Abstract
Small-intestinal growth and function are critical for optimal animal growth and health and play a major role in nutrient digestion and absorption, energy and nutrient expenditure, and immunological competence. During fetal and perinatal development, the small intestine is affected by the maternal environment and nutrient intake. In ruminants, altered small-intestinal mass, villi morphology, hypertrophy, hyperplasia, vascularity, and gene expression have been observed as a result of poor gestational nutrition or intrauterine growth restriction. Although many of these data come from fetal stages, data have also demonstrated that nutrition during mid- and late gestation affects lamb small-intestinal growth, vascularity, digestive enzyme activity, and gene expression at 20 and 180 d of age as well. The small intestine is known to be a highly plastic tissue, changing with nutrient intake and physiological state even in adulthood, and the maternal small intestine adapts to pregnancy and advancing gestation. In ruminants, the growth, vascularity, and gene expression of the maternal small intestine also adapt to the nutritional plane and specific nutrient intake such as high selenium during pregnancy. These changes likely alter both pre- and postnatal nutrient delivery to offspring. More research is necessary to better understand the role of the offspring and maternal small intestines in whole-animal responses to developmental programming, but programming of this plastic tissue seems to play a dynamic role in gestational nutrition impacts on the whole animal.
Keywords: fetal programming, gene expression, pregnancy, ruminant, selenium, small intestine, vascular development
Introduction
Small-intestinal growth, development, and vascularization are often overlooked but essential processes that drive nutrient metabolism, immunological competence, neonatal survival, and postnatal growth. The small intestine not only serves as the main site for the digestion and absorption of nutrients (or postruminal digestion and absorption in ruminants) but is also a major energy and nutrient sink because of its high metabolic activity and rapid turnover (1, 2). In addition, the small intestine is a critical site of immune challenge because of its constant interaction with foreign substances (3).
The small intestine is a dynamic, highly plastic tissue that is known to change or adapt to variations in an animal’s diet, physiological state, or environment (4, 5). Changes in small-intestinal mass (6, 7), cellularity (8), and oxygen consumption (9, 10) have been demonstrated during feed restriction and in response to specific nutrients in ruminants (11, 12) and other species. These changes occur via tissue response to nutrient presence in the lumen, immunomodulatory factors, hormones, growth factors, local cell communication, and microbial and host interactions (4, 13–15).
Organ systems, including the small intestine, may also be programmed in utero. The Barker hypothesis states that the environment an animal is exposed to early in life not only affects its development but also has lifelong impacts on its health and performance (16) and is often referred to as fetal or developmental programming. The effects of early environment, especially in utero, have become a major area of study in human and animal nutrition, physiology, and epidemiology research, as evidenced by the hundreds of reviews on the subject. In livestock, intrauterine growth restriction (IUGR)6 results in impaired fetal development, low birth-weight offspring, and decreased long-term production (17–19). Developmental programming is a more accurate term than fetal programming, especially considering that a growing body of evidence clearly demonstrates environmental influences during the early postnatal period can also have long-term implications (20–22).
Programming of growth and development in livestock may be driven by many factors but often occurs in response to compromised nutrient supply to developing offspring, a primary cause of which is inadequate or improper maternal nutrition (23). Gestating ruminant livestock are likely to experience large oscillations in nutrient supply because of extensive management systems and the wide range of feedstuffs of varying quality used to feed ruminants throughout the world. Moreover, sheep are an established model of human pregnancy (24) and give us insight into the effects of poor gestational nutrition on human infant outcomes and mechanisms of developmental programming.
Because the small intestine is both critical to animal growth, health, and production and responds to its luminal and extraluminal environment, early-life effects on small-intestinal development likely play an important role in the observed programming of later animal health and performance, including the acquisition of nutrients during the pre- and postnatal periods. In addition, impacts of gestational nutrition on the maternal small intestine itself change nutrient delivery to offspring both in utero and during lactation. This review examines the impacts of nutrition during pregnancy on the small intestine of both the offspring and dam and focuses on knowledge gained from ruminant livestock models.
Fetal Small-Intestinal Growth and Development
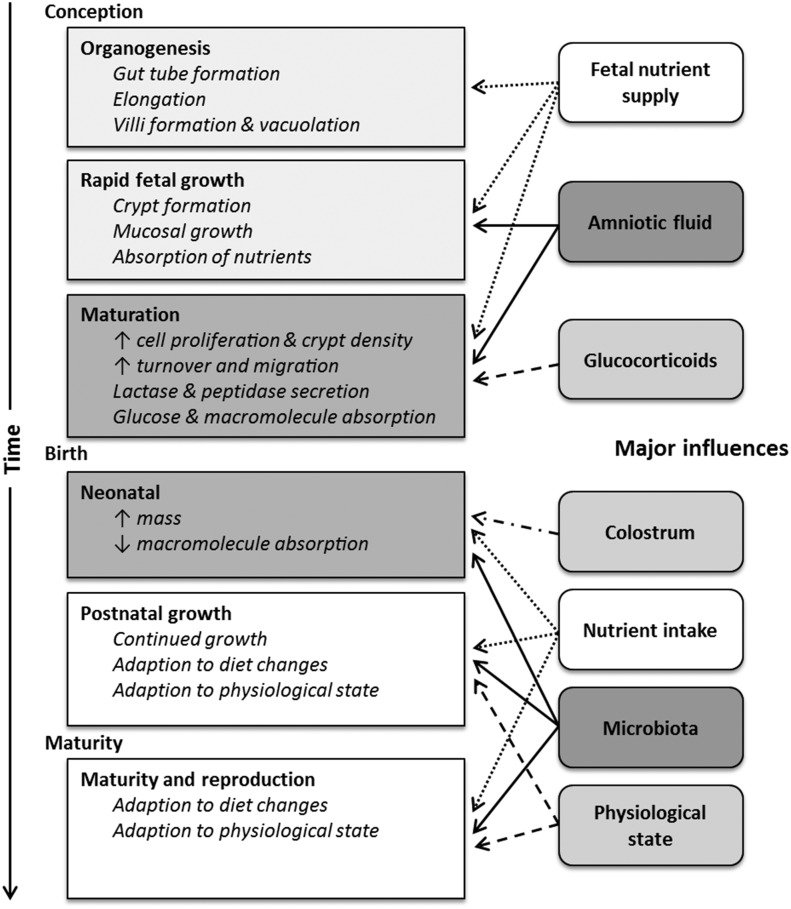
There are multiple developmental windows for the small intestine during fetal, perinatal, and neonatal periods (25–27). Organogenesis generally occurs during early- to midgestation and is followed by rapid fetal growth in the last third of gestation and then prepared for the transition from the uterine to outside environment during the perinatal period (27). In addition to these windows, the small intestine continues to develop postnatally and even into maturity, when it remains plastic and responds to physiological state, diet, and other factors. The windows of small-intestinal growth and development and specific influences for each periods are illustrated in Figure 1.
FIGURE 1.
Windows of small-intestinal growth and development and their influences. The timing of these events varies with species, but in general organogenesis occurs during early- to midgestation, rapid fetal growth occurs in mid- to late gestation, and maturation occurs during late gestation immediately before birth. ↓, decreased; ↑, increased.
