Abstract
Since the 1970s, the positive effects of dietary fiber on health have increasingly been recognized. The collective term “dietary fiber” groups structures that have different physiologic effects. Since 1995, some dietary fibers have been denoted as prebiotics, implying a beneficial physiologic effect related to increasing numbers or activity of the gastrointestinal microbiota. Given the complex composition of the microbiota, the demonstration of such beneficial effects is difficult. In contrast, an exploration of the metabolites of dietary fiber formed as a result of its fermentation in the colon offers better perspectives for providing mechanistic links between fiber intake and health benefits. Positive outcomes of such studies hold the promise that claims describing specific health benefits can be granted. This would help bridge the “fiber gap”—that is, the considerable difference between recommended and actual fiber intakes by the average consumer.
Keywords: short chain fatty acids, fiber, microbiome, prebiotics, colon, health claims
Introduction
The term “dietary fiber” was first used to denote the nondigestible constituents of the plant cell wall (1). In the 1970s, much interest was generated with regard to dietary fiber in conjunction with hypotheses postulating an inverse relation between its consumption and the incidence of Western diseases such as colon cancer and heart disease (2). These hypotheses were based on the low prevalence of Western diseases among those consuming traditional high-fiber diets in Africa and the higher incidence of Western diseases when a more Western diet was adopted by the more wealthy and those living in urbanized communities (3). Both epidemiologic and intervention studies have since revealed beneficial effects of dietary fiber intake on metabolic disorders. The European Union explicitly recognizes that, depending on their structure and properties, fibers have “one or more beneficial physiological effects such as decreasing intestinal transit time, increasing stool bulk, being fermentable by colonic microflora, reducing blood total and LDL cholesterol levels, reducing post-prandial blood glucose and reducing blood insulin levels” (4).
At the same time, advice to increase the intake of dietary fiber from grains, vegetables, and fruits has become part of current nutritional policies and recommendations around the world. The 2010 Dietary Guidelines for Americans (5) stipulates that an intake of fiber corresponding to 14 g/1000 calories is adequate and recommends intakes of 25 g/d for women and 38 g/d for men. Figure 1 shows a typical food label in the United States, which relates percentage of daily values of dietary fiber to calorie intake. The European Food Safety Authority (EFSA) considers a daily intake of 25 g dietary fiber sufficient for normal laxation and notes that in adults there is evidence of health benefits (e.g., reduced risk of coronary artery disease and type 2 diabetes and improved weight maintenance) associated with the consumption of diets rich in fiber-containing foods with dietary fiber intakes >25 g/d (6).
FIGURE 1.
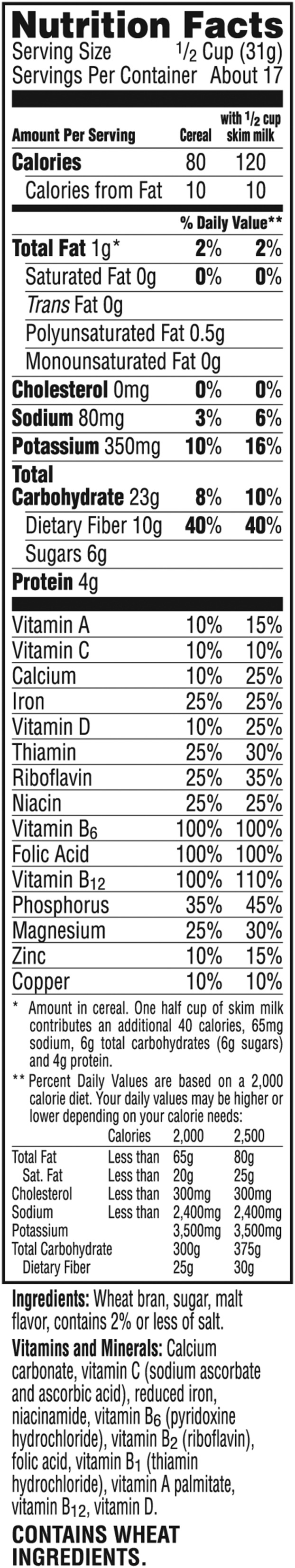
Example of a nutrition facts label (Kellogg’s All Bran Original) showing daily values for a dietary fiber intake of 25 or 30 g depending on total daily calorie intake. Used with permission from Kellogg’s.
As an accompanying measure, in the interest of international trade, the Codex Alimentarius Commission (7) has put forward a definition for dietary fiber that has been adopted by many countries. In the European Union (4), fiber is now defined (and labeled) as follows: “carbohydrate polymers with three or more monomeric units, which are neither digested nor absorbed in the human small intestine and belong to the following categories: (i) edible carbohydrate polymers naturally occurring in foods as consumed, (ii) edible carbohydrate polymers which have been obtained from food raw materials by physical, enzymatic, or chemical means and which have a beneficial physiological effect demonstrated by generally accepted scientific evidence, and (iii) edible synthetic carbohydrate polymers which have a beneficial physiological effect demonstrated by generally accepted scientific evidence.” Ingredient manufacturers have been driving development for the second and third categories. Notable examples of the latter categories are inulin-type fructans and polydextrose and soluble corn fiber. Their fermentation by colonic microbiota leads to products that are active locally in the gut or can be absorbed from the large bowel to exert a systemic influence in various parts of the body (8). In any case, the term “dietary fiber” comprises a complex group of substances with very diverse structures, properties, and impacts. Because of this, the content of dietary fiber in food systems cannot be used for specific health claims.
