Abstract
Background:
Hypotension induced by combined spinal epidural anesthesia in parturient with hypertensive disorders of pregnancy (HDP) can easily compromise blood supply to vital organs including uteroplacental perfusion and result in fetal distress. The aim of this study was to investigate whether the goal-directed fluid therapy (GDFT) with LiDCOrapid system can improve well-being of both HDP parturient and their babies.
Methods:
Fifty-two stable HDP parturient scheduled for elective cesarean delivery were recruited. After loading with 10 ml/kg lactated Ringer's solution (LR), parturient were randomized to the GDFT and control group. In the GDFT group, individualized fluid therapy was guided by increase in stroke volume (ΔSV) provided via LiDCOrapid system. The control group received the routine fluid therapy. The primary endpoints included maternal hypotension and the doses of vasopressors administered prior to fetal delivery. The secondary endpoints included umbilical blood gas abnormalities and neonatal adverse events.
Results:
The severity of HDP was similar between two groups. The total LR infusion (P < 0.01) and urine output (P < 0.05) were higher in the GDFT group than in the control group. Following twice fluid challenge tests, the systolic blood pressure, mean blood pressure, cardiac output and SV in the GDFT group were significantly higher, and the heart rate was lower than in the control group. The incidence of maternal hypotension and doses of phenylephrine used prior to fetal delivery were significantly higher in the control group than in the GDFT group (P < 0.01). There were no differences in the Apgar scores between two groups. In the control group, the mean values of pH in umbilical artery/vein were remarkably decreased (P < 0.05), and the incidences of neonatal hypercapnia and hypoxemia were statistically increased (P < 0.05) than in the GDFT group.
Conclusions:
Dynamic responsiveness guided fluid therapy with the LiDCOrapid system may provide potential benefits to stable HDP parturient and their babies.
Keywords: Cesarean Delivery, Fluid Therapy, Hypertensive Disorders of Pregnancy, LiDCOrapid System, Parturient
INTRODUCTION
Hypertensive disorder of pregnancy (HDP), a multisystem disorder unique to pregnancy, is one of the leading causes of maternal morbidity and mortality.[1] It, complicating 3–8% of pregnancies, is responsible for fetal growth restriction and preterm birth.[2] Elevated blood pressure (BP) is considered to compensate the reduced maternal-fetal blood flow due to systematic arteriole spasm.[3] Major maternal complications associated with HDP are placental abruption, hemolysis, elevated liver enzymes, low platelets syndrome, disseminated intravascular coagulation, neurologic deficits, pulmonary edema and acute renal failure.[4] Widespread endothelial dysfunction can also occur in the placenta that ultimately leads to placental ischemic injury or even infarction.[5]
Perioperative management of parturient with HDP is a great challenge. HDP is prone to peripheral edema while the intravascular volume is paradoxically insufficient due to increased capillary permeability and decreased oncotic pressure. Plasma volume in parturient is decreased,[6] and indeed, it has been reported that 600–800 ml/m2 of plasma volume deficit occurred in such patients when compared to normal pregnant woman.[7] Severe preeclampsia may be accompanied with cardiac dysfunction, reduced oncotic pressure, elevated hydrostatic pressure and pulmonary capillary leak, all of which lead to pulmonary edema.[8] Parturient with severe preeclampsia is poorly tolerant to overhydration if the ventricular dysfunction is present and is also sensitive to sympathetic blockade.
Cesarean delivery, which is usually applied to terminate pregnancy in such parturient, is currently popular to be performed under combined spinal epidural anesthesia (CSEA). Singh et al.[4] suggested that spinal anesthesia could be safely used for lower segment cesarean delivery in stable eclamptic patients to avoid risks of general anesthesia.