Evidence of Developmental Programming of the Offspring Small Intestine
Intrauterine growth restriction
As summarized in Table 1, the effects of IUGR on the small intestine have been studied in many species. Small-intestinal effects have been observed using various models of IUGR, including carunclectomy-induced placental insufficiency (28), carunclectomy combined with maternal nutrient restriction (29), uterine location (30), and IUGR determined by divergent birth weight within the litter (32). These effects generally include reduced mass and/or length of the small intestine (Table 1). Despite this, small-intestinal mass relative to body weight was not always altered by IUGR (28, 32), raising the question of whether reduced small-intestinal mass proportional to body size is truly biologically relevant. Decreased villus and crypt density (28), villus height and/or width (28, 30, 34), crypt depth (28, 29), and mucosal size (28, 29, 32) suggest that this reduced mass may also be accompanied by reduced functional area and development. In addition, decreases in proliferation (30) and enterocyte differentiation (29) suggest altered crypt-proliferative dynamics, which could indicate a less advanced developmental stage or programming that causes decreased small-intestinal mass. Although the effects of IUGR on the small intestine have been better characterized prenatally or immediately after birth, these effects persist postnatally to day 5 in pigs (34). Thus, despite a similar small-intestinal mass relative to body weight in many studies, functional changes have been observed in IUGR offspring.
TABLE 1.
Summary of the effects of intrauterine growth restriction on the small intestine from selected studies1
| Reference | Species | Age measured2 | Small-intestinal mass or length response | Additional small-intestinal responses |
| 28 | Sheep | Day 140 gestation | ↓Mass; ↓length | ↓Villus and crypt density; ↓villus height and crypt depth; ↓mucosal thickness |
| 29 | Sheep | Day 90 gestation | ↓Mass; ↓relative mass | ↓Mucosal circumference and area; ↓crypt depth; abnormal enterocyte differentiation |
| 30 | Rabbits | Day 31 gestation | Not determined | ↓Villus height; ↓ proliferation; ↑ epidermal growth factor mRNA |
| 31 | Rats | Birth–12 wk | ↓Mass (to 4 wk); ↓length (to 12 wk) | ↑Maltase (at birth); ↑lactase (at birth) |
| 32 | Pigs | Birth | ↓Mass; ↓length | ↓Mucosal weight; ↓IGF-1 mRNA expression |
| 33 | Pigs | Birth | ↓Mass; ↓relative mass | Altered proteome |
| 34 | Pigs | Birth–5 d | ↓Mass (to 2 d); ↓length (to 5 d) | ↓Villus height (to 2 d); ↓villus width (at 2 d); ↑adherent bacterial number; altered transcriptome |
IGF-1, insulin-like growth factor 1; ↓, decreased; ↑, increased.
Approximate gestation lengths: sheep = 150 d, rabbits = 31 d.
Gene expression of the small intestine was also altered by IUGR in these models. Piglets identified as IUGR had altered jejunal protein expression, including 7 downregulated (e.g., creatine kinase β-type and serum albumin precursor) and 4 upregulated (e.g., desmin and scavenger-receptor protein) proteins (33). Altered ileal gene expression was also observed in IUGR compared with normal piglets, although these expressions were affected by the day of sampling (birth compared with postnatal days 2 or 5). At each time point, genes differentially expressed included those involved in macromolecular metabolism, biosynthesis, and cellular metabolism (34). Small-intestinal insulin-like growth factor 1 mRNA was decreased (32) in IUGR pigs at birth, but epidermal growth factor mRNA was increased in IUGR rabbits in late gestation (30) in other studies.
Although many of the reported effects of IUGR on the small intestine seem to be negative, this is not always the case. For example, Qiu et al. (31) showed that jejunal lactase and maltase were greater for IUGR rats than control at birth (although this did not extend past the immediate postnatal period) and suggested that increased digestive enzyme production at birth was an adaptive mechanisms that allowed IUGR neonates to have increased digestive capacity. In another study (34), ileal adherent bacterial numbers were increased for IUGR pigs at postnatal day 2, indicating that IUGR can alter bacterial colonization of the small intestine postnatally. Luminal bacterial populations were unchanged in this study, suggesting that changes in the small intestine may bring about further alterations in adherent populations. It is unclear, however, whether gene expression and villus morphology differences observed at day 2 were caused by the effect of IUGR on small-intestinal development or by bacterial colonization.
Maternal nutrient manipulation during gestation
The effect of maternal nutrition during specific stages of gestation rather than IUGR in general on the small intestine has not been as extensively studied, but data exist from ruminant models. Research to date indicates that both the nutritional plane (Table 2) and specific nutrient intake of the dam can affect the fetal small intestine and persist into postnatal life. The timing of these maternal nutritional insults is important because of the developmental windows outlined in Figure 1.
TABLE 2.
Summary of effects of maternal nutrition on the ruminant offspring small intestine from selected studies1
| Reference | Species | Treatments | Age measured2 (d) | Small-intestinal mass response | Additional small-intestinal responses |
| 35 | Cattle | CON vs. RES (days 30–125 gestation) | 125 Gestation | NS | ↑Proliferation in RES |
| 35 | Cattle | CON vs. RES (days 30–125) and realimented (days 126–245) | 245 Gestation | NS | ↑Total vascularity in RES and realimented |
| 36 | Cattle | CON vs. RES vs. RES + AA supplement (days 45 to 185 gestation) | ∼450 Postnatal | NS | ↑GUCY1B3 mRNA in RES + AA |
| 37 | Sheep | CON vs. RES (days 50–130 gestation) | 130 Gestation | NS | ↓Protein concentration in RES; ↑oxygen consumption in RES |
| 38, 39 | Sheep | CON vs. RES (days 64–135 gestation) | 135 Gestation | ↓In RES | ↓Total vascularity in RES; ↓protein:DNA in RES; ↓GUCY1B3 mRNA in RES |
| 40, 41 | Sheep | CON vs. RES (day 40 gestation to birth) | 20 Postnatal | NS | ↓Total vascularity in RES; ↓capillary surface density in RES; ↑capillary size in RES; ↑GLP-2 mRNA in RES; ↓postnatal weight gain in RES |
| 42 | Sheep | CON vs. RES (day 50 gestation to birth) | 180 Postnatal | NS | ↓Capillary size in RES; ↓total proliferation in RES; ↓GLP-2 mRNA in RES |
| 40, 41 | Sheep | CON vs. OVR (day 40 gestation to birth) | 20 Postnatal | NS | ↑DNA concentration in OVR |
| 42 | Sheep | CON vs. OVR (day 50 gestation to birth) | 180 Postnatal | NS | ↓Total proliferating cells in OVR |
AA, amino acids; CON, control nutritional plane (near nutrient requirements); GLP-2, glucagon-like peptide 2; GUCY1B3, soluble guanylate cyclase (NO receptor) 1 β3; NS, not significant (P > 0.10); OVR, overnutrition; RES, nutrient restriction; RES + AA, nutrient restriction with protein supplementation to meet essential AA of control; ↓, decreased; ↑, increased.
