Abstract
The purpose of this review is to draw attention to the limited information available on food intake (FI) control in children and adolescents 7–17 y of age, which is essential for developing food policies and guidelines in this population. Although environmental factors have been the overwhelming focus of research on the causative factors of obesity, research focusing on the physiologic control of appetite in children and adolescents is a neglected area of research. To present this message, a review of FI regulation and the role of food and food components in signaling processes are followed by an examination of the role of hormones during puberty in intake regulation. To examine the interaction of environment and physiology on FI regulation, the effects of exercise, television programs, and food advertisements are discussed. In conclusion, although limited, this literature review supports a need for children and adolescents to be a greater focus of research that would lead to sound nutrition policies and actions to reduce chronic disease. A focus on the environment must be balanced with an understanding of physiologic and behavioral changes associated with this age group.
Keywords: food intake regulation, children, macronutrients, puberty, hormones, physical activity, food advertisements
Introduction
The increased prevalence of obesity in children and adolescents is due to a complex interplay between environmental and behavioral factors that affect the physiologic regulation of energy balance. Food factors include an inexpensive food supply, ready availability and consumption of high-energy–dense foods, food variety, and increased portion sizes (1–3). Environmental factors include time spent in sedentary pursuits, the structure of the built environment, schools and day care, parenting styles, and peer pressure in children and adolescents. Many public health actions have been trying to address these issues, yet obesity in children has not decreased (3).
Although environmental factors have been the overwhelming focus of research on the causative factors of obesity (3–5), research focusing on the physiologic control of appetite in children is a neglected area. Physiologic factors affecting intake control during childhood and adolescence include age, sex, pubertal stage, body fatness, and the macronutrient composition of food. It is unclear whether obesity develops because physiologic mechanisms of food intake (FI)6 control are compromised first or if these are simply overridden by the environment and become compromised (1). Understanding how these determinants interact to disrupt the delicate balance between energy intake and energy expenditure is necessary to prevent overweight and obesity in children. It is also needed as the foundation for evidence-based food policies and decisions being made to change the food environment for children.
The purpose of this review is to draw attention to the limited information available on FI control in children and adolescents 7–17 y of age and for food policies and guidelines in this population. As noted in the other reports from this symposium, infants and toddlers have been a focus of dietary advice encompassing a crucial stage of development (6). However, to preserve health and prevent development of chronic disease, a continued understanding throughout childhood and adolescence is required.
The emerging message is that the physiologic regulation of FI continues to evolve during childhood, differing from adulthood and involving environmental factors that have the potential to override and permanently alter physiologic mechanisms, leading to a lifelong struggle to achieve a healthy energy balance. To present this message, a review of FI regulation and the role of food and food components in the signaling processes is followed by an examination of the role of hormones during puberty in intake regulation. To show the interaction of environment and physiology on FI regulation, the effects of exercise, television programs, and food advertisements are discussed.
FI Regulation (Overview)
Research on the regulation of FI has intensified with the increased prevalence of overweight and obesity in all age groups. The majority of research has focused on dietary and pharmaceutical treatments in adults. However, little attention has been given to understanding the regulation of FI and body weight in normal-weight and obese children, or during puberty, and how this regulation is affected by environmental factors.
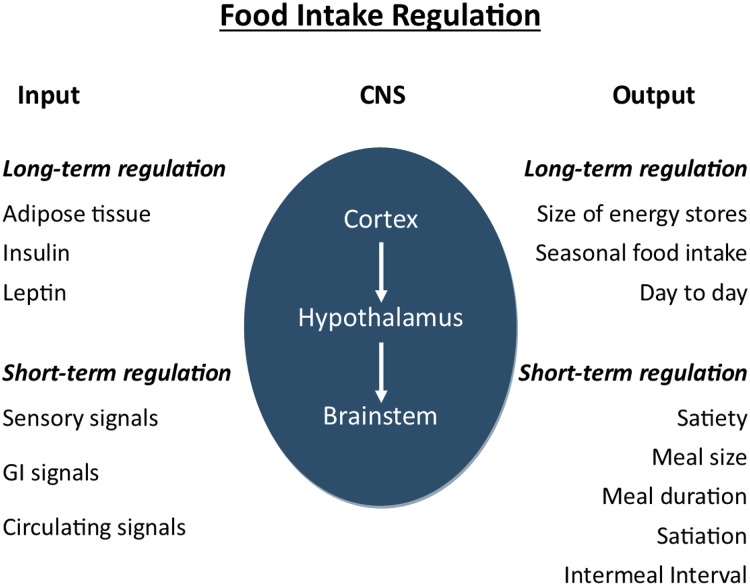
FI regulation (Figure 1) is a precise biological process that involves the integration of complex homeostatic mechanisms in the central nervous system (CNS) arising from peripherally derived signals (7, 8). These signals include sensory properties of foods, mechanical and chemical receptors in the gastrointestinal tract, gut hormones, and circulating metabolites (9, 10). The hypothalamus, brainstem, and cortex integrate these signals and translate them into information regulating meal size and duration; interval to the next meal; the amount of food consumed throughout the day or over several days, weeks, and months; and possibly the composition of food, as well as the intake of total energy. This long-term regulation of FI is mediated by leptin and insulin, which are secreted in proportion to the adipose tissue mass, exerting their action in the hypothalamus. They also act synergistically with gut hormones in the regulation of short-term FI.
FIGURE 1.
Physiology of food intake regulation. Adapted with permission from reference 7. CNS, central nervous system; GI, gastrointestinal.
At present, the relative importance of each of these signals contributing to satiety is unclear. Energy imbalance could theoretically arise from errors in the control of many aspects of short-term intake, as well as in the long-term regulation of intake. Furthermore, it is possible the plasticity of the regulatory system before and during puberty could lead to a decreased sensitivity to satiety signals, thus contributing to obesity. For example, some research shows that compensation for the energy content of preloads consumed before test meals is highly variable among children, being less precise in older children than in younger children (11).