The Notion of Prebiotics
Some dietary fibers, such as inulin-type fructans and galacto-oligosaccharides, are also viewed as prebiotics. Prebiotics have been defined as nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improve host health (9). Three criteria are used to recognize a compound as prebiotic: “(i) resistance to gastric acidity, hydrolysis by mammalian enzymes and gastrointestinal absorption, (ii) fermentation by intestinal microbiota, and (iii) selective stimulation of the growth and/or activity of intestinal bacteria associated with health and wellbeing” (10). However, the term “associated” does not necessarily implicate a causal relation. Whether the microbes themselves are directly involved in the associated benefits or whether the effects are due to microbial metabolites remains to be proven (10).
Where Are We Today?
Since the launch of the prebiotic concept ∼20 y ago and the definition for dietary fiber formulated 10 y ago, inulin and its fructo-oligosaccharides and galacto-oligosaccharides have been studied as potential prebiotics (11) as have arabinoxylan oligosaccharides from wheat bran (12). Inulin and fructo-oligosaccharides have entered the food market as ingredients, and dossiers have been filed with EFSA on the prebiotic concept. Producers of prebiotics claim the effects have a “beneficial effect on intestinal microflora, gut integrity, digestion, intestinal bifidobacteria, prebiotic effect, prebiotic, and intestinal health” (13). Given the set of criteria in claim examinations, the EFSA panel concluded that “the evidence [presented in the dossiers] … does not establish that increasing numbers of gastro-intestinal microbiota is a beneficial physiological effect” and that “a cause and effect relationship has not been established between the consumption of the … food constituents which are the subject of the health claims and a beneficial physiological effect related to increasing numbers of gastro-intestinal microbiota” (13). As a result, those substances under current legislation cannot be labeled as prebiotics and thus only as dietary fiber, but with no associated mention of health benefits. The difficulties associated with efforts to obtain health claims thus remain (14).
Prebiotics and Fermentable Fibers: From Microbial Composition to Microbial Metabolism
During the past 2 decades, next-generation sequencing techniques have revolutionized the way in which we study microbial communities (15). Their application has shown that changes in microbiota composition after prebiotic administration are not limited to increased levels of Bifidobacterium and Lactobacillus species and therefore challenges the concept that prebiotics only affect a minor part of the microbial ecosystem. A prebiotic feeding study in mice revealed that changes in 102 distinct taxa (16) and other fermentable fibers, not considered as prebiotics, also affect the composition of the intestinal microbiota. In healthy men, polydextrose and soluble corn fiber supplementation significantly shifted the abundance of the dominant phyla Bacteroidetes and Firmicutes (17).
That carbohydrate prebiotics have a much more extensive impact on the microbial community than previously anticipated and that other fermentable dietary fibers induce similar modifications in microbiota composition leads one to question the distinction between such prebiotics and fermentable fibers. It seems no longer appropriate to maintain the classification as either prebiotics or nonprebiotics because both types of fermentable substrates produce similar effects. In addition, the broad modulation of the microbiota definitely makes it even more difficult to show links between changes in microbiota composition and health benefits than believed earlier. In addition, because the microbiota has a significant degree of functional redundancy (18), with different bacteria metabolizing the same substrates and producing similar metabolites, it seems less important to know “who is there” than to know “what they do.”
How to Study Health Effects and Obtain Health Claims?
One of the most prominent functional changes induced by the administration of prebiotics or other fermentable dietary fibers is an increased production of SCFAs. The recognition of SCFAs both as energy sources and signaling molecules on G protein–coupled receptors or in inhibition of histone deacetylation has greatly increased the interest in these bacterial metabolites because they are beginning to provide a mechanistic link between fermentable fiber and health benefits. For instance, SCFAs have been shown to regulate energy homeostasis and glucose metabolism (19) and to affect the immune system by regulating the release of cytokines (20, 21) and by inducing regulatory T cells in the colon (22). However, the mere demonstration of functional changes in the microbial ecosystem—such as fiber-induced increases in SCFA production—does not suffice any more than does the demonstration of compositional changes—such as increases in bifidobacteria—for allowing regulatory bodies to grant health claims. What is required is the demonstration of changes in relevant hard endpoints.
