Abstract
Background
We evaluated a simplified method for converting Unified Parkinson’s Disease Rating Scale Part III Motor Examination total scores (UPDRS III) to the Movement Disorders Society’s revised version of the scores.
Methods
Parkinson’s disease patients in the Arizona Study of Aging and Neurodegenerative Disorders were assessed with both scales. The accuracy of the predicted scores was assessed using regression modeling, classical intraclass correlation coefficients, and the Bland-Altman method.
Results
There was strong correlation between the two scores. Adding seven points to a UPDRS III total score performed approximately as well as previously published conversion formulas (intraclass correlation 0.96). The adjusted score is expected to be within three points of the Movement Disorders Society-UPDRS III score 50% of the time, and within nine points 95% of the time.
Conclusions
Simply adding seven points to a UPDRS III total score provides a good approximation of the Movement Disorders Society-UPDRS III total score.
Keywords: Parkinson’s, UPDRS, MDS, motor, score
Introduction
In 2008 the Movement Disorders Society (MDS) revised the Unified Parkinson’s Disease Rating Scale (UPDRS) that had been in use since 1987.1, 2 The MDS-UPDRS has a four-part structure similar to the original UPDRS. Part III of both scales focuses on the motor examination. From a research standpoint, Part III is an important outcome measure in many clinical trials. Clinicians also employ the Part III in daily clinical practice to objectively track motor performance and progression over time. Six items were added to the Part III Motor Examination scale, so that the maximum possible total score for Part III increased from 108 points to 132 points. In addition, the anchors for the item scores were changed and some items were renamed. Calibration formulas for three Hoehn and Yahr stage categories were reported so that archival UPDRS III total scores from cohort studies could be combined with MDS-UPDRS III total scores in the same analyses.3 However, these calibration formulas are cumbersome to use for manual review of the longitudinal trajectory of an individual patient in a busy clinical practice. A reliable and simple conversion metric to adjust the scores of patients who have been monitored with UPDRS III to the new MDS-UPDRS III may be useful for the widespread adoption of the new MDS-UPDRS. We assessed the accuracy of a simplified method for converting UPDRS III total scores to equivalent MDS-UPDRS III total scores.
Methods
Data were obtained from the Arizona Study of Aging and Neurodegenerative Disorders.4, 5 This study began collecting UPDRS motor exam data in 1997. Beginning in 2014, participants with Parkinson’s disease were assessed with both the UPDRS and the MDS-UPDRS. The same examiner scored the subject at the same visit using the two different versions of the scale. The association between UPDRS III total scores and MDS-UPDRS III total scores was assessed by using regression modeling. Predicted MDS-UPDRS III total scores were calculated by using previously published conversion formulas (Hoehn and Yahr Stage I–II: 1.2× + 2.3; Stage III: 1.2× + 1.0; Stage IV–V: 1.1× + 7.5).3 Simplified predicted MDS-UPDRS III total scores were calculated by adding the mean difference in scores to each UPDRS III total score. Agreement between MDS-UPDRS III total scores and converted scores was assessed by using the classical intraclass correlation coefficient (ICC) and the Bland-Altman method.
Results
Thirty-eight participants with Parkinson’s disease were assessed with both UPDRS III and MDS-UPDRS III scales. Age ranged from 61 to 96 years with a mean of 76.4 years (SD 8.3), and 14 (39%) were women. There were 20 subjects at Hoehn and Yahr Stage I–II, 13 at Stage III, and 5 at Stage IV–V. Thirty-three (87%) were assessed in the practically defined off state. UPDRS III total scores ranged from 4 to 73 points with a mean of 27 (SD 16) points, and MDS-UPDRS III total scores ranged from 7 to 86 points with a mean of 34 (SD 17) points.