Appetite-regulating signals arise primarily through gastric activation. Gastric activation occurs at the sight, smell, or mere thought of food (cephalic phase), through the stimulation of stretch- and chemical receptors after food is being ingested (gastric phase), and upon the arrival of partially digested proteins and amino acids in the duodenum (intestinal phase) (12). These appetite-regulating signals include anorexic hormones or appetite-suppressing signals from the small intestine, including cholecystokinin, glucagon-like peptide (GLP) 1 and 2, bombesin, gastrin-releasing peptide, neuromedin B, glucagon, apo A-IV, amylin, somatostatin, enterostatin, and peptide YY (PYY) (3–36), and from the stomach, leptin and the only known appetite-stimulating signal, ghrelin (13, 14). Many of the actions of gastrointestinal hormones are expressed via their receptors in the CNS (15). Some enter the central circulation via brainstem and hindbrain, “leaky areas” in the blood–brain barrier, or send signals through vagal afferents to the hypothalamus. The release of gut hormones is also macronutrient-dependent, explaining, at least in part, differences in the satiating and satiety effect of macronutrients. For example, in humans, protein is a stronger secretagogue of cholecystokinin and GLP-1 than are carbohydrate and fat (14, 16).
Postabsorptive signals are generated after nutrients have been digested and have entered the circulation, where they stimulate satiety centers in the brain by endocrine and metabolic actions. The glucostatic, aminostatic, and lipostatic hypotheses of energy intake control have been the main theories describing how absorbed nutrients generate and influence satiety signals in the CNS (7).
Dietary Components and FI Regulation
The composition of food, in addition to energy, contributes to both the short- and long-term regulation of FI. One of the challenges here is to understand their relative importance and how to optimize their interaction with intake regulatory systems for the purpose of achieving optimal energy balance.
Carbohydrates
All macronutrients provide energy; however, studies of their influence on FI in adults have made it clear that their effects cannot be predicted from their caloric content alone. Each macronutrient exerts specific satiating effects independent of their caloric value (7, 17), as described in the following.
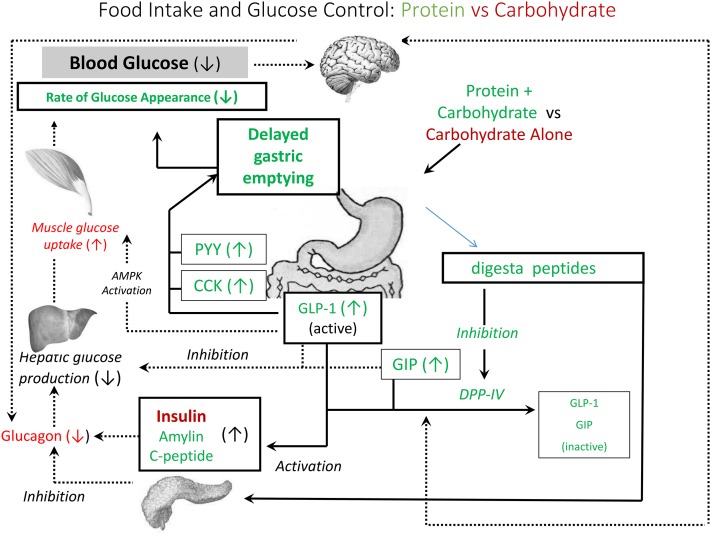
Carbohydrates are the primary macronutrient source most commonly consumed, averaging ∼50% of total energy intake (18). Carbohydrate consumption increases blood glucose, which is associated with increased satiety and reduced FI (7), consistent with the glucostatic theory of FI regulation as proposed 65 y ago by Mayer (19). However, in addition to glucose, the release of insulin and gut hormones, including GLP-1 and cholecystokinin, elicit carbohydrate-induced satiety by affecting gastric emptying rate and stimulating satiety signals in the CNS (Figure 2) (20). Satiety signals arising from sugars are unique compared with those arising from other carbohydrates, because sweet-tasting products also lead to sensory specific satiety (21).
FIGURE 2.
Interaction of protein and carbohydrate in the regulation of food intake and postprandial glycemia. Glucose control by carbohydrate is shown in red. Glucose control by protein is shown in green. AMPK, AMP-activated protein kinase; CCK, cholecystokinin; DPP-IV, dipeptidyl peptidase-4; GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide; PYY, peptide YY.
In children and adults, compensation for energy consumed as carbohydrates may be dependent on the quality and timing of the preload before the measurement of FI. Mealtime intake regulation for previously consumed calories is more accurate if the duration between the preload and the meal is short. For example, preschool children aged 2.5–5 y compensated accurately at a lunch buffet given 20–30 min later for the energy content of premeal carbohydrate puddings of varying energy densities (22). Similarly, snacks of raisins, grapes, and almonds, eaten freely or in fixed amounts, suppressed FI at a meal 30 min later in 8–12-y-old boys and girls (23). However, children aged 4–6 y did not compensate at a lunch buffet after a high carbohydrate (82%) yogurt preload when the time interval between the preload and test meal was extended to 90 min (24).
The effect of ingestion of beverage calories is also time-dependent. Sugars, when consumed in liquid form shortly before meals, do not bypass regulatory systems in adults. Adults readily decrease FI to compensate for the calories consumed if they are within 30–60 min of the later meal (25, 26). Similarly, preschool children aged 3–5 y demonstrated almost perfect caloric compensation in a test meal given 30 and 60 min post-preload ingestion of beverages containing sucrose, low glucose maltodextrin, or a combination of the 2 (average of 1.25 g/kg of body weight) (27). In contrast, caloric beverages at mealtime add calories to the mealtime occasion and have no impact on the amount of food eaten, suggesting that thirst might be driving the need for fluids and is the reason caloric content is not registered (26).
Poorer self-regulation of FI in children has been associated with increased adiposity (28), but this observation may be limited by failure to provide test treatments on the basis of body weight. However, this may depend on the composition of the source of calories. In one report, both normal-weight and obese boys aged 9–14 y reduced their FI 30 min after consumption of a glucose drink (50 g), suggesting that satiety response to carbohydrates is not affected by obesity (29).
Protein
In contrast to many studies in adults, relatively little is known about the effect of protein on FI in children and adolescents. Short-term satiety is increased by dietary protein when compared with carbohydrate or fat in adults, as indicated by both quantitative and subjective measures (14, 30). In adults, the effect of protein on FI is dependent on source, dose, form (solid vs. liquid), the presence of other macronutrients, and time to the next meal. Although little studied, there is also evidence that protein is more satiating than carbohydrate in children. Young children aged 5–6 y given a high carbohydrate (∼67 g carbohydrate) or a high protein (∼46 g protein) meal, consumed less energy from an afternoon snack after the high protein meal (31).
When protein and carbohydrates were given separately, normal-weight boys aged 9–14 y decreased FI after glucose (50 g) and a whey protein (50 g) drinks at a pizza meal 30 min later. In contrast, in obese boys of the same age, only 50 g of glucose but not 50 g of whey protein suppressed FI 30 min later (29), suggesting that the effect of protein on FI is body weight–dependent, although the effect of carbohydrate may not be.