Approximate gestation lengths: cattle = 285 d, sheep = 150 d.
Fetal.
Nutrient restriction during early- and midgestation does not seem to affect fetal small-intestinal growth, and fetuses from nutrient-restricted ewes have had small-intestinal masses that were similar to their control counterparts (35, 43, 44). Nutrient restriction during early- and midgestation increased jejunal crypt-region proliferation at gestational day 125 in fetal calves, however (35). Small-intestinal development may have been delayed by nutrient restriction, although this seems unlikely because the mass did not differ from the control. In addition, when nutrient-restricted cows were realimented in this study, total vascularity of the fetal small intestine was increased at gestational day 245 (35). These data suggest that nutrient restriction increased the efficiency of the fetal small intestine, perhaps like the “thrifty phenotype” hypothesis (45) that has been postulated to describe fetal development as increasing survival in the face of a negative environment or poor nutrition (46).
Maternal nutrient restriction of ewes in mid- and late gestation decreased small-intestinal mass and jejunal hypertrophy (protein:DNA) (38) despite a lack of differences in jejunal proliferation (39). In these studies, lambs from nutrient-restricted ewes had decreased total jejunal microvascular volume concurrently with reduced jejunal mRNA expression of soluble guanylate cyclase 1 β3, a NO receptor involved in vasodilation and angiogenesis (39). Conversely, small-intestinal mass of fetal lambs from ewes that were nutrient-restricted during the last 3 wk of gestation was unaffected (47), suggesting that longer periods of maternal nutrient restriction are necessary to affect the fetal small intestine. In a recent study (37), nutrient restriction during mid- and late gestation increased oxygen consumption per unit of small intestine in late-term fetal lambs, although the reasons for this increase in energy use are unclear.
Postnatal.
Changes in maternal nutrition in late gestation may negatively affect gut maturation during this time as well, although fetal small-intestinal measurements may not sufficiently detect such changes. Cortisol and fetal swallowing of amniotic fluid both play an important role in the small-intestinal maturation process (4, 48). For example, vascular endothelial growth factor (VEGF) expression in the fetal small intestine, which is important for angiogenesis of the growing tissue, is likely cortisol-dependent in sheep (49). Maternal cortisol levels are often changed by the gestational plane of nutrition (26, 50), and nutrient content of the amnion has been altered by nutrient restriction in ewes (51), suggesting that maternal nutrition may have an even greater impact during final prenatal maturation. Small-intestinal function is particularly important in livestock species that rely upon the transfer of passive immunity from immunoglobulins in colostrum (e.g., cattle and sheep). Colostrum also contains a cadre of growth factors, hormones, and nutrients that are crucial for small-intestinal development (48, 52–54), and its production in ewes has been decreased by both nutrient restriction and overnutrition (55, 56), which could also have further implications in perinatal small-intestinal maturation.
There are few data from ruminant developmental programming models investigating small-intestinal parameters postnatally. To our knowledge, only 1 study (36) has investigated the impact of maternal nutrition during early- and midgestation in cattle (Table 2). In this study, few small-intestinal differences existed in calves aged ∼450 d. This may not be surprising given the timing of the nutritional treatments and long period between these treatments and tissue collection. Interestingly, soluble guanylate cyclase 1 β3 expression in the jejunum was altered even at this late postnatal age despite a lack of growth or vascularity differences.
Two studies have investigated postnatal lamb small-intestinal growth and vascularity after mid- and late gestation nutrient restriction or overnourishment (Table 2). These data demonstrate that lambs aged 20 d have continued alterations in jejunal hyperplasia, vascularity, and gene expression, even when they were fed a common artificial colostrum and milk replacer after birth and managed together (41). Moreover, jejunal proliferation, vascularity, and gene expression were also affected by gestational nutrition in lambs aged 180 days in a similar model (42), demonstrating that changes to the small intestine may persist well into life. In both 20- and 180-d-old lambs, glucagon-like peptide 2 expression was altered, although in opposite ways (Table 2). Glucagon-like peptide 2 is very important for small-intestinal development, including in growth and vascularization (57), making it a likely mechanism for small-intestinal changes observed in these studies.
It has also been demonstrated that the maternal intake of specific nutrients such as selenium during gestation can affect fetal small-intestinal development. Fetuses from ewes fed supranutritional selenium throughout gestation had increased jejunal hypertrophy (38) and decreased jejunal VEGF mRNA expression (39). In addition, the form and amount of maternal selenium supplementation during gestation have affected fetal jejunal hypertrophy (58). Even when lambs were fed similar postnatal diets, high selenium during gestation has continued to affect lamb jejunal measures at days 20 (41) and 180 (42), suggesting long-term impacts of this micronutrient prenatally or compensation by offspring after normal selenium intakes postnatally.
Maternal Small-Intestinal Adaptations
The maternal gastrointestinal tract is responsible for acquiring nutrients to be delivered to the gravid uterus and is also extremely nutrient- and energy-demanding itself; therefore, it exerts multiple controls over nutrient delivery to the uteroplacenta during pregnancy. Along with the mammary gland and uteroplacenta, the gastrointestinal tract is a key nutrient-transferring tissue that controls nutrient delivery to offspring pre- and postnatally (59). Although much is known about the role of the uteroplacenta in developmental programming (23, 60), the gastrointestinal tract and small intestine in particular have not been investigated to the same extent in most species. In fact, more data exist for ruminants during pregnancy than for many other species because of the research summarized in this review.
The small intestine remains plastic into adulthood and undergoes changes not only with fluctuating diet type and quantity but also with physiological state. Because the small intestine has such a critical role in both nutrient acquisition and utilization, it responds to the dynamic, nutritionally demanding processes of gestation and lactation in the reproducing female. When nutrient intake is altered during these periods, the maternal small intestine undergoes even more adaptations, likely to optimize nutrient availability for maintenance, fetal growth, and/or lactogenesis.
Adaptation to pregnancy and lactation
During pregnancy, energy requirements of the dam increase in an exponential manner for most species; this increasing nutrient demand of pregnancy is typically coupled with increasing nutrient intake until just before parturition (61). In addition, systemic blood flow and volume increase dramatically through increased cardiac output and decreased total peripheral resistance in pregnant females (62). The resulting increase in organ workload during pregnancy, especially for the gastrointestinal tract (62, 63), necessitates either increased gastrointestinal organ mass or functional capacity, perhaps through greater absorptive capacity per unit of tissue (61).
Adaptations of the small intestine to pregnancy seem to result from both pregnancy itself and the stage of gestation. In multiparous ewes, both actual and relative (gram per kilogram of body weight) small-intestinal mass increased as a result of pregnancy despite similar total gastrointestinal tract mass (64). In addition, capillary area density and total vascularity of the small intestine were increased with pregnancy, suggesting greater capacity for blood flow and nutrient uptake (65). Primiparous heifer small-intestinal mass was less responsive in another study (66), although jejunal proliferation was decreased and jejunal DNA concentration was increased by pregnancy (67). This suggests that pregnant heifers had decreased cell turnover rates to maintain mass with less proliferation and greater cell numbers, which could indicate a reduction of small-intestinal nutrient and energy use. Moreover, small-intestinal energy use, determined by in vitro oxygen consumption and scaled for organ mass, was less for pregnant than nonpregnant heifers at gestational days 40 and 270 (67). Research investigating ruminant small-intestinal absorptive capacity and digestive enzyme activity has been limited, but primiparous rat research has demonstrated increased enzyme-specific activity resulting from pregnancy (68). These data suggest that pregnancy induces differential changes depending on parity and age of the dam, which may also depend on whether the dam has reached a mature body weight. Because young females are still growing, pregnancy may slow small-intestinal growth, which would result in decreased small-intestinal mass and reduced nutrient demands of this tissue.