However, this anesthetic regimen may lead to maternal hypotension and subsequent uteroplacental hypoperfusion.[9] The hemodynamic instability, which HDP parturient are sensitive to, is mainly produced by profound peripheral vasodilation and subsequent reduce in cardiac output (CO) following CSEA[10] and hence in turn deteriorates uteroplacental hypoperfusion.[11] Thus, adequate fluid therapy, which is aimed at the optimization of maternal organ perfusion and prevent CSEA induced uteroplacental hypoperfusion, should be of importance. Robson et al.[11] have reported that the uteroplacental perfusion is dependent on maternal CO in parturient under CSEA. Accordingly, the use of goal-directed fluid therapy (GDFT) with the LiDCO system targeted at optimizing maternal stroke volume (SV) may be beneficial.
GDFT, a recent advance in perioperative fluid management, enables to optimize hemodynamics and oxygen delivery.[12] Perioperative GDFT has been proven to reduce the risk of postoperative organ dysfunction and morbidity.[13] LiDCOrapid system,[14] a noninvasive method to achieve SV optimization, is in principle to maintain the plateau of the Frank-Starling curve by SV variation or increase in SV (∆SV). LiDCOrapid with CNAP develops an arterial waveform noninvasively by applying oscillometric pulse pressure to the finger signal and shifting the amplified curve to the oscillometric pressure. The PulseCO algorithm, which is validated by LiDCOplus,[15,16] is employed in LiDCOrapid system and used to estimate SV using an autocorrelation formula and a patient-specific calibration factor calculated by in-vivo data.[17] In this study, we will evaluate if GDFT strategy used with the LiDCOrapid system (LiDCO Ltd., Cambridge, UK) could improve maternal hemodynamics and neonatal health outcomes for stable HDP parturient undergoing CSEA.
METHODS
Patients
After obtaining approval from Ethical Committee and written informed consent from parturient, 52 parturient with stable HDP presenting for elective cesarean delivery were recruited to this study. Parturient with HDP includes gestational hypertension and preeclampsia; and the severity of HDP of patients was diagnosed by obstetricians accordingly.[3,8] All the parturient were followed up for 12 weeks following delivery and confirmed the diagnosis by obstetricians. Inclusion criteria were term parturient with late onset stable HDP. The late onset HDP[18] means the onset of hypertension is after 34 weeks gestation. The definitions[4] of stable HDP include: (1) Conscious, cooperative, responding parturient, (2) no convulsive episode for at least 12 h, (3) no papilledema, (4) no other signs of raised intracranial pressure, (5) parturient receiving magnesium sulfate or antihypertensive therapy, (6) without any other systemic complications such as congestive heart failure, pulmonary edema, acute renal failure or hepatic failure, (7) urine output more than 0.5 ml·kg−1·h−1, (8) platelet count more than 100 × 109 cells/L. All the recruited parturient were randomly allocated by the computer generated random-digit to the control (n = 26) and GDFT group (n = 26) [Figure 1]. Parturient with the following situations were excluded from this study: American Society of Anesthesiology III–V, twin/multiple pregnancies, contraindications for spinal anesthesia, known fetal abnormalities and coexisting maternal diseases except HDP, early onset HDP, gestational weeks <37 weeks, unstable HDP, and patients who were not willing to participate in the study. In total, 20 patients were excluded from this study.
Figure 1.
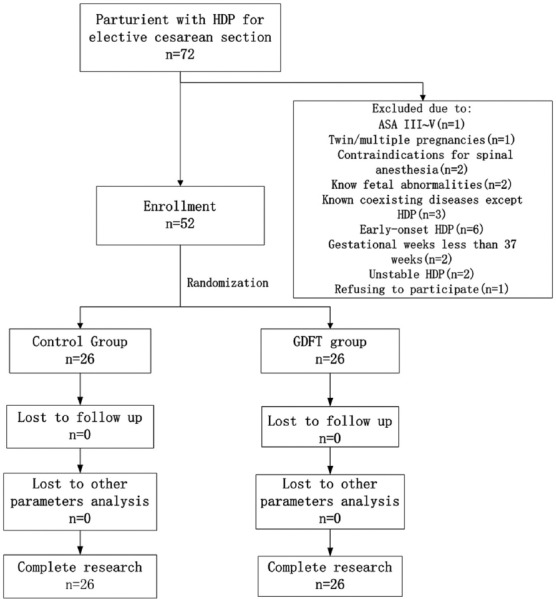
Patients’ recruitment flow chart. Patients were excluded from our study for the following reasons: 1 for ASA physical level of III–V; 1 for twin pregnancy; 2 for contraindications for spinal anesthesia; 2 for known fetal abnormalities; 3 for known coexisting diseases except HDP; 6 for early-onset HDP, 2 for gestational weeks <37 weeks, 2 for unstable HDP, and 1 for refusing to participate. GDFT: Goal-directed fluid therapy; ASA: American Society of Anesthesiology; HDP: Hypertensive disorders of pregnancy.