Fat
Over the past 4 decades, dietary fat has been associated with a high prevalence of obesity (33). Consequently, dietary guidelines have recommended reducing fat intake. Studies report that dietary fat intake is not related to obesity (34), and the relation between dietary fat and the development of obesity is still unclear (35). In adults, experimental studies show that protein is more satiating than carbohydrate, which is more satiating than fat (36, 37), indicating that fat may contribute to excess energy intake. The effect of fat on intake regulatory mechanisms suggests the opposite, because it stimulates release of cholecystokinin, slows gastric emptying and provides a signal to the CNS (38), and regulates food transit in the small intestine via the vagus nerve (39, 40), which may explain why some studies have found that fat is more satiating than carbohydrates (41). Furthermore, outcomes of studies of fat and appetite vary depending on FA composition, or if fat is studied alone or within a meal and if individuals consume a habitual high- or low-fat diet. Meals containing 42% of total energy as fat from MUFAs produced a lower satiety response than did meals high in PUFAs and SFAs (42). Safflower oil preloads in water suppress short-term FI less than an equivalent energy preload containing glucose in young adults consuming a later meal (43). However, another report suggests that such comparisons are affected by habitual diet. Individuals who habitually consume a high-fat diet (46.7% energy) display higher baseline hunger, but also a greater suppression of hunger, in response to a meal than do individuals on a lower-fat diet (29.9% energy) (44).
The effects of fat on satiety in children and adolescents are few but, as in adults, responses may be influenced by many similar factors, including body weight (29), as well as physiologic and hormonal changes through puberty (32). In contrast to adults, preschool children aged 2–5 y showed excellent compensation for energy density differences produced by manipulating the fat content of ice cream (45) and yogurt (46) or by replacing dietary fat by a nonenergy fat substitute (47). Similarly, a recent study reported similar FI at a meal with a fixed amount of meat with full-fat french fries vs. baked fries (48). However, in another study, children aged 4–6 y did not compensate for a yogurt preload high in fat (71%) at a lunch buffet 90 min later (24). In addition, there is one report showing that obese children have a higher preference for fatty foods (49), perhaps because of a weaker response to the satiating properties of fat. Consistent with this hypothesis, small high-fat meals (450 kcal) maintained higher ghrelin and lower PYY responses in obese children than in 7–11-y-old normal-weight pre- and peripubertal children, suggesting that obese children are more vulnerable to overeating if given high-fat foods or meals (50). However, the serving size of the meals was small, not provided on a body weight basis, and neither appetite nor FI was measured. This leaves uncertainty about the role of fat in satiation and hormonal responses after meals eaten to satiation in children and adolescents, and merits further investigation.
Food combinations in the regulation of FI
An understanding of the mechanism of action of each of the macronutrients in FI control has proven useful, but it overlooks the benefit of food combinations as usually consumed in meals in determining FI and metabolic control. For example, carbohydrates affect glucose regulation and satiety primarily by their direct effects on insulin (Figure 2). However, when consumed with protein, meals trigger the release of many hormones and bioactive peptides, including PYY, cholecystokinin, GLP-1, and gastric inhibitory polypeptide, which signal satiety, reduce stomach emptying, and contribute to reduced blood glucose concentrations (30).
Based on the physiology of FI control, it is clear that methods describing the physiologic effects of macronutrients in isolation may lead to misleading mealtime advice. This can be easily illustrated by postprandial glucose responses in studies investigating the consumption of carbohydrates with a protein or at a meal. Mealtime advice for carbohydrates is often based on their effect on blood glucose as defined by the glycemic index (GI) (51). The GI measures the incremental AUC for 2 h blood glucose response after consumption of a fixed amount (usually 50 g) of available carbohydrates in a test food. The GI of foods, compared to glucose as the reference value, is arbitrarily classified as low (≤55), intermediate (56–69), and high (≥70). A similar approach has been taken to characterize the satiety index (SI) of carbohydrates. In these studies, participants rank their subjective feelings of hunger over 2 h after consuming foods containing a fixed amount of carbohydrate. Using these methods, the GIs of boiled potatoes, pasta, or rice were 65–91, 43–55, and 60–86, and the SI values were 323, 119, and 138, respectively, showing an inverse relation between GI and SI (52). This relation is consistent with the physiologic role of glucose in FI regulation (53). However, both the GI and SI are based on the consumption of a fixed amount (e.g., 50 g) of available carbohydrate, which may not be representative of the amount of carbohydrates commonly consumed within a meal or predictive of a postmeal glycemic or satiety response if eaten with protein.
Because potatoes fall into the category of high-GI foods, many dietary guidelines recommend decreasing their consumption, which fails to recognize not only that within a meal they are consumed with protein foods, but also that they are nutrient-rich vegetables (54). As a result, over the past 40 y, the consumption of potatoes has decreased by 41% (54). Fried potatoes have been removed from school cafeterias, and fast food restaurants are under pressure to remove or reduce serving sizes because of observational studies linking their consumption to increased risk of obesity (55). However, the unintended consequence of this has not been the expected decrease in high GI carbohydrates but rather increased consumption of energy-dense, nutrient-low starchy foods, such as rice and pasta, many of which are high-GI, with no discernible decrease in the upward trajectory of the number overweight and obese individuals.
There is an abundance of evidence that the GI of a fixed portion of these carbohydrates is readily modified by the addition of a protein source (54). For example, to provide 50 g available carbohydrate, consuming a baked potato with 62 g cheddar cheese reduced the GI from 93 to 39 (56). Similarly, serving mashed potatoes containing 50 g of carbohydrate with oil, chicken breast, and salad as a meal reduced the GI of the potato from 108 to 54 (57). The high GI of rice is also markedly reduced by other food components in a usual meal. Two recent reports that examined the effect of rice on postprandial glycemia after a representative meal with fixed portions concluded that evaluation of white rice as a high GI food should not guide mealtime advice for adults (58, 59). The addition of tofu and egg, along with vegetables and oil (58), or chicken, oil, and vegetables (59) fully attenuated the postprandial glycemia caused by rice.
In addition, protein in an ad libitum meal has the potential to modify postprandial glycemia by reducing the amount of carbohydrate eaten at the meal. Yet, to our knowledge, only one study has reported the effect on energy intake and postprandial glucose of ad libitum access to these carbohydrate sources when consumed by men along with a fixed portion (150 g) of pork steak. FI after an ad libitum potato meal was 31% and 23% lower than with pasta and rice meals, respectively, reflecting the satiating value of potatoes, possibly triggered by their high glycemic characteristics (60). Moreover, no differences between the treatments were found in FI at an ad libitum meal served 4 h later; thus, the potato meal reduced cumulative FI the most over the study period. The consumption of the potato meal also resulted in lower concentrations of blood glucose and insulin than did the rice and pasta meals, which is consistent with the lower carbohydrate intake.