If one can show if and to what extent similar effects on clinically relevant variables can be obtained after the administration of fermentable fiber and the administration of the equivalent amount of SCFAs produced from those fibers, one would be able to provide a direct link between fermentable fiber, colonic SCFA production, and health benefits that goes beyond the level of an association. The major obstacle is the quantification of the in vivo colonic SCFA production, which is problematic due to the inaccessibility of the colon, the absorption of SCFAs by colonocytes, and the extensive metabolism in colonocytes and liver. However, application of stable isotope technology allows for selective and sensitive monitoring in plasma of those SCFAs that originate from the colon and offers a unique opportunity to study the nutrikinetics of SCFAs. Stable isotopes can safely be administered to humans. Thus, growing crops under 13C atmosphere and isolating 13C-labeled fiber fractions from them, which are then used for quantification of in vivo SCFA production, provides a way to relate fiber intake to SCFA production. Such experiments for practical and cost reasons should be preceded by both thorough characterization of the fibers under study in terms of monosaccharide composition, structure, physicochemical properties, and possibly co-passengers as well as by in vitro experiments that measure SCFA production as well as many other outcomes, some of which are microbiota and metabolome related.
The above would then provide grounds for health claims based on why the fermentation of specific, well-defined fermentable fibers—either prebiotic or not—is beneficial. In a not-too-distant future, the field may evolve such that distinctions between different fibers can be made, in line with scientific developments. It thus seems of immediate benefit to all stakeholders involved to focus on specific beneficial effects of fermentable dietary fibers without necessarily trying to show that increased numbers of gastrointestinal microorganisms are a marker for a beneficial physiologic effect. The possibility of showing benefits in terms of health-related endpoints of different fermentable fibers that leads to the granting of specific health claims would be extremely valuable in bridging the so-called fiber gap (8)—that is, the considerable difference between the recommended and actual fiber intake by the average consumer—as well as in promoting specific health outcomes that are generally recognized by the scientific community.
Acknowledgments
We thank Anne Birkett (WK Kellogg Institute for Food and Nutrition Research, Battle Creek, Michigan) for helpful comments. All authors read and approved the final manuscript.
References
- 1.Hipsley EH. Dietary “fibre” and pregnancy toxaemia. BMJ 1953;2:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkitt DP, Walker AR, Painter NS. Dietary fiber and disease. JAMA 1974;229:1068–74. [PubMed] [Google Scholar]
- 3.Burkitt DP. Some diseases characteristic of modern Western civilization. BMJ 1973;1:274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Commission Directive 2008/100/EC of 28 October 2008 Amending Council Directive 90/496/EEC on Nutrition Labelling for Foodstuffs as Regards Recommended Daily Allowances, Energy Conversion Factors and Definitions. Off J Eur Union 2008;285:9–11.
- 5. US Department of Agriculture; US Department of Health and Human Services. Dietary guidelines for Americans. 7th ed Washington (DC): US Government Printing Office; 2010. [Google Scholar]
- 6.Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J 2010;8(1462):1–77. [Google Scholar]
- 7.Joint FAO/WHO Food Standards Programme. Secretariat of the CODEX Alimentarius Commission: CODEX Alimentarius (CODEX) guidelines on nutrition labeling CAC/GL 2-1985 as last amended 2010. Rome (Italy): FAO; 2010. [Google Scholar]
- 8.Jones JM. CODEX-aligned dietary fiber definitions help to bridge the 'fiber gap’. Nutr J 2014;13(34);1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota—introducing the concept of prebiotics. J Nutr 1995;125:1401–12. [DOI] [PubMed] [Google Scholar]
- 10.Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 2004;17:259–75. [DOI] [PubMed] [Google Scholar]
- 11.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, et al. . Prebiotic effects: metabolic and health benefits. Br J Nutr 2010;104(Suppl 2):S1–63. [DOI] [PubMed] [Google Scholar]
- 12.Broekaert WF, Courtin CM, Verbeke K, Van de Wiele T, Verstraete W, Delcour JA. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr 2011;51:178–94. [DOI] [PubMed] [Google Scholar]
- 13.Scientific opinion on the substantiation of health claims related to various food(s)/food constituents(s) and increasing numbers of gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905), and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 2010;8(1809):1–16.
- 14.Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 2015;12:303–10. [DOI] [PubMed] [Google Scholar]
- 15.Hanage WP. Microbiome science needs a healthy dose of skepticism. Nature 2014;512:247–8. [DOI] [PubMed] [Google Scholar]
- 16.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GM, Neyrinck M, Possemiers S, Van Holle A, François P, de Vos WM, et al. . Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011;60:2775–86. Erratum in: Diabetes 2011;60:3307. Muccioli, Giulio M [corrected to Muccioli, Giulio G]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holscher HD, Caporaso JG, Hooda S, Brulc JM, Fahey GC, Swanson KS. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr 2015;101:55–64. [DOI] [PubMed] [Google Scholar]
- 18.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, et al. . Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci USA 2009;106:5859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 20.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol 2007;13:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Li J, Liu Y, Xiao N, Suo H, Xie K, Yang CL, Wu C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-kappaB pathway in raw264.7 cells. Inflammation 2012;35:1676–84. [DOI] [PubMed] [Google Scholar]
- 22.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