As gestation progresses, small-intestinal mass, relative mass (grams per kilogram of body weight), and individual section mass may change in ruminants, although these data are variable. In primiparous heifers, total relative small-intestinal mass increased during early- to midgestation (66), but small-intestinal mass of primiparous ewe lambs decreased from early- to midgestation then remained steady to late gestation in another study (69). Small-intestinal mass increased from mid- to late gestation in multiparous ewes (64) but did not change from mid- to late gestation in multiparous cows (35). Vascularization of the small intestine also seems to change during pregnancy in ruminants. In primiparous ewes, the number of jejunal capillaries per tissue area decreased from early- to midgestion and mid- to late gestation, whereas jejunal capillary size increased from mid- to late gestation (69). Alternatively, jejunal vascularity increased from mid- to late gestation in mature ewes (65) and beef cows (35). Small-intestinal oxygen consumption also increased linearly during gestation in multiparous ewes (67), suggesting increased organ workload as pregnancy progresses.
Mechanisms of small-intestinal changes during pregnancy are largely unknown. Although some effects must result from increased intake and nutrient flux, additional factors probably exist as well. Hormones and growth factors, including angiogenic and vasoactive factors, likely also affect the small intestine during the many endocrine-related and metabolic changes of pregnancy. For example, 17β-estradiol increased jejunal proliferation of ovariectomized ewes in a study (70). Because estradiol increases greatly during pregnancy, it may be 1 of the contributing factors in small-intestinal adaptation to pregnancy.
During lactation, the small intestine increases in size and function (71–73), fueled at least in part by a voluntary increase in nutrient intake. Small-intestinal mass in rodents can increase up to 200% during lactation based on the lactational demand of their offspring, allowing for increased nutrient uptake despite similar or reduced mass-specific rates of nutrient transport (73, 74). Additional small-intestinal adaptations have been observed in rodent models, including increased villus height (74), ovalbumin uptake (75), enzyme-specific activities (68), and calcium absorption (73). It is unknown how small-intestinal changes during pregnancy affect these further adaptations during pregnancy and whether gestational changes also serve as lactation for lactational nutrient demands. If the maternal small intestine cannot adapt during lactation because of changes in pregnancy, nutrient acquisition and therefore lactation yield will likely be reduced, impairing postnatal nutrient delivery to offspring.
Adaptation to nutrient manipulation
Nutritional plane.
Small-intestinal growth and function are known to change with nutrient intake, so it should come as no surprise that they change with the nutritional plane during pregnancy. Most of the studies cited herein include treatments that vary in nutrient intake and bulk density of feed, both of which affect the small intestine (76). The studies that investigated the impacts of nutritional plane during gestation on ruminant small-intestinal mass, proliferation, vascularity, and gene expression are summarized in Table 3.
TABLE 3.
Summary of effects of gestational nutrition on the ruminant dam small intestine from selected studies1
| Reference | Species, parity | Treatments | Stage measured2 (d) | Small-intestinal mass response | Additional small-intestinal responses |
| 35 | Cattle, multiparous | CON vs. RES (days 30–125 gestation) | 125 Gestation | NS | ↓RNA:DNA in RES |
| 35 | Cattle, multiparous | CON vs. RES (days 30–125) and realimented (days 126–245) | 245 Gestation | NS | ↓RNA:DNA in RES |
| 64, 65 | Sheep, multiparous | CON vs. RES (days 50–90) | 90 Gestation | ↓In RES | ↓DNA concentration in RES; ↑capillary area density in RES |
| 77 | Sheep, first | CON vs. RES (days 50–90) | 130 Gestation | NS | NS |
| 77 | Sheep, first | CON vs. RES (days 50–130) | 130 Gestation | ↓In RES | ↓DNA concentration in RES |
| 64, 65 | Sheep, multiparous | CON vs. RES (days 50–130) | 130 Gestation | ↓In RES | ↑DNA concentration in RES; ↑capillary area density in RES |
| 37 | Sheep, first | CON vs. RES (days 50–130) | 130 Gestation | ↓In RES | ↑Oxygen consumption in RES |
| 77 | Sheep, first | CON vs. RES (days 90–130) | 130 Gestation | ↓In RES | ↑RNA concentration in RES |
| 38, 39 | Sheep, first | CON vs. RES (days 64–135) | 135 Gestation | ↓In RES | ↓Total vascularity in RES; ↓capillary area density in RES; ↓capillary size in RES; ↑ VEGFR-1 and -2 mRNA in RES; ↑NRP1 and NRP2 mRNA in RES |
| 78 | Sheep, first | CON vs. RES (day 40 to parturition) | 0 Postpartum | NS | ↓RNA concentration and RNA:DNA in RES; ↓capillary surface density in RES; ↓mucosal density in RES |
| 78 | Sheep, first | CON vs. RES (day 40 to parturition) | 20 Postpartum | NS | ↑Proliferation in RES; ↓capillary surface density in RES |
| 69 | Sheep, first | CON vs. OVR (days 0–50) | 50 Gestation | NS | ↑RNA concentration and RNA:DNA in OVR |
| 69 | Sheep, first | CON vs. OVR (days 0–90) | 90 Gestation | ↑In OVR | ↑RNA concentration and RNA:DNA in OVR |
| 69 | Sheep, first | CON vs. OVR (days 0–130) | 130 Gestation | ↓In OVR | ↑RNA concentration in OVR |
| 78 | Sheep, first | CON vs. OVR (day 40 to parturition) | 0 Postpartum | ↑In OVR | ↓RNA concentration and RNA:DNA in OVR; ↑total vascularity in OVR; ↑VEGFR-1 mRNA in OVR; ↑NOS3 mRNA in OVR |
| 78 | Sheep, first | CON vs. OVR (day 40 to parturition) | 20 Postpartum | NS | ↓Proliferation in OVR; ↑total vascularity in OVR |
CON, control nutritional plane; NOS3, endothelial NO synthase 3; NRP1, neuropilin 1; NRP2, neuropilin 2; NS, not significant (P > 0.05); OVR, overnutrition; RES, nutrient restriction; VEGFR-1, vascular endothelial growth factor 1 receptor; VEGFR-2, vascular endothelial growth factor 2 receptor; ↓, decreased; ↑, increased.
Approximate gestation lengths: cattle = 285 d, sheep = 150 d.