A preliminary report in children also confirms the observation that consuming potatoes with a protein source results in an earlier termination of FI. Normal-weight children aged 11–13 y consuming meals composed of 100 g of meatballs with free access to pasta, rice, and potatoes (baked and fried) ate 30% fewer calories at a meal with boiled and mashed potatoes than at all other meals and produced similar levels of postprandial glycemia. Furthermore, after the meal with full-fat french fries, their caloric intake was not different from baked fries, pasta, or rice, but their postmeal insulin was 30% lower. Fried potatoes also have been shown to reduce postmeal glycemia compared with boiled and mashed potatoes (48). Previous studies moreover have demonstrated an inverse association between glycemic response and satiety, suggesting that high-fat meals may lower postmeal glycemia and thus increase satiety (61).
In summary, it is clear that there is a need for a more thorough understanding of the effects of mealtime composition on FI and metabolic responses in children for the development of food policies and guidelines in this population.
Puberty and Hormonal Regulation of FI in Children
At present, there is no evidence that “errors” in physiologic mechanisms of FI regulation account for overweight or obesity in children. Children’s energy intake is concordant with sex-specific changes in body composition, peak growth velocity and the development of puberty (62, 63). In later puberty, FI is higher in boys than in girls (64). Furthermore, both boys and girls consume more calories in late compared with early puberty, but this is attributable primarily to an increase in body weight (64). However, to understand the relation between satiety and satiation, it is necessary to learn about the associations between food characteristics and satiety signals. Furthermore, puberty may be a vulnerable time for physiologic systems to be permanently altered by the environment. Sex hormones are well known to play a role in appetite control in adults (65), but their interaction with FI and food behavior in children passing through puberty has received only limited attention.
Estrogen and testosterone are the primary sex steroids produced during pubertal development in girls and boys. A role for estrogen and testosterone in FI regulation has been derived primarily from animal studies. Estradiol, the most important form of estrogen, has a suppressive effect on FI in adult rats (66, 67). Consistent with this, energy intake in women is decreased when estrogen is at higher concentrations in the follicular compared with the luteal phase of the menstrual cycle (68). In contrast to estradiol, less is known about testosterone’s role on FI. In adult rats, testosterone injections have a stimulatory effect on FI (65), and orchiectomy in rats decreases FI, an effect reversed by physiologic doses of testosterone (69). A recent report found testosterone concentrations at fasting to be inversely related to fasting ghrelin, and they decreased 60 min after boys consumed a drink containing glucose and whey protein (70). Consistent with this observation, testosterone administration to short peripubertal boys aged 8–12.5 y led to a marked decline in circulating concentrations of ghrelin and leptin (71). However, it remains unclear whether these relations between testosterone and FI-regulating hormones are indicative of the role of testosterone in regulating short-term FI.
Although there are potentially many hormones related to FI, insulin, PYY, ghrelin, and leptin have been shown to interact with sex hormone production. Insulin resistance is a normal physiologic event during puberty (72), increasing by Tanner stage 2, remaining stable between stages 2 and 4, and returning to approximate prepubertal levels by the end of puberty (Tanner stage 5). Ghrelin is a potent stimulator of release of growth hormone (73) and, like leptin, it plays a permissive role in pubertal onset and development (74). Mean ghrelin concentrations in boys in Tanner stages 4 and 5 are about 40% lower than in Tanner stage 1 and are inversely correlated with testosterone concentrations (75). Fasting blood concentrations of both acylated and unacylated ghrelin are higher in prepubertal (Tanner stage 1) than in pubertal (Tanner stages 2–5) normal-weight and obese children, and are inversely related to testosterone and estradiol (75, 76).
In summary, not only is the activity of sex hormones increasing during puberty but also the actions of other hormones regulating FI are modified by their interactions with the sex hormones. Thus it can be hypothesized that puberty is a crucial time of development of FI regulatory mechanisms, which can offer a plausible explanation for why some children become overweight or obese. Of course, in addition to the complex hormonal changes occurring during the advancement of puberty, the environment also may play a role in determining the development of lifelong FI regulatory mechanisms. This is shown by the effects of activity, television programs, and food advertisements on children.
Environmental Factors Affecting FI
The physiologic regulation of FI in children can be overridden by a plethora of environmental factors that are correlated with a risk of obesity. In adults, nonfood-related factors, such as reduced physical activity, alcohol consumption, smoking, socioeconomic status, and mental illness negatively affect body weight. Food-related environmental factors, such as a food’s salience, variety, package or portion size, and palatability, also relate to the way food is provided or presented. Moreover, the mealtime environment and the amount eaten is dependent on how food is obtained; the eating atmosphere; the social interactions that occur with family, friends and peers; and the distractions that may be taking place, including television viewing (5). Nonfood-related, food-related, and mealtime factors may each contribute to how much energy is consumed at a meal, and these factors can also operate together to affect FI. Compared with studies in adults, research with children and adolescents from 7–17 y of age is very limited, leading to the unfortunate assumption that what applies to adults applies to children and can be used to develop dietary guidance for children.
Activity and FI regulation in children
Although there is a large body of evidence showing that declining levels of physical activity are associated with a higher BMI (77), the benefits of physical activity and exercise as a form of structured physical activity for achieving body weight are still under debate. Countless physical activity and dietary programs have been designed for the adult population to counteract the obesity epidemic, without much success (78). However, studies in children are much more limited but are needed for developing evidence-based policies. Physical activity programs, such as the Take Ten program, have been promoted in schools as a means to combat obesity (79). The program encourages teachers to facilitate 10 min bouts of physical activity throughout the school day in order to accumulate a total energy expenditure of 27–30 kcal/d or 300 kcal/wk (80, 81). One of the first studies aimed at validating the program found that 9–14-y-old boys expending 60–80 kcal by exercising for ≤12 min at a low to moderate intensity at the ventilatory threshold increased appetite and prospective food consumption scores (82); however, FI was not measured. The authors hypothesized that FI would be increased with aerobic activity, counteracting the effects on weight loss, because subjective appetite and prospective consumption scores often correlate with FI in adults (83). However, follow-up studies at the ventilatory threshold and 25% above the ventilatory threshold (84) and for durations (at 15 min and 45 min) (85) found no effect on FI at an ad libitum meal 30 min after exercise, even with increased appetite ratings. Another study investigated the effects of exercise on substrate utilization and the subsequent relation with FI (86). Carbohydrate and fat oxidation was modified by a glucose preload and exercise at 44–49% maximum oxygen consumption, but, again, the study found no increase in FI, even with varying substrate utilization. Adult studies indicate that short-term FI is only affected by high-intensity activity, above 70% maximal oxygen uptake, which consistently suppresses appetite (88, 89) and FI (90). However, little is known about the effect of high-intensity activities and FI in children and adolescents. One study found that FI is supressed at high intensities (75% maximal oxygen uptake), but only in obese children when compared with lean children (91). Thus, the overall evidence, although scarce, does not point toward an increase of FI in children to correct for the energy deficit of exercise in the amount that can be promoted in schools, and supports programs such as Take Ten.
