] [PubMed] [Google Scholar]
- 50.Meyerhof W. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol 2005;154:37–72. [DOI] [PubMed] [Google Scholar]
- 51.Lipchock SV, Mennella JA, Spielman AI, Reed DR. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am J Clin Nutr 2013;98:1136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr 2008;28:367–88. [DOI] [PubMed] [Google Scholar]
- 53.Bell KI, Tepper BJ. Short-term vegetable intake by young children classified by 6-n-propylthoiuracil bitter-taste phenotype. Am J Clin Nutr 2006;84:245–51. [DOI] [PubMed] [Google Scholar]
- 54.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 2003;299:1221–5. [DOI] [PubMed] [Google Scholar]
- 55.Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol 2005;15:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim U, Wooding S, Ricci D, Jorde LB, Drayna D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat 2005;26:199–204. [DOI] [PubMed] [Google Scholar]
- 57.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 2005;115:e216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turnbull B, Matisoo-Smith E. Taste sensitivity to 6-n-propylthiouracil predicts acceptance of bitter-tasting spinach in 3–6-y-old children. Am J Clin Nutr 2002;76:1101–5. [DOI] [PubMed] [Google Scholar]
- 59.Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite 2002;38:3–12. [DOI] [PubMed] [Google Scholar]
- 60.Fisher JO, Mennella JA, Hughes SO, Liu Y, Mendoza PM, Patrick H. Offering "dip" promotes intake of a moderately-liked raw vegetable among preschoolers with genetic sensitivity to bitterness. J Acad Nutr Diet 2012;112:235–45. [DOI] [PubMed] [Google Scholar]
- 61.Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol Behav 2006;87:304–13. [DOI] [PubMed] [Google Scholar]
- 62.Duffy VB, Bartoshuk LM. Food acceptance and genetic variation in taste. J Am Diet Assoc 2000;100:647–55. [DOI] [PubMed] [Google Scholar]
- 63.Mennella JA, Reed DR, Roberts KM, Mathew PS, Mansfield CJ. Age-related differences in bitter taste and efficacy of bitter blockers. PLoS ONE 2014;9:e103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mennella JA, Reed DR, Mathew PS, Roberts KM, Mansfield CJ. “A spoonful of sugar helps the medicine go down”: bitter masking by sucrose among children and adults. Chem Senses 2015;40:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharafi M, Hayes JE, Duffy VB. Masking vegetable bitterness to improve palatability depends on vegetable type and taste phenotype. Chemosens Percept 2013;6:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Capaldi ED, Privitera GJ. Decreasing dislike for sour and bitter in children and adults. Appetite 2008;50:139–45. [DOI] [PubMed] [Google Scholar]
- 67.Mennella JA. The chemical senses and the development of flavor preferences in humans. In: Hale TW, Hartmann PE, editors. Textbook on human lactation. Amarillo (TX): Hale Publishing; 2007. p. 403–14. [Google Scholar]
- 68.Mennella JA, Johnson A, Beauchamp GK. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses 1995;20:207–9. [DOI] [PubMed] [Google Scholar]
- 69.Mennella JA, Beauchamp GK. Smoking and the flavor of breast milk. N Engl J Med 1998;339:1559–60. [DOI] [PubMed] [Google Scholar]
- 70.Mennella JA, Beauchamp GK. Maternal diet alters the sensory qualities of human milk and the nursling's behavior. Pediatrics 1991;88:737–44. [PubMed] [Google Scholar]
- 71.Mennella JA, Beauchamp GK. The transfer of alcohol to human milk. Effects on flavor and the infant's behavior. N Engl J Med 1991;325:981–5. [DOI] [PubMed] [Google Scholar]
- 72.Mennella JA, Beauchamp GK. The human infants’ responses to vanilla flavors in mother’s milk and formula. Infant Behav Dev 1996;19:13–9. [Google Scholar]
- 73.Mennella JA, Beauchamp GK. Experience with a flavor in mother's milk modifies the infant's acceptance of flavored cereal. Dev Psychobiol 1999;35:197–203. [DOI] [PubMed] [Google Scholar]
- 74.Schaal B, Marlier L, Soussignan R. Human foetuses learn odours from their pregnant mother's diet. Chem Senses 2000;25:729–37. [DOI] [PubMed] [Google Scholar]
- 75.Désage M, Schaal B, Soubeyrand J, Orgeur P, Brazier JL. Gas chromatographic-mass spectrometric method to characterise the transfer of dietary odorous compounds into plasma and milk. J Chromatogr B Biomed Appl 1996;678:205–10. [DOI] [PubMed] [Google Scholar]
- 76.Hausner H, Bredie WL, Mǿlgaard C, Petersen MA, Mǿller P. Differential transfer of dietary flavour compounds into human breast milk. Physiol Behav 2008;95:118–24. [DOI] [PubMed] [Google Scholar]
- 77.Barker E. Sensory evaluation of human milk [MS thesis]. Manitoba (Canada): University of Manitoba;1980.