In general, nutritional plane alteration during early gestation alone does not seem to affect the mass of the ruminant small intestine (69, 79), although overnutrition during this period increased indexes of jejunal hypertrophy (69). Nutrient restriction during early- and midgestation or midgestation only is more variable, however, and either has decreased (44, 64, 79) or not affected (35, 80) maternal small-intestinal mass when measured immediately after the nutrient restriction. Dams were able to rebound when nutrient restriction was followed by realimentation in late gestation, and small-intestinal mass was not different from the control near term (77, 79).
In most studies, small-intestinal mass has followed the nutritional plane during both mid- and late gestation or late gestation only when measured at the end of the restriction period (Table 3). Changes in small-intestinal mass were not always proportional to body weight, however. Many changes in cellularity have been observed in these studies (Table 3), indicating that both hypertrophy and hyperplasia may play a role in growth differences, even when no change in mass was observed. Despite differences in mass and cellularity, no differences have been observed in jejunal crypt cell proliferation as a result of the nutritional plane (38, 65, 77, 78). This is likely because tissues were collected from ewes after long periods (40–80 d) of nutrient restriction in these studies. Alterations in the proliferative rate necessary to change small-intestinal mass occurred much earlier during nutrient restriction, and the tissue had most likely reached steady state by late gestation. Small-intestinal adaptation has been detected as soon as 5–14 d (6, 8, 81, 82) after dietary changes and thus supports this hypothesis. Not much is known about the impacts of gestational nutrition on small-intestinal energy use, but 1 study (37) reported that oxygen consumption was increased per unit of tissue in nutrient-restricted ewes. This was determined in late gestation and may not indicate energy use throughout the nutrient restriction (mid- and late gestation); nonetheless, it does raise interesting questions about small-intestinal metabolism of nutrient-restricted pregnant dams.
Jejunal vascularity has responded to the nutritional plane during gestation in several studies in ewes and in general increases or decreases with the nutritional plane (Table 3) (58). This may be in an age-dependent manner, however. In primiparous ewes, capillary area density and size decreased with nutrient restriction, resulting in a decrease in total vascularity when scaled to small-intestinal mass (38). Small-intestinal mass was also decreased by nutrient restriction, further reducing total vascularization of these ewes. Conversely, mature ewes had increased capillary area density after nutrient restriction, allowing for similar total vascularity to control-fed ewes despite having less small-intestinal mass (65). Mature ewes seemed to be able to compensate for decreased small-intestinal mass by increasing vascular density, whereas the small-intestinal vascularity of primiparous dams may be inhibited by nutrient restriction because these animals and their tissues are still growing.
The mechanisms of adaptation to the altered nutritional plane during gestation in both growth and vascularity of the ruminant small intestine are not well known, but angiogenic and vasoactive factor gene expression may play a role. VEGF and NO system expression has been altered in ewes (Table 3) (58), although some of these data are contradictory. Jejunal mRNA expression of VEGF and its vascular endothelial growth receptors (VEGFR-1 and VEGFR-2) were greater for nutrient-restricted ewes in late gestation (39, 78), suggesting that angiogenic factor upregulation occurred in the face of reduced small-intestinal growth and vascularization (38). Jejunal expression of VEGF and endothelial NO synthase 3 have also been increased after overnutrition during pregnancy (78). In vitro systems have demonstrated that VEGF delivery to the small intestine increases vascularity (83), suggesting that the small intestine of both nutrient-restricted and overnourished ewes may use VEGF or its receptors to modulate vascularization during nutritional insults. It is important to point out that it is uncertain whether angiogenic factors influenced vascularization changes earlier in the nutrient restriction period because gene expression was determined at 1 time point only.
Impacts of gestational nutrition on small-intestinal adaptation to lactation have not been greatly studied. One ewe study (78) indicated that the small intestine adapts quickly to an increased nutritional plane during early lactation because small-intestinal mass increased rapidly when ewes that were nutrient-restricted, fed to requirements, or overnourished during gestation were all fed to meet their nutrient requirements (similar intake to overnutrition treatment during gestation) for the first 20 d of lactation. In this study, ewes that had been nutrient-restricted or control-fed during pregnancy had increased jejunal proliferation at day 20 of lactation, which allowed them to have a small-intestinal mass that was similar to the previously overnourished ewes. Compensatory growth in the gastrointestinal tract came at a cost, however, because milk yield was reduced in ewes that were previously nutrient-restricted (55). This was likely both negatively affected by the decreased size and potentially absorptive capacity of the small intestine as well as the diversion of nutrients from the mammary gland to rebuilding the gastrointestinal tract and other tissues. Lactating mice that had undergone small-intestinal resection had a disproportionate increase in small-intestinal mass (212–313% compared with 64%) and length (66–71% compared with 34%) compared with virgin controls (84), indicating that lactation signals a greater increase in tissue growth in females with limited small-intestinal capacity. Taken together, these data suggest that negative effects of gestational nutrition on the small intestine likely affect postnatal nutrient delivery by altering milk production, even when dams are fed to meet the nutrient demands of lactation.
Specific nutrients.
There have been few published studies to date to our knowledge that have investigated the effect of specific nutrient intake on the maternal small intestine during gestation. Results have been variable in a series of studies that set out to determine the impacts of supranutritional selenium in ewes during gestation. Diets high in selenium that were fed during gestation have had no effect on (58, 77), increased (38), or decreased (78) primiparous ewe small-intestinal mass. When small-intestinal mass was increased, no effects of selenium on cellularity measures, proliferation, or vascularity were observed (38). Alternatively, supranutritional selenium decreased DNA concentration in other studies (58, 77), with the proliferative rate of crypt cells unaffected (77) or increased by selenium (58). VEGF and NO system expression has been affected by high selenium, whereas supranutritional selenium has reduced the mRNA of VEGF and its receptors (39, 78). Selenium has been hypothesized to decrease cancerous tumor growth and vascularization (85); thus, actions of selenium on proliferation and vascularity of the small intestine may have similar mechanisms. When high selenium was removed from the diet during lactation, small-intestinal mass of ewes increased within the first 20 d to that of control-fed ewes (78). It is unclear what caused differences in responses to high selenium in these studies, although the selenium source and amount of supplementation seem to alter small-intestinal response (39, 58) and thus likely influenced results.