Mealtime television and food advertisements
Mealtime television and food advertisements in children’s programs have been associated with overweight and obesity in children, and dietary guidance and policies have focused on food advertisements, suggesting that they are the most problematic aspect of television for children. Television viewing during meals or snacks accounts for about one-quarter of total daily energy intake in children (92). Proposed causes for the obesity epidemic are not only lower activity levels but also an increased preference for energy-dense foods and sweet beverages, with increased time spent viewing television (93); snacking, but with lower intake of fruits and vegetables (94); and distracted eating (95).
However, the impact of distraction by television during a meal on satiety and satiation has been reported only recently and suggests that distraction alone is more likely to lead to excess FI than food advertisements in television programs In addition, sex and puberty may be factors in these responses. When boys watched a favorite television program, both satiety and satiation before and during a meal were reduced. Boys aged 9– 4 y old who watched a nonfood related television program (“The Simpsons”) during the meal had both decreased satiation as measured by FI at the test meal and decreased satiety after the glucose drink consumed 30 min earlier, leading to overall 24% higher energy intakes than when television was not watched (96). Similarly, peri- but not postpubertal girls watching the television program “Hannah Montana” at mealtime had lower caloric compensation after consumption of a glucose drink. However, their FI was not affected by television during a pizza meal (97).
Food behavior and choice also may be adversely influenced by television advertisements for food products (98). Children who watched the most hours of television were found to be consumers of those foods most advertised on television (99). To test the effects of food commercials on food choices in children directly, 7–11-y-old students were divided into random groups and watched a 14 min episode of “Disney’s Recess” with or without food commercials. The food commercial group watched 4 food commercials lasting 30 s long during 2 designated advertising breaks. The commercials promoted common snack and breakfast foods (a high-sugar cereal, waffle sticks with syrup, fruit rollups, and potato chips) with the use of a fun message tailored to children (100). Children watching food advertisements ate a considerable greater amount of the snacks (45%) than did children not watching food commercials (101). However, whatever may be the origin of food preferences, it does not necessarily lead to energy imbalance. Preschool children, when given food choices in test meals, accomplished caloric compensation at the meal for calories consumed shortly before the meal by selectively reducing intake of nonpreferred foods and maintaining consumption of highly preferred foods (45).
Taken together, these data may suggest that a food policy preventing food advertisements in television programs for children would be effective in addressing the obesity epidemic. However, the following reports challenge the view. Food advertisements in television at mealtime reduced FI in boys, had no effect in normal-weight girls, but increased FI in overweight/obese girls (102). Whether snack food advertisements in a television program would increase selection of highly preferred foods and decrease intake of fruits and vegetables is untested. However, many attempts have been made to increase fruit and vegetable consumption by children in this age group, largely unsuccessfully (103). For example, when children were exposed to healthy fast food advertisements embedded in cartoons, they increased their liking for fast foods, but did not cause them to make a healthier choice (98).
Currently, evidence of television’s adverse effect on the mealtime FI of children rests more with its effect on distracted eating and less with the food advertisements in contains. Furthermore, there is some evidence that girls respond differently from boys and that age is a factor. However, for effective food policy aimed at regulating food advertisements, much more understanding through randomized, controlled studies is needed. For now, the simple solution seems to be to recommend that children and adolescents not watch television while eating.
Conclusions
In conclusion, although limited, this literature review supports a need for children and adolescents to be a greater focus of research that leads to the development of sound nutrition policies and actions to reduce chronic disease. A focus on the environment must be balanced with an understanding of physiology and behaviors relevant to this age group.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CNS, central nervous system; FI, food intake; GI, glycemic index; GLP, glucagon-like peptide; PYY, peptide YY; SI, satiety index.
References
- 1.Ebbeling CB, Sinclair KB, Pereira MA, Garcia-Lago E, Feldman HA, Ludwig DS. Compensation for energy intake from fast food among overweight and lean adolescents. JAMA 2004;291:2828–33. [DOI] [PubMed] [Google Scholar]
- 2.Rolls BJ, Roe LS, Meengs JS. Reductions in portion size and energy density of foods are additive and lead to sustained decreases in energy intake. Am J Clin Nutr 2006;83:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011;34:717–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5. [DOI] [PubMed] [Google Scholar]
- 5.Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu Rev Nutr 2004;24:455–79. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics. Committee on Nutrition, Kleinman RE. Pediatric nutrition: policy of the American Academy of Pediatrics. 7th edition. ed.