Protocols
After arrival in the operating room, the standard monitoring including noninvasive BP, electrocardiography and pulse oximetry (SpO2) were applied. LiDCOrapid Pulse Contour Analysis System was also established and calibrated to measure SV, CO and increase in SV (∆SV) in all patients, but it was only used for guiding fluid therapy in the GDFT group. Before IV-drip was started, each patient was allowed to rest without any disturbance for 5 min. The routine hemodynamic parameters were measured for three times with 2 min gap between and the mean of which was recorded as the baseline values. Oxygen was administered through nasal cannula at 5 L/min from the start of anesthesia until baby delivery.
The standard CSEA protocol was intrathecal injection of 0.5% bupivacaine 7.5 mg (1.5 ml, isobaric, 1.0 ml/10 s) at the L2–3 intervertebral spaces in the lateral decubitus position, and epidural catheter was then inserted cephalad as a rescue for spinal anesthesia or for postoperative pain relief. Patients were immediately placed supine with 15° left lateral tilt following spinal anesthesia. The cephalic spread of sensory level was assessed bilaterally by loss of pinprick perception. The sensory block was aimed to reach around above T5 but lower than T3. Fetal heart rate (HR) was monitored by external cardiotocography until prepping of the abdomen.
Starting from the skin prepping of CSEA, each patient was coloaded with lactated Ringer's solution (LR) of 10 ml/kg. The ideal body weight (patient's height - 110) was used to reduce the impact of varying body weight during pregnancy. In both groups, maintenance LR was infused at a rate of 2 ml·kg−1·h−1 including any drug therapy.[19] In the GDFT group, following coloading with LR of 10 ml/kg, LR of 3 ml/kg was infused to test the fluid responsiveness to guide the individual fluid therapy. The infusion duration of fluid challenge test was 3 min. An ∆SV of more than 10% was defined as the positive fluid response. If the first fluid response was positive, a further fluid challenge with the same loading volume was repeated 1 min later until the negative response was obtained. In control group, only maintenance fluid infusion was administered without any fluid challenge. In both groups, equal volume hydroxyethyl starch (HES, 130/0.4, Voluven) was administered to replace the volume (usually <800 ml) of blood loss during surgery.
The definition of maternal hypotension was a decrease of >20% of the baseline systolic BP (SBP).[20] If hypotension occurred, phenylephrine or ephedrine depending on patient's HR was injected intravenously. If their HR was above 60 beat/min, phenylephrine was administered with an increment of 25 μg; otherwise ephedrine was given with an increment of 6 mg. Further details of intraoperative fluid management protocol are shown in Figure 2.
Figure 2.