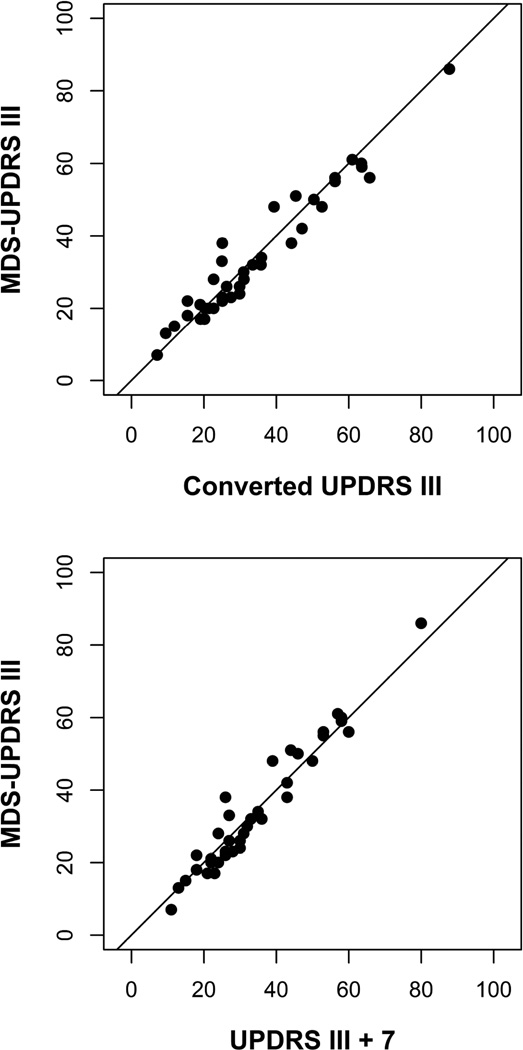
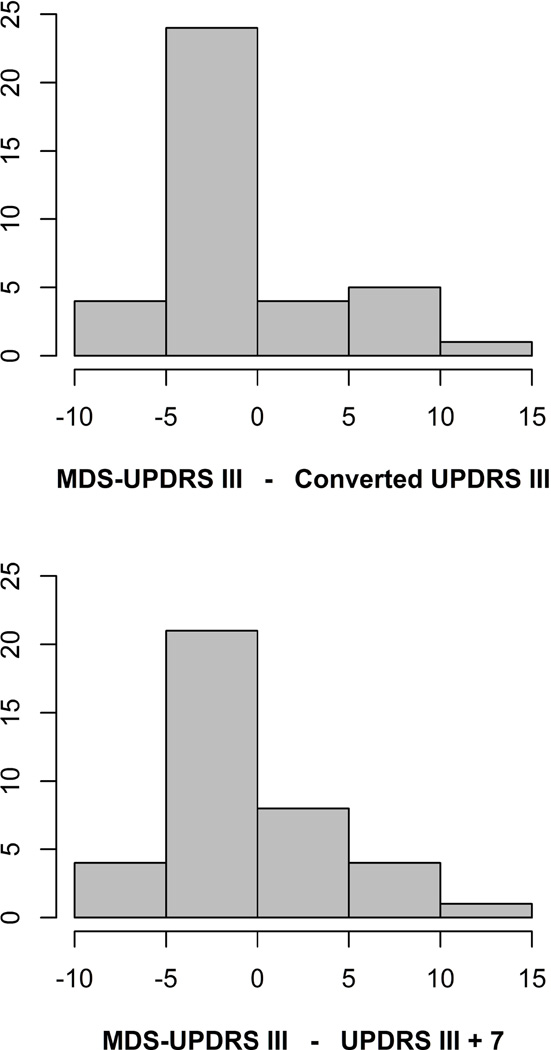
The association between MDS-UPDRS III total scores and predicted scores based on the previously published conversion formulas is shown in Figure 1. There was excellent agreement between the predicted scores and the MDS-UPDRS III total scores (ICC 0.967, 95% CI 0.83 to 1.00). The predicted score is expected to be within ten points of the MDS-UPDRS III total score 95% of the time (Bland-Altman limits of agreement −9.6 to 8.5). The distribution of differences between the MDS-UPDRS III total scores and the predicted scores based on the previously published conversion formulas is shown in Figure 2.