- 7.Anderson GH, Aziz A, Abou Samra R. Physiology of food intake regulation: interaction with dietary components. Nestle Nutr Workshop Ser Pediatr Program 2006;58:133–43, discussion 43–5. [DOI] [PubMed] [Google Scholar]
- 8.Chambers AP, Sandoval DA, Seeley RJ. Integration of satiety signals by the central nervous system. Curr Biol 2013;23:R379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahan-Mihan A, Luhovyy BL, El Khoury D, Anderson GH. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 2011;3:574–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 2007;117:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecil JE, Palmer CN, Wrieden W, Murrie I, Bolton-Smith C, Watt P, Wallis DJ, Hetherington MM. Energy intakes of children after preloads: adjustment, not compensation. Am J Clin Nutr 2005;82:302–8. [DOI] [PubMed] [Google Scholar]
- 12.Johnson LR. Physiology of the gastrointestinal tract. Fifth edition. ed, 2012. [Google Scholar]
- 13.Jahan-Mihan A, Smith C, Anderson GH. The Effect of Protein Source in Diets Fed during Gestation and Lactation on Food Intake Regulation in Male Offspring of Wistar Rats. Am J Physiol Regul Integr Comp Physiol 2011:300(5):R1175–84. [DOI] [PubMed] [Google Scholar]
- 14.Panahi S, El Khoury D, Kubant R, Akhavan T, Luhovyy BL, Goff HD, Anderson GH. Mechanism of action of whole milk and its components on glycemic control in healthy young men. J Nutr Biochem 2014;25:1124–31. [DOI] [PubMed] [Google Scholar]
- 15.Hoyda TD, Smith PM, Ferguson AV. Gastrointestinal hormone actions in the central regulation of energy metabolism: potential sensory roles for the circumventricular organs. Int J Obes (Lond) 2009;33: Suppl 1:S16–21. [DOI] [PubMed] [Google Scholar]
- 16.Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol 2012;303:G129–40. [DOI] [PubMed] [Google Scholar]
- 17.Ortinau LC, Hoertel HA, Douglas SM, Leidy HJ. Effects of high-protein vs. high- fat snacks on appetite control, satiety, and eating initiation in healthy women. Nutr J 2014;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr 2011;93:836–43. [DOI] [PubMed] [Google Scholar]
- 19.Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci 1955;63:15–43. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–71. [DOI] [PubMed] [Google Scholar]
- 21.Bellisle F, Drewnowski A, Anderson GH, Westerterp-Plantenga M, Martin CK. Sweetness, satiation, and satiety. J Nutr 2012;142:1149S–54S. [DOI] [PubMed] [Google Scholar]
- 22.Birch LL, Deysher M. Caloric compensation and sensory specific satiety: evidence for self regulation of food intake by young children. Appetite 1986;7:323–31. [DOI] [PubMed] [Google Scholar]
- 23.Patel BP, Luhovyy B, Mollard R, Painter JE, Anderson GH. A premeal snack of raisins decreases mealtime food intake more than grapes in young children. Appl Physiol Nutr Metab 2013;38(4):382–9. [DOI] [PubMed] [Google Scholar]
- 24.Zandstra EH, Mathey MF, Graaf C, van Staveren WA. Short-term regulation of food intake in children, young adults and the elderly. Eur J Clin Nutr 2000;54:239–46. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GH, Woodend D. Consumption of sugars and the regulation of short-term satiety and food intake. Am J Clin Nutr 2003;78:843S–9S. [DOI] [PubMed] [Google Scholar]
- 26.Panahi S, El Khoury D, Luhovyy BL, Goff HD, Anderson GH. Caloric beverages consumed freely at meal-time add calories to an ad libitum meal. Appetite 2013;65:75–82. [DOI] [PubMed] [Google Scholar]
- 27.Birch LL, McPhee L, Sullivan S. Children’s food intake following drinks sweetened with sucrose or aspartame: time course effects. Physiol Behav 1989;45:387–95. [DOI] [PubMed] [Google Scholar]
- 28.Alviña M, Araya H. Rapid carbohydrate digestion rate produced lesser short-term satiety in obese preschool children. Eur J Clin Nutr 2004;58:637–42. [DOI] [PubMed] [Google Scholar]
- 29.Bellissimo N, Desantadina MV, Pencharz PB, Berall GB, Thomas SG, Anderson GH. A comparison of short-term appetite and energy intakes in normal weight and obese boys following glucose and whey-protein drinks. Int J Obes(Lond) 2008;32:362–71. [DOI] [PubMed] [Google Scholar]
- 30.Anderson GH, Luhovyy B, Akhavan T, Panahi S. Milk proteins in the regulation of body weight, satiety, food intake and glycemia. Nestle Nutr Workshop Ser Pediatr Program 2011;67:147–59. [DOI] [PubMed] [Google Scholar]
- 31.Araya H, Hills J, Alvina M, Vera G. Short-term satiety in preschool children: a comparison between high protein meal and a high complex carbohydrate meal. Int J Food Sci Nutr 2000;51:119–24. [DOI] [PubMed] [Google Scholar]
- 32.Patel BP, Anderson GH, Vien S, Bellissimo N, McCrindle BW, Hamilton JK. Obesity, sex and pubertal status affect appetite hormone responses to a mixed glucose and whey protein drink in adolescents. Clin Endocrinol (Oxf) 2014;81:63–70. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Ludwig DS. The 2015 US Dietary Guidelines: Lifting the ban on total dietary fat. JAMA 2015;313:2421–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willett WC. Dietary fat plays a major role in obesity: no. Obes Rev 2002;3(2):59–68. [DOI] [PubMed] [Google Scholar]
- 35.Seidell JC. Dietary fat and obesity: an epidemiologic perspective. Am J Clin Nutr 1998; 67(3, Suppl)546S–50S. [DOI] [PubMed] [Google Scholar]
- 36.Anderson GH. Regulation of food intake. Edtion ed. In: Shils ME, Olson JA, Shike M, eds. Modern nutrition in health and disease. Malvern: Lea & Febiger, 1994:524–36. [Google Scholar]
- 37.Rolls BJ, Hetherington M, Burley VJ. The specificity of satiety: The influence of foods of different macronutrient content on the development of satiety. Physiol Behav 1988;43:145–53. [DOI] [PubMed] [Google Scholar]
- 38.Read N, French S, Cunningham K. The role of the gut in regulating food intake in man. Nutr Rev 1994;52:1–10. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg D, Smith GP, Gibbs J. Cholecystokinin and the satiating effect of fat. Gastroenterology 1992;102:1801–3. [DOI] [PubMed] [Google Scholar]
- 40.Ross RA, Rossetti L, Lam TK, Schwartz GJ. Differential effects of hypothalamic long chain fatty acid infusions on suppression of hepatic glucose production. Am J Physiol Endocrinol Metab 2010:299(4);E633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulshof T, De Graaf C, Weststrate JA. The effects of preloads varying in physical state and fat content on satiety and energy intake. Appetite 1993;21:273–86. [DOI] [PubMed] [Google Scholar]