- 78.Park SY, Paik HY, Skinner JD, Ok SW, Spindler AA. Mothers’ acculturation and eating behaviors of Korean American families in California. J Nutr Educ Behav 2003;35:142–7. [DOI] [PubMed] [Google Scholar]
- 79.Skinner JD, Carruth BR, Wendy B, Ziegler PJ. Children’s food preferences: a longitudinal analysis. J Am Diet Assoc 2002;102:1638–47. [DOI] [PubMed] [Google Scholar]
- 80.Provenza FD, Launchbauch KL. Foraging on the edge of chaos. In: Launchbaugh KL, Sanders KD, Mosley JC, editors. Grazing behavior of livestock and wildlife. Idaho Forest, Wildlife and Range Experiment Station. Bulletin No.70. Moscow (ID): University of Idaho; 1999. p. 1–12. [Google Scholar]
- 81.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001;107:E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Todrank J, Heth G, Restrepo D. Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proc Biol Sci 2011;278:1949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nicklaus S, Boggio V, Chabanet C, Issanchou S. A prospective study of food preferences in childhood. Food Qual Prefer 2004;15:805–18. [Google Scholar]
- 84.Galloway AT, Lee Y, Birch LL. Predictors and consequences of food neophobia and pickiness in young girls. J Am Diet Assoc 2003;103:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mennella JA, Nicklaus S, Jagolino AL, Yourshaw LM. Variety is the spice of life: strategies for promoting fruit and vegetable acceptance during infancy. Physiol Behav 2008;94:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forestell CA, Mennella JA. Early determinants of fruit and vegetable acceptance. Pediatrics 2007;120:1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerrish CJ, Mennella JA. Flavor variety enhances food acceptance in formula-fed infants. Am J Clin Nutr 2001;73:1080–5. [DOI] [PubMed] [Google Scholar]
- 88.Caton SJ, Ahern SM, Remy E, Nicklaus S, Blundell P, Hetherington MM. Repetition counts: repeated exposure increases intake of a novel vegetable in UK pre-school children compared to flavour-flavour and flavour-nutrient learning. Br J Nutr 2013;109:2089–97. [DOI] [PubMed] [Google Scholar]
- 89.Sullivan SA, Birch LL. Infant dietary experience and acceptance of solid foods. Pediatrics 1994;93:271–7. [PubMed] [Google Scholar]
- 90.Forestell CA, Mennella JA. More than just a pretty face. The relationship between infant's temperament, food acceptance, and mothers’ perceptions of their enjoyment of food. Appetite 2012;58:1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Remy E, Issanchou S, Chabanet C, Nicklaus S. Repeated exposure of infants at complementary feeding to a vegetable puree increases acceptance as effectively as flavor-flavor learning and more effectively than flavor-nutrient learning. J Nutr 2013;143:1194–200. [DOI] [PubMed] [Google Scholar]
- 92.Lundy B, Field T, Carraway K, Hart S, Malphurs J, Rosenstein M, Pelaez-Nogueras M, Coletta F, Ott D, Hernandez-Reif M. Food texture preferences in infants versus toddlers. Early Child Dev Care 1998;146:69–85. [Google Scholar]
- 93.Paul IM, Savage JS, Anzman SL, Beiler JS, Marini ME, Stokes JL, Birch LL. Preventing obesity during infancy: a pilot study. Obesity (Silver Spring) 2011;19:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hetherington MM, Schwartz C, Madrelle J, Croden F, Nekitsing C, Vereijken CM, Weenen H. A step-by-step introduction to vegetables at the beginning of complementary feeding. The effects of early and repeated exposure. Appetite 2015;84:280–90. [DOI] [PubMed] [Google Scholar]
- 95.Birch LL, Marlin DW. I don't like it; I never tried it: effects of exposure on two-year-old children's food preferences. Appetite 1982;3:353–60. [DOI] [PubMed] [Google Scholar]
- 96.Birch LL, McPhee L, Shoba BC, Pirok E, Steinberg L. What kind of exposure reduces children’s food neophobia? Looking vs. tasting. Appetite 1987;9:171–8. [DOI] [PubMed] [Google Scholar]
- 97.Rozin P. The acquisition of food habits and preferences. In: Mattarazzo JD, Weiss SM, Herd JA, Miller NE, Weiss SM, editors. Behavioral health: a handbook of health enhancement and disease prevention. New York: John Wiley and Sons; 1984. p. 590–607. [Google Scholar]
- 98.Ekman P, Friesen W. The facial action coding system. Palo Alto (CA): Consulting Psychologists Press; 1978. [Google Scholar]
- 99.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 2000;24:173–98. [DOI] [PubMed] [Google Scholar]
- 100.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 1996;20:1–25. [DOI] [PubMed] [Google Scholar]
- 101.Forestell CA, LoLordo VM. Palatability shifts in taste and flavour preference conditioning. Q J Exp Psychol B 2003;56:140–60. [DOI] [PubMed] [Google Scholar]