Figure 1.
Figure 2.
None of the 38 MDS-UPDRS III total scores were lower than the corresponding UPDRS III total score. The mean difference was 7 points. The association was linear, and the slope was nearly one (β 1.08). So adding a constant would provide a good approximation of the MDS-UPDRS III total score.
Figure 1 also shows the association between the MDS-UPDRS III total scores and predicted scores based on adding seven points to the UPDRS III total score. The simplified conversion performed as well in this sample as the more complicated regression formulas (ICC 0.965, 95% CI 0.82 to 1.00). The adjusted UPDRS III total score is expected to be within three points of the MDS-UPDRS III total score 50% of the time, within five points of the MDS-UPDRS III total score 75% of the time, and within nine points of the MDS-UPDRS III total score 95% of the time (Bland-Altman limits of agreement −8.8 to 8.4). The distribution of differences between the MDS-UPDRS III total scores and the predicted scores based on adding seven points to the UPDRS III total scores is shown in Figure 2.
Discussion
Goetz et al. presented formulas to calibrate Parts II and III of the original UPDRS to the new MDS-UPDRS.3 They showed that the Bland-Altman limits of agreement for converting from UPDRS III total score to MDS-UPDRS III total score were −8.6 to 7.6, indicating that the predicted score is expected to be within nine points of the MDS-UPDRS III total score 95% of the time. While this method is highly accurate, it does not lend itself for easy adoption in a busy clinical practice.
Merello et al. also showed excellent correlation between the motor sections of the UPDRS and MDS-UPDRS (r .965).6 In addition, they found that prediction of long-term levodopa response using a 24% change in MDS-UPDRS III total score was equivalent to predication of long-term levodopa response using a 30% change in UPDRS III total score. However, their study did not address a means to convert UPDRS III scores to their equivalent MDS-UPDRS III scores.
Our study shows that simply adding seven points to a UPDRS III total score provides a good approximation of the MDS-UPDRS III total score. The accuracy of the simplified method, as measured by the Bland-Altman limits of agreement, was within 3% of the accuracy previously reported using conversion formulas. The simplified adjustment is expected to be within three points of the MDS-UPDRS III total score 50% of the time, within five points of the MDS-UPDRS III total score 75% of the time, and within nine points of the MDS-UPDRS III total score 95% of the time. For research studies, the formulas reported by Goetz et al. would still provide the best available accuracy. However, our simplified conversion method may be more useful in a routine clinical setting to monitor and track the progression of motor signs in patients with Parkinson’s disease, and is recommended for manual review of the longitudinal trajectories of individual patients.
References
- 1.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 2.Fahn S, Elton RL. UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease II. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 3.Goetz CG, Stebbins GT, Tilley BC. Calibration of Unified Parkinson’s Disease Rating Scale Scores to Movement Disoder Society-Unified Parkinson’s Disease Rating Scale Scores. Movement Disorders. 27(10):1239–1242. doi: 10.1002/mds.25122. [DOI] [PubMed] [Google Scholar]
- 4.Beach TG, Adler CH, Sue LI, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35(4):354–389. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: Clinicopathologic study. Neurology. 2014;83(5):406–412. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merello M, Gerschcovich ER, Ballesreror D, Cerquetti D. Correlation between the Movement Disorders Society Unified Parkinson’s Disease rating scale (MDS-UPDRS) and the Unified Parkinson’s Disease rating scale (UPDRS) during L-dopa acute challenge. Parkinsonism and Related Disorders. 2011;17(9):705–707. doi: 10.1016/j.parkreldis.2011.07.002. [DOI] [PubMed] [Google Scholar]