- 42.Kozimor A, Chang H, Cooper JA. Effects of dietary fatty acid composition from a high fat meal on satiety. Appetite 2013;69:39–45. [DOI] [PubMed] [Google Scholar]
- 43.Woodend DM, Anderson GH. Effect of sucrose and safflower oil preloads on short term appetite and food intake of young men. Appetite 2001;37:185–95. [DOI] [PubMed] [Google Scholar]
- 44.Cooling J, Blundell J. Are high-fat and low-fat consumers distinct phenotypes? Differences in the subjective and behavioural response to energy and nutrient challenges. Eur J Clin Nutr 1998;52:193–201. [DOI] [PubMed] [Google Scholar]
- 45.Birch LL, McPhee LS, Bryant JL, Johnson SL. Children’s lunch intake: effects of midmorning snacks varying in energy density and fat content. Appetite 1993;20:83–94. [DOI] [PubMed] [Google Scholar]
- 46.Johnson SL, McPhee L, Birch LL. Conditioned preferences: young children prefer flavors associated with high dietary fat. Physiol Behav 1991;50:1245–51. [DOI] [PubMed] [Google Scholar]
- 47.Birch LL, Johnson SL, Jones MB, Peters JC. Effects of a nonenergy fat substitute on children’s energy and macronutrient intake. Am J Clin Nutr 1993;58:326–33. [DOI] [PubMed] [Google Scholar]
- 48.Akilen R, Deljoomanesh N, Al-Dabous K, Arshad M, Smith C, Hamilton J, Anderson H. The effects of potatoes and other carbohydrate side dishes on meal time food intake, blood glucose and satiety response in lean healthy children. Experimental Biology: The FASEB Journal, 2015:29(Supp 1);597–2. [Google Scholar]
- 49.Ricketts CD. Fat preferences, dietary fat intake and body composition in children. Eur J Clin Nutr 1997;51:778–81. [DOI] [PubMed] [Google Scholar]
- 50.Lomenick JP, Melguizo MS, Mitchell SL, Summar ML, Anderson JW. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide YY secretion in prepubertal children. J Clin Endocrinol Metab 2009;94:4463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6. [DOI] [PubMed] [Google Scholar]
- 52.Holt SH, Miller JC, Petocz P, Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr 1995;49:675–90. [PubMed] [Google Scholar]
- 53.Anderson GH, Woodend D. Effect of glycemic carbohydrates on short-term satiety and food intake. Nutr Rev 2003;61:S17–26. [DOI] [PubMed] [Google Scholar]
- 54.Anderson GH, Soeandy CD, Smith CE. White vegetables: glycemia and satiety. Adv Nutr 2013;4:356S–67S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henry CJ, Lightowler HJ, Kendall FL, Storey M. The impact of the addition of toppings/fillings on the glycaemic response to commonly consumed carbohydrate foods. Eur J Clin Nutr 2006;60:763–9. [DOI] [PubMed] [Google Scholar]
- 57.Hätönen KA, Virtamo J, Eriksson JG, Sinkko HK, Sundvall JE, Valsta LM. Protein and fat modify the glycaemic and insulinaemic responses to a mashed potato-based meal. Br J Nutr 2011;106:248–53. [DOI] [PubMed] [Google Scholar]
- 58.Kameyama N, Maruyama C, Matsui S, Araki R, Yamada Y, Maruyama T. Effects of consumption of main and side dishes with white rice on postprandial glucose, insulin, glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 responses in healthy Japanese men. Br J Nutr 2014;111:1632–40. [DOI] [PubMed] [Google Scholar]
- 59.Sun L, Ranawana DV, Leow MK, Henry CJ. Effect of chicken, fat and vegetable on glycaemia and insulinaemia to a white rice-based meal in healthy adults. Eur J Nutr 2014;53:1719–26. [DOI] [PubMed] [Google Scholar]
- 60.Erdmann J, Hebeisen Y, Lippl F, Wagenpfeil S, Schusdziarra V. Food intake and plasma ghrelin response during potato-, rice- and pasta-rich test meals. Eur J Nutr 2007;46:196–203. [DOI] [PubMed] [Google Scholar]
- 61.Leeman M, Ostman E, Bjorck I. Glycaemic and satiating properties of potato products. Eur J Clin Nutr 2008;62:87–95. [DOI] [PubMed] [Google Scholar]
- 62.Bitar A, Vernet J, Coudert J, Vermorel M. Longitudinal changes in body composition, physical capacities and energy expenditure in boys and girls during the onset of puberty. Eur J Nutr 2000;39:157–63. [DOI] [PubMed] [Google Scholar]
- 63.Sun M, Gower BA, Bartolucci AA, Hunter GR, Figueroa-Colon R, Goran MI. A longitudinal study of resting energy expenditure relative to body composition during puberty in African American and white children. Am J Clin Nutr 2001;73:308–15. [DOI] [PubMed] [Google Scholar]
- 64.Shomaker LB, Tanofsky-Kraff M, Savastano DM, Kozlosky M, Columbo KM, Wolkoff LE, Zocca JM, Brady SM, Yanovski SZ, Crocker MK, et al. Puberty and observed energy intake: boy, can they eat! Am J Clin Nutr 2010;92:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 2006;361:1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butera PC. Estradiol and the control of food intake. Physiol Behav 2010;99:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 2011;104:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, Wishart JM, Jones KL, Horowitz M, Feinle-Bisset C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol 2009;297:G602–10. [DOI] [PubMed] [Google Scholar]
- 69.Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol 1999;276:R1366–73. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz A, Patel BP, Vien S, McCrindle BW, Anderson GH, Hamilton J. Acute decrease in serum testosterone after a mixed glucose and protein beverage in obese peripubertal boys. Clin Endocrinol (Oxf) 2014;83(3):332–8. [DOI] [PubMed] [Google Scholar]
- 71.Lebenthal Y, Gat-Yablonski G, Shtaif B, Padoa A, Phillip M, Lazar L. Effect of sex hormone administration on circulating ghrelin levels in peripubertal children. J Clin Endocrinol Metab 2006;91:328–31. [DOI] [PubMed] [Google Scholar]
- 72.Moran A, Jacobs DR Jr, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–44. [DOI] [PubMed] [Google Scholar]
- 73. Peino R, Baldelli R, Rodriguez-Garcia J, Rodriguez-Segade S, Kojima M, Kangawa K, Arvat E, Ghigo E, Dieguez C, Casanueva FF. Ghrelin-induced growth hormone secretion in humans. Eur J Endocrinol 2000;143(6):R11–4. [DOI] [PubMed]
- 74.Tena-Sempere M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitam Horm 2008;77:285–300. [DOI] [PubMed] [Google Scholar]