Future Directions
The small intestine is a dynamic, rapidly changing tissue that is crucial for animal growth and health. Further research is necessary to better understand the role of the maternal small intestine in providing nutrients to the fetus and postnatal offspring and to advance knowledge of the effects of maternal nutrition on the programming of offspring small-intestinal growth and function. In addition, research in the role of epigenetics and the microbiome in programming of the small intestine is in its infancy and can provide a wealth of knowledge. A better understanding of the effects of gestational nutrition on the maternal and offspring small intestine will allow for the development of management and therapeutic strategies to optimize the efficiency of livestock production and will lead to increased understanding of human impacts.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
IUGR, intrauterine growth restriction; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
References
- 1.Ferrell CL. Contribution of visceral organs to animal energy expenditures. J Anim Sci 1988;66(Suppl 3):23–34. [Google Scholar]
- 2.McBride BW, Kelly JM. Energy cost of absorption and metabolism in the ruminant gastrointestinal tract and liver: a review. J Anim Sci 1990;68:2997–3010. [DOI] [PubMed] [Google Scholar]
- 3.Shanahan F. The intestinal immune system. In: Johnson LR, ed. Physiology of the gastrointestinal tract. New York: Raven Press; 1994. p. 643–84. [Google Scholar]
- 4.Trahair JF, Sangild PT. Studying the development of the small intestine: philosophical and anatomical perspectives. In: Zabielski R, Gregory PC, Westrom B, eds. Biology of the intestine in growing animals. Amsterdam: Elsevier Science; 2002. p. 1–54. [Google Scholar]
- 5.Johnson DE, Johnson KA, Baldwin RL. Changes in liver and gastrointestinal tract energy demands in response to physiological workload in ruminants. J Nutr 1990;120:649–55. [DOI] [PubMed] [Google Scholar]
- 6.Burrin DG, Ferrell CL, Britton RA, Bauer ML. Level of nutrition and visceral organ size and metabolic activity in sheep. Br J Nutr 1990;64:439–48. [DOI] [PubMed] [Google Scholar]
- 7.Nozière P, Attaix D, Bocquier F, Doreau M. Effects of underfeeding and refeeding on weight and cellularity of splanchnic organs in ewes. J Anim Sci 1999;77:2279–90. [DOI] [PubMed] [Google Scholar]
- 8.Burrin DG, Britton RA, Ferrell CL, Bauer ML. Level of nutrition and visceral organ protein synthetic capacity and nucleic acid content in sheep. J Anim Sci 1992;70:1137–45. [DOI] [PubMed] [Google Scholar]
- 9.Freetly HC, Ferrell CL, Jenkins TG, Goetsch AL. Visceral oxygen consumption during chronic feed restriction and realimentation in sheep. J Anim Sci 1995;73:843–52. [DOI] [PubMed] [Google Scholar]
- 10.Burrin DG, Ferrell CL, Eisemann JH, Britton RA, Nienaber JA. Effect of level of nutrition on splanchnic blood flow and oxygen consumption in sheep. Br J Nutr 1989;62:23–34. [DOI] [PubMed] [Google Scholar]
- 11.Soto-Navarro SA, Lawler TL, Taylor JB, Reynolds LP, Reed JJ, Finley JW, Caton JS. Effect of high-selenium wheat on visceral organ mass, and intestinal cellularity and vascularity in finishing beef steers. J Anim Sci 2004;82:1788–93. [DOI] [PubMed] [Google Scholar]
- 12.Swanson KC, Redmer DA, Reynolds LP, Caton JS. Ruminally undegraded intake protein in sheep fed low-quality forage: effect on weight, growth, cell proliferation, and morphology of visceral organs. J Anim Sci 1999;77:198–205. [DOI] [PubMed] [Google Scholar]
- 13.Ferraris RP. Regulation of intestinal nutrient transport. In: Johnson LR, ed. Physiology of the gastrointestinal tract. New York: Raven Press; 1994. p. 1821–44. [Google Scholar]
- 14.Levine GM. Regulation of intestinal mucosal growth. In: Morisset J, Solomon TE, eds. Growth of the gastrointestinal tract: gastrointestinal hormones and growth factors. Boca Raton (FL): CRC Press; 1991. p. 175–89. [Google Scholar]
- 15.Burrin DG. Trophic factors and regulation of gastrointestinal tract and liver development. In: Polin RA, Fox WW, Abman SH, eds. Fetal and neonatal physiology. Philadelphia: Saunders; 2004. p. 1095–100. [Google Scholar]
- 16.Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993;341:938–41. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr 2000;71(5 Suppl):1344S–52S. [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Bazer FW, Wallace JM, Spencer TE. Intrauterine growth retardation: implications for the animal sciences. J Anim Sci 2006;84:2316–37. [DOI] [PubMed] [Google Scholar]
- 19.Caton JS, Hess BW. Maternal plane of nutrition: impacts on fetal outcomes and postnatal offspring responses. In: Hess BW, DelCurto T, Bowman JGP, Waterman RC, eds. Proceedings of the 4th Grazing Livestock Nutrition Conference; 2010 Jul 9–10; Champaign (IL) Western Section American Society of Animal Science; 2010. p. 104–22. [Google Scholar]
- 20.Greenwood PL, Cafe LM. Prenatal and pre-weaning growth and nutrition of cattle: long-term consequences for beef production. Animal 2007;1:1283–96. [DOI] [PubMed] [Google Scholar]
- 21.Patel MS, Srinivasan M, Laychock SG. Metabolic programming: role of nutrition in the immediate postnatal life. J Inherit Metab Dis 2009;32:218–28. [DOI] [PubMed] [Google Scholar]
- 22.Berge P. Long-term effects of feeding during calfhood on subsequent performance in beef cattle (a review). Livest Prod Sci 1991;28:179–201. [Google Scholar]
- 23.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Hammer CJ, Maddock Carlin KR, Grazul-Bilska AT, Redmer DA. Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. J Anim Sci 2010;88:E61–72. [DOI] [PubMed] [Google Scholar]
- 24.Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology 2008;69:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J 2006;47:73–82. [DOI] [PubMed] [Google Scholar]
- 26.Symonds ME, Stephenson T, Gardner DS, Budge H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev 2007;19:53–63. [DOI] [PubMed] [Google Scholar]
- 27.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. [DOI] [PubMed] [Google Scholar]
- 28.Avila CG, Harding R, Rees S, Robinson PM. Small intestinal development in growth-retarded fetal sheep. J Pediatr Gastroenterol Nutr 1989;8:507–15. [DOI] [PubMed] [Google Scholar]
- 29.Trahair JF, DeBarro TM, Robinson JS, Owens JA. Restriction of nutrition in utero selectively inhibits gastrointestinal growth in fetal sheep. J Nutr 1997;127:637–41. [DOI] [PubMed] [Google Scholar]
- 30.Cellini C, Xu J, Arriaga A, Buchmiller-Crair T. Effect of epidermal growth factor infusion on fetal rabbit intrauterine growth retardation and small intestinal development. J Pediatr Surg 2004;39:891–7. [DOI] [PubMed] [Google Scholar]