- 102.Mennella JA, Trabulsi JC. Complementary foods and flavor experiences: setting the foundation. Ann Nutr Metab 2012;60(Suppl2):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Birch LL. Effects of peer models’ food choices and eating behaviors on preschoolers’ food preferences. Child Dev 1979;51:489–96. [Google Scholar]
- 104.Sullivan SA, Birch LL. Pass the sugar, pass, the salt: experience dictates preference. Dev Psychol 1990;26:546–51. [Google Scholar]
- 105.Zeinstra GG, Koelen MA, Kok FJ, de Graaf C. Cognitive development and children's perceptions of fruit and vegetables; a qualitative study. Int J Behav Nutr Phys Act 2007;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mathias KC, Rolls BJ, Birch LL, Kral TV, Hanna EL, Davey A, Fisher JO. Serving larger portions of fruits and vegetables together at dinner promotes intake of both foods among young children. J Acad Nutr Diet 2012;112:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guenther PM, Dodd KW, Reedy J, Krebs-Smith SM. Most Americans eat much less than recommended amounts of fruits and vegetables. J Am Diet Assoc 2006;106:1371–9. [DOI] [PubMed] [Google Scholar]
- 108.Worobey H, Ostapkovich K, Yudin K, Worobey J. Trying versus liking fruits and vegetables: correspondence between mothers and preschoolers. Ecol Food Nutr 2010;49:87–97. [DOI] [PubMed] [Google Scholar]
- 109.Birch LL, Anzman-Frasca S, Paul IM. Starting early: obesity prevention during infancy. Nestle Nutr Inst Workshop Ser 2012;73:81–94. [DOI] [PubMed] [Google Scholar]
- 110.Savage JS, Fisher JO, Birch LL. Parental influence on eating behavior: conception to adolescence. J Law Med Ethics 2007;35:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hart CN, Raynor HA, Jelalian E, Drotar D. The association of maternal food intake and infants’ and toddlers’ food intake. Child Care Health Dev 2010;36:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fisher JO, Mitchell DC, Smiciklas-Wright H, Birch LL. Parental influences on young girls’ fruit and vegetable, micronutrient, and fat intakes. J Am Diet Assoc 2002;102:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roe LS, Meengs JS, Birch LL, Rolls BJ. Serving a variety of vegetables and fruit as a snack increased intake in preschool children. Am J Clin Nutr 2013;98:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Foterek K, Hilbig A, Alexy U. Associations between commercial complementary food consumption and fruit and vegetable intake in children. Results of the DONALD study. Appetite 2015;85:84–90. [DOI] [PubMed] [Google Scholar]
- 115.Christian MS, Evans CE, Nykjaer C, Hancock N, Cade JE. Evaluation of the impact of a school gardening intervention on children's fruit and vegetable intake: a randomised controlled trial. Int J Behav Nutr Phys Act 2014;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mennella JA, Beauchamp GK. The role of early life experiences in flavor perception and delight. In: Dube L, Dagher A, Drewnowski A, LeBel J, Richard JP, Yada RY, editors. Obesity prevention. The role of society and brain on individual behavior. London: Elsevier; 2010. p. 203–18. [Google Scholar]
- 117.Trabulsi JC, Mennella JA. Diet, sensitive periods in flavour learning, and growth. Int Rev Psychiatry 2012;24:219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mangold JE, Hill DL. Extensive reorganization of primary afferent projections into the gustatory brainstem induced by feeding a sodium-restricted diet during development: less is more. J Neurosci 2007;27:4650–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moskowitz HW, Kumaraiah V, Sharma KN, Jacobs HL, Sharma SD. Cross-cultural differences in simple taste preferences. Science 1975;190:1217–8. [DOI] [PubMed] [Google Scholar]
- 120.Cooke LJ, Haworth CM, Wardle J. Genetic and environmental influences on children's food neophobia. Am J Clin Nutr 2007;86:428–33. [DOI] [PubMed] [Google Scholar]
- 121.Faith MS, Heo M, Keller KL, Pietrobelli A. Child food neophobia is heritable, associated with less compliant eating, and moderates familial resemblance for BMI. Obesity (Silver Spring) 2013;21:1650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cassells EL, Magarey AM, Daniels LA, Mallan KM. The influence of maternal infant feeding practices and beliefs on the expression of food neophobia in toddlers. Appetite 2014;82:36–42. [DOI] [PubMed] [Google Scholar]
- 123.Perry RA, Mallan KM, Koo J, Mauch CE, Daniels LA, Magarey AM. Food neophobia and its association with diet quality and weight in children aged 24 months: a cross sectional study. Int J Behav Nutr Phys Act 2015;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]