- 75.Pomerants T, Tillmann V, Jurimae J, Jurimae T. Relationship between ghrelin and anthropometrical, body composition parameters and testosterone levels in boys at different stages of puberty. J Endocrinol Invest 2006;29:962–7. [DOI] [PubMed] [Google Scholar]
- 76.Bellone S, Prodam F, Savastio S, De Rienzo F, Demarchi I, Trovato L, Petri A, Rapa A, Aimaretti G, Bona G. Acylated and unacylated ghrelin levels in normal weight and obese children: influence of puberty and relationship with insulin, leptin and adiponectin levels. J Endocrinol Invest 2012;35:191–7. [DOI] [PubMed] [Google Scholar]
- 77.Remmers T, Sleddens EF, Gubbels JS, de Vries SI, Mommers M, Penders J, Kremers SP, Thijs C. Relationship between physical activity and the development of body mass index in children. Med Sci Sports Exerc 2014;46:177–84. [DOI] [PubMed] [Google Scholar]
- 78.Skender ML, Goodrick GK, Del Junco DJ, Reeves RS, Darnell L, Gotto AM, Foreyt JP. Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc 1996;96:342–6. [DOI] [PubMed] [Google Scholar]
- 79.Donnelly JE, Lambourne K. Classroom-based physical activity, cognition, and academic achievement. Prev Med 2011;52: Suppl 1:S36–42. [DOI] [PubMed] [Google Scholar]
- 80.Peregrin T. Take 10! Classroom-based program fights obesity by getting kids out of their seats. J Am Diet Assoc 2001;101:1409. [DOI] [PubMed] [Google Scholar]
- 81.Stewart JA, Dennison DA, Kohl HW, Doyle JA. Exercise level and energy expenditure in the TAKE 10! in-class physical activity program. J Sch Health 2004;74:397–400. [DOI] [PubMed] [Google Scholar]
- 82.Bellissimo N, Thomas SG, Goode RC, Anderson GH. Effect of short-duration physical activity and ventilation threshold on subjective appetite and short-term energy intake in boys. Appetite 2007;49:644–51. [DOI] [PubMed] [Google Scholar]
- 83.Drapeau V, Blundell J, Therrien F, Lawton C, Richard D, Tremblay A. Appetite sensations as a marker of overall intake. Br J Nutr 2005;93:273–80. [DOI] [PubMed] [Google Scholar]
- 84.Tamam S, Bellissimo N, Patel BP, Thomas SG, Anderson GH. Overweight and obese boys reduce food intake in response to a glucose drink but fail to increase intake in response to exercise of short duration. Appl Physiol Nutr Metab 2012;37(3):520–9. [DOI] [PubMed] [Google Scholar]
- 85.Bozinovski NC, Bellissimo N, Thomas SG, Pencharz PB, Goode RC, Anderson GH. The effect of duration of exercise at the ventilation threshold on subjective appetite and short-term food intake in 9 to 14 year old boys and girls. Int J Behav Nutr Phys Act 2009;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hunschede S, El Khoury D, Antoine-Jonville S, Smith C, Thomas S, Anderson GH. Acute changes in substrate oxidation do not affect short-term food intake in healthy boys and men. Appl Physiol Nutr Metab 2015;40(2):168–77. [DOI] [PubMed] [Google Scholar]
- 87.King NA, Appleton K, Rogers PJ, Blundell JE. Effects of sweetness and energy in drinks on food intake following exercise. Physiol Behav 1999;66:375–9. [DOI] [PubMed] [Google Scholar]
- 88.Blundell JE, King NA. Exercise, appetite control, and energy balance. Nutrition 2000;16:519–22. [DOI] [PubMed] [Google Scholar]
- 89.Sim AY, Wallman KE, Fairchild TJ, Guelfi KJ. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int J Obes (Lond) 2014;38:417–22. [DOI] [PubMed] [Google Scholar]
- 90.Ueda SY, Yoshikawa T, Katsura Y, Usui T, Fujimoto S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J Endocrinol 2009;203:357–64. [DOI] [PubMed] [Google Scholar]
- 91.Thivel D, Metz L, Julien A, Morio B, Duche P. Obese but not lean adolescents spontaneously decrease energy intake after intensive exercise. Physiol Behav 2014;123:41–6. [DOI] [PubMed] [Google Scholar]
- 92.Matheson DM, Killen JD, Wang Y, Varady A, Robinson TN. Children’s food consumption during television viewing. Am J Clin Nutr 2004;79:1088–94. [DOI] [PubMed] [Google Scholar]
- 93.Coon KA, Tucker KL. Television and children’s consumption patterns. A review of the literature. Minerva Pediatr 2002;54:423–36. [PubMed] [Google Scholar]
- 94.Fainardi V, Scarabello C, Brunella I, Errico MK, Mele A, Gelmetti C, Sponzilli I, Chiari G, Volta E, Vitale M, et al. Sedentary lifestyle in active children admitted to a summer sport school. Acta Biomed 2009;80:107–16. [PubMed] [Google Scholar]
- 95.Bickham DS, Blood EA, Walls CE, Shrier LA, Rich M. Characteristics of screen media use associated with higher BMI in young adolescents. Pediatrics 2013;131:935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bellissimo N, Pencharz PB, Thomas SG, Anderson GH. Effect of television viewing at mealtime on food intake after a glucose preload in boys. Pediatr Res 2007;61:745–9. [DOI] [PubMed] [Google Scholar]
- 97.Patel BP, Bellissimo N, Thomas SG, Hamilton JK, Anderson GH. Television viewing at mealtime reduces caloric compensation in peripubertal, but not postpubertal, girls. Pediatr Res 2011;70:513–7. [DOI] [PubMed] [Google Scholar]
- 98.Boyland EJ, Whalen R. Food advertising to children and its effects on diet: a review of recent prevalence and impact data. Pediatr Diabetes 2015;16:331–7. [DOI] [PubMed] [Google Scholar]
- 99.Utter J, Scragg R, Schaaf D. Associations between television viewing and consumption of commonly advertised foods among New Zealand children and young adolescents. Public Health Nutr 2006;9:606–12. [DOI] [PubMed] [Google Scholar]
- 100.Powell LM, Szczypka G, Chaloupka FJ, Braunschweig CL. Nutritional content of television food advertisements seen by children and adolescents in the United States. Pediatrics 2007;120:576–83. [DOI] [PubMed] [Google Scholar]
- 101.Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol 2009;28(4):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson GH, Khodabandeh S, Patel B, Luhovyy BL, Bellissimo N, Mollard RC. Mealtime exposure to food advertisements while watching television increases food intake in overweight and obese girls but has a paradoxical effect in boys. Appl Physiol Nutr Metab 2015;40(2):162–7. [DOI] [PubMed] [Google Scholar]
- 103.Kim SA, Grimm KA, May AL, Harris DM, Kimmons J, Foltz JL. Strategies for pediatric practitioners to increase fruit and vegetable consumption in children. Pediatr Clin North Am 2011;58(6):1439–53. [DOI] [PubMed] [Google Scholar]