- 31.Qiu XS, Huang TT, Shen ZY, Deng HY, Ke ZY. Effect of early nutrition on intestine development of intrauterine growth retardation in rats and its correlation to leptin. World J Gastroenterol 2005;11:4419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ. Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate 2005;88:66–72. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Chen L, Li D, Yin Y, Wang X, Li P, Dangott LJ, Hu W, Wu G. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr 2008;138:60–6. [DOI] [PubMed] [Google Scholar]
- 34.D’Inca R, Kloareg M, Gras-Le Guen C, Le Hueron-Luron I. Intrauterine growth restriction modifies the developmental pattern of intestinal structure transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr 2010;140:925–31. [DOI] [PubMed] [Google Scholar]
- 35.Meyer AM, Reed JJ, Vonnahme KA, Soto-Navarro SA, Reynolds LP, Ford SP, Hess BW, Caton JS. Effects of stage of gestation and nutrient restriction during early to mid-gestation on maternal and fetal visceral organ mass and indices of jejunal growth and vascularity in beef cows. J Anim Sci 2010;88:2410–24. [DOI] [PubMed] [Google Scholar]
- 36.Meyer AM, Hess BW, Paisley SI, Du M, Caton JS. Small intestinal growth measures are correlated with feed efficiency in market weight cattle, despite minimal effects of maternal nutrition during early to mid-gestation. J Anim Sci 2014;2:3855–67; [DOI] [PubMed] [Google Scholar]
- 37.Prezotto LD, Lemley CO, Camacho LE, Doscher FE, Meyer AM, Caton JS, Awda BJ, Vonnahme KA, Swanson KC. Effects of nutrient restriction and melatonin supplementation on maternal and foetal hepatic and small intestinal energy utilization. J Anim Physiol Anim Nutr (Berl) 2014;98:797–807. [DOI] [PubMed] [Google Scholar]
- 38.Reed JJ, Ward MA, Vonnahme KA, Neville TL, Julius SL, Borowicz PP, Taylor JB, Redmer DA, Grazul-Bilska AT, Reynolds LP, et al. . Effects of selenium supply and dietary restriction on maternal and fetal body weight, visceral organ mass, cellularity estimates, and jejunal vascularity in pregnant ewe lambs. J Anim Sci 2007;85:2721–33. [DOI] [PubMed] [Google Scholar]
- 39.Neville TL, Redmer DA, Borowicz PP, Reed JJ, Ward MA, Johnson ML, Taylor JB, Soto-Navarro SA, Vonnahme KA, Reynolds LP, et al. . Maternal dietary restriction and selenium supply alters mRNA expression of angiogenic factors in maternal intestine, mammary gland, and fetal jejunal tissues during late gestation in pregnant ewe lambs. J Anim Sci 2010;88:2692–702. [DOI] [PubMed] [Google Scholar]
- 40.Meyer AM, Reed JJ, Neville TL, Taylor JB, Hammer CJ, Reynolds LP, Vonnahme KA, Caton JS. Effects of plane of nutrition and selenium supply during gestation on ewe and neonatal offspring performance, body composition, and serum selenium. J Anim Sci 2010;88:1786–800. [DOI] [PubMed] [Google Scholar]
- 41.Meyer AM, Neville TL, Reed JJ, Taylor JB, Reynolds LP, Redmer DA, Hammer CJ, Vonnahme KA, Caton JS. Maternal nutritional plane and selenium supply during gestation impact visceral organ mass and intestinal growth and vascularity of neonatal lamb offspring. J Anim Sci 2013;91:2628–39. [DOI] [PubMed] [Google Scholar]
- 42.Yunusova RD, Neville TL, Vonnahme KA, Hammer CJ, Reed JJ, Taylor JB, Redmer DA, Reynolds LP, Caton JS. Impacts of maternal selenium supply and nutritional plane on visceral tissues and intestinal biology in 180 day-old offspring in sheep. J Anim Sci 2013;91:2229–42. [DOI] [PubMed] [Google Scholar]
- 43.Luther J, Aitken R, Milne J, Matsuzaki M, Reynolds L, Redmer D, Wallace J. Maternal and fetal growth, body composition, endocrinology, and metabolic status in undernourished adolescent sheep. Biol Reprod 2007;77:343–50. [DOI] [PubMed] [Google Scholar]
- 44.Scholljegerdes EJ, Vonnahme KA, Molle JDC, Nayigihugu V, Lake SL, Atkinson RL, Ludden PA, Miller LR, Ford SP, Hess BW. Effects of maternal undernutrition from early- to mid-gestation on visceral organs of the ewe and fetus. Proc West Sect Am Soc Anim Sci 2004;55:344–8. [Google Scholar]
- 45.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992;35:595–601. [DOI] [PubMed] [Google Scholar]
- 46.Wells JCK. The thrifty phenotype as an adaptive maternal effect. Biol Rev Camb Philos Soc 2007;82:143–72. [DOI] [PubMed] [Google Scholar]
- 47.Charlton V, Johengen M. Effects of intrauterine nutritional supplementation on fetal growth restriction. Biol Neonate 1985;48:125–42. [DOI] [PubMed] [Google Scholar]
- 48.Sangild PT, Fowden AL, Trahair JF. How does the foetal gastrointestinal tract develop in preparation for enteral nutrition after birth. Livest Prod Sci 2000;66:141–50. [Google Scholar]
- 49.Holmes K, Charnock Jones SD, Forhead AJ, Giussani DA, Fowden AL, Licence D, Kempster S, Smith GCS. Localization and control of expression of VEGF-A and the VEGFR-2 receptor in fetal sheep intestines. Pediatr Res 2008;63:143–8. [DOI] [PubMed] [Google Scholar]
- 50.Lemley CO, Meyer A, Neville T, Hallford D, Camacho L, Maddock-Carlin K, Wilmoth T, Wilson M, Perry G, Redmer D. Dietary selenium and nutritional plane alter specific aspects of maternal endocrine status during pregnancy and lactation. Domest Anim Endocrinol 2014;46:1–11. [DOI] [PubMed] [Google Scholar]
- 51.Kwon H, Ford SP, Bazer FW, Spencer TE, Nathanielsz PW, Nijland MJ, Hess BW, Wu G. Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids. Biol Reprod 2004;71:901–8. [DOI] [PubMed] [Google Scholar]
- 52.Berni Canani R, Passariello A, Buccigrossi V, Terrin G, Guarino A. The nutritional modulation of the evolving intestine. J Clin Gastroenterol 2008;42:S197–200. [DOI] [PubMed] [Google Scholar]
- 53.Xu RJ. Development of the newborn GI tract and its relation to colostrum/milk intake: a review. Reprod Fertil Dev 1996;8:35–48. [DOI] [PubMed] [Google Scholar]
- 54.Quigley JD III, Drewry JJ. Nutrient and immunity transfer from cow to calf pre- and postcalving. J Dairy Sci 1998;81:2779–90. [DOI] [PubMed] [Google Scholar]
- 55.Meyer AM, Reed JJ, Neville TL, Thorson JF, Maddock-Carlin KR, Taylor JB, Reynolds LP, Redmer DA, Luther JS, Hammer CJ, et al. . Nutritional plane and selenium supply during gestation impact yield and nutrient composition of colostrum and milk in primiparous ewes. J Anim Sci 2011;89:1627–39. [DOI] [PubMed] [Google Scholar]
- 56.Swanson TJ, Hammer CJ, Luther JS, Carlson DB, Taylor JB, Redmer DA, Neville TL, Reynolds LP, Caton JS, Vonnahme KA. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J Anim Sci 2008;86:2415–23. [DOI] [PubMed] [Google Scholar]
- 57.Dubé PE, Brubaker PL. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab 2007;293:E460–5. [DOI] [PubMed] [Google Scholar]
- 58.Neville TL, Ward MA, Reed JJ, Soto-Navarro SA, Julius SL, Borowicz PP, Taylor JB, Redmer DA, Reynolds LP, Caton JS. Effects of level and source of dietary selenium on maternal and fetal body weight, visceral organ mass, cellularity estimates, and jejunal vascularity in pregnant ewe lambs. J Anim Sci 2008;86:890–901. [DOI] [PubMed] [Google Scholar]
- 59.Vonnahme KA, Lemley CO, Caton JS, Meyer AM. Impacts of maternal nutrition on vascularity of nutrient transferring tissues during gestation and lactation. Nutrients 2015;7:3497–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vonnahme KA, Lemley CO, Shukla P, O’Rourke ST. 2011 and 2012 Early Careers Achievement Awards: placental programming: how the maternal environment can impact placental function. J Anim Sci 2013;91:2467–80. [DOI] [PubMed] [Google Scholar]
- 61.Stock MK, Metcalfe J. Maternal physiology during gestation. Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven Press; 1994. p. 947–83. [Google Scholar]
- 62.Thornburg KL, Bagby SP, Giraud GD. Maternal adaptation to pregnancy. In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM, eds. Knobil and Neill’s physiology of reproduction. St Louis (MO): Elsevier; 2006. p. 2899–923. [Google Scholar]
- 63.Ferrell CL. Energy metabolism. In: Church DC, ed. The ruminant animal: digestive physiology and nutrition. Englewood Cliffs (NJ): Prentice Hall; 1988. p. 250–68. [Google Scholar]
- 64.Scheaffer AN, Caton JS, Redmer DA, Reynolds LP. The effect of dietary restriction, pregnancy, and fetal type in different ewe types on fetal weight, maternal body weight, and visceral organ mass in ewes. J Anim Sci 2004;82:1826–38. [DOI] [PubMed] [Google Scholar]
- 65.Scheaffer AN, Caton JS, Redmer DA, Arnold DR, Reynolds LP. Effect of dietary restriction, pregnancy, and fetal type on intestinal cellularity and vascularity in Columbia and Romanov ewes. J Anim Sci 2004;82:3024–33. [DOI] [PubMed] [Google Scholar]
- 66.Scheaffer AN, Caton JS, Bauer ML, Reynolds LP. Influence of pregnancy on body weight, ruminal characteristics, and visceral organ mass in beef heifers. J Anim Sci 2001;79:2481–90. [DOI] [PubMed] [Google Scholar]
- 67.Scheaffer AN, Caton JS, Bauer ML, Redmer DA, Reynolds LP. The effect of pregnancy on visceral growth and energy use in beef heifers. J Anim Sci 2003;81:1853–61. [DOI] [PubMed] [Google Scholar]
- 68.Burdett K, Reek C. Adaptation of the small intestine during pregnancy and lactation in the rat. Biochem J 1979;184:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caton JS, Reed JJ, Aitken R, Milne JS, Borowicz PP, Reynolds LP, Redmer DA, Wallace JM. Effects of maternal nutrition and stage of gestation on body weight, visceral organ mass, and indices of jejunal cellularity, proliferation, and vascularity in pregnant ewe lambs. J Anim Sci 2009;87:222–35. [DOI] [PubMed] [Google Scholar]
- 70.O’Neil MR, Lardy GP, Reynolds LP, Caton JS, Vonnahme KA. Impacts of linseed meal and estradiol-17B on mass, cellularity, angiogenic factors, and vascularity of the jejunum. J Anim Sci 2008;86:3014–22. [DOI] [PubMed] [Google Scholar]
- 71.Gibb MJ, Ivings WE, Dhanoa MS, Sutton JD. Changes in body components of autumn-calving Holstein-Friesian cows over the first 29 weeks of lactation. Anim Prod 1992;55:339–60. [Google Scholar]
- 72.Reynolds CK, Durst B, Lupoli B, Humphries DJ, Beever DE. Visceral tissue mass and rumen volume in dairy cows during the transition from late gestation to early lactation. J Dairy Sci 2004;87:961–71. [DOI] [PubMed] [Google Scholar]
- 73.Hammond KA. Adaptation of the maternal intestine during lactation. J Mammary Gland Biol Neoplasia 1997;2:243–52. [DOI] [PubMed] [Google Scholar]
- 74.Casirola DM, Ferraris RP. Role of the small intestine in postpartum weight retention in mice. Am J Clin Nutr 2003;78:1178–87. [DOI] [PubMed] [Google Scholar]
- 75.Harmatz PR, Bloch KJ, Brown M, Walker WA, Kleinman RE. Intestinal adaptation during lactation in the mouse I. Enhanced intestinal uptake of dietary protein antigen. Immunology 1989;67:92–5. [PMC free article] [PubMed] [Google Scholar]
- 76.Rompala RE, Hoagland TA, Meister JA. Effect of dietary bulk on organ mass, fasting heat production and metabolism of the small and large intestine in sheep. J Nutr 1988;118:1553–7. [DOI] [PubMed] [Google Scholar]
- 77.Carlson DB, Reed JJ, Borowicz PP, Taylor JB, Reynolds LP, Neville TL, Redmer DA, Vonnahme KA, Caton JS. Effects of dietary selenium supply and timing of nutrient restriction during gestation on maternal growth and body composition of pregnant adolescent ewes. J Anim Sci 2009;87:669–80. [DOI] [PubMed] [Google Scholar]
- 78.Meyer AM, Reed JJ, Neville TL, Taylor JB, Reynolds LP, Redmer DA, Vonnahme KA, Caton JS. Effects of nutritional plane and selenium supply during gestation on visceral organ mass and indices of intestinal growth and vascularity in primiparous ewes at parturition and during early lactation. J Anim Sci 2012;90:2733–49. [DOI] [PubMed] [Google Scholar]
- 79.Camacho LE, Lemley CO, Van Emon ML, Caton JS, Swanson KC, Vonnahme KA. Effects of maternal nutrient restriction followed by realimentation during early and midgestation on beef cows. I. Maternal performance and organ weights at different stages of gestation. J Anim Sci 2014;92:520–9. [DOI] [PubMed] [Google Scholar]
- 80.Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland MJ, Skinner DC, Nathanielsz PW, et al. . Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod 2003;69:133–40. [DOI] [PubMed] [Google Scholar]
- 81.Rompala RE, Hoagland TA. Effect of level of alimentation on visceral organ mass and the morphology and Na+, K+ adenosinetriphosphatase activity of intestinal mucosa in lambs. J Anim Sci 1987;65:1058–63. [DOI] [PubMed] [Google Scholar]
- 82.Drouillard JS, Klopfenstein TJ, Britton RA, Bauer ML, Gramlich SM, Wester TJ, Ferrell CL. Growth, body composition, and visceral organ mass and metabolism in lambs during and after metabolizable protein or net energy restrictions. J Anim Sci 1991;69:3357–75. [DOI] [PubMed] [Google Scholar]
- 83.Rocha FG, Sundback CA, Krebs NJ, Leach JK, Mooney DJ, Ashley SW, Vacanti JP, Whang EE. The effect of sustained delivery of vascular endothelial growth factor on angiogenesis in tissue-engineered intestine. Biomaterials 2008;29:2884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammond KA, Lam M, Kent Lloyd KC, Diamond J. Simultaneous manipulation of intestinal capacities and nutrient loads in mice. Am J Physiol 1996;271:G969–79. [DOI] [PubMed] [Google Scholar]
- 85.Zeng H, Combs GF Jr. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasions. J Nutr Biochem 2008;19:1–7. [DOI] [PubMed] [Google Scholar]