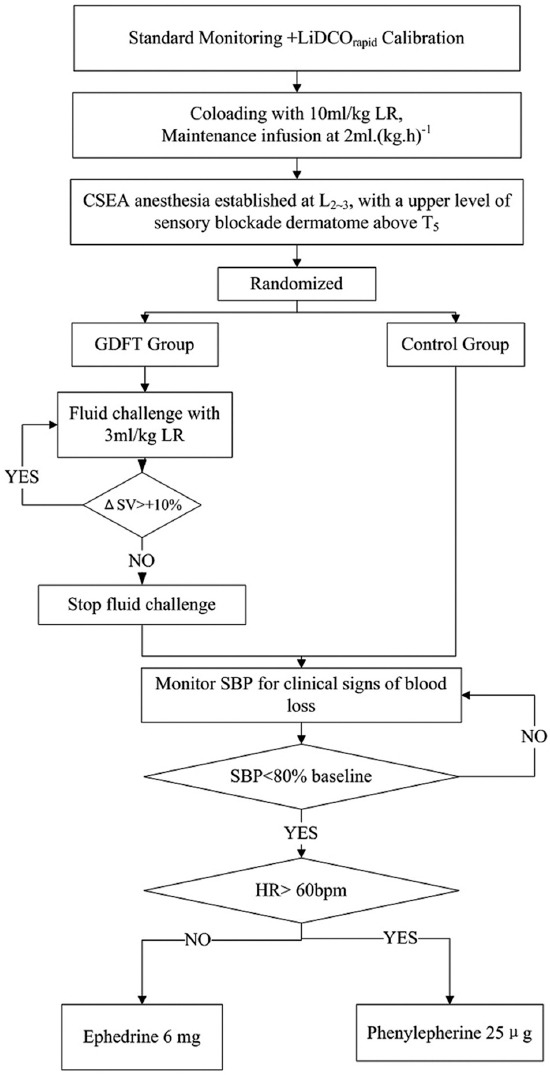
Protocol for intraoperative management. LR: Lactated Ringer's solution; CSEA: Combined spinal epidural anesthesia; GDFT: Goal-directed fluid therapy; ΔSV: Increase in stroke volume; SV: Stroke volume; HR: Heart rate.
Data collection and study endpoints
In both groups, the demographic data, fasting duration, cephalic spread of sensory blockade, frequency and total doses of epidural anesthesia rescue, induction-delivery interval, uterine incision-delivery interval, volume of fluids infused, and blood loss were recorded. The increased body weight during pregnancy, neonatal birthweight, gestational age, maximum SBP and diastolic BP (DBP), level of albumin and urine protein in 24 h, duration of hypertension, the severity of HDP, and the level of edema were also recorded. Edema was classified in standard grade by a physician as follows:[21] (1 = A normal foot and leg contour with a barely perceptible pit; 2 = Fairly normal lower extremity contours with a moderately deep pit; 3 = Obvious foot and leg swelling with a deep pit; 4 = Severe foot and leg swelling that distorts the normal contours with a deep pit).
Maternal hemodynamic parameters, including SBP, mean BP (MBP), HR, SpO2, CO and SV, were recorded at the baseline (T1), following coloading with LR of 10 ml/kg (T2), 4 min following LR coloading (following first fluid challenge test) (T3), 8 min following LR coloading (following second fluid challenge test) (T4), stable blockade level obtained (thoracic level 5–3) (T5), immediate after delivery (T6), placental expulsion (T7), and discharge from operating room (T8). Prior to delivery, the incidences of maternal hypotension, nausea and vomiting, and the total used doses of vasopressors were recorded. Immediately following delivery, blood samples were taken from umbilical artery and vein in a doubly clamped umbilical cord segment, and subsequently analyzed with a blood gas analyzer (ABL800 FLEX, Radiometer Medical ApS, Denmark). Apgar scores were evaluated at 1 and 5 min following delivery. The character of amniotic fluid was graded on a scale of 0–2 (0 = Clear, 1 = Meconium tinged, 2 = Thick meconium). The normal limits of umbilical arterial were defined as pH >7.20, PCO2 <55 mmHg, and PO2 >15 mmHg[22] and venous blood gases were defined as pH >7.28, PCO2 <46 mmHg, and PO2 >21 mmHg.[23]
The primary endpoints of this study were the incidence of maternal hypotension and the used doses of vasopressors prior to delivery. The secondary endpoints were umbilical blood gas abnormalities and neonatal adverse events.
Statistical analysis
Following preliminary study (n = 20), the incidences of hypotension were 10% in the GDFT group and 60% in the control group individually. A sample size of 14 was needed in each group to have a power of 95%, differences (hypotension) of 30% or more detected between the groups. Twenty-six patients per group were recruited to compensate for any exclusion.
Statistical analysis was done with SPSS software (version 11.5, SPSS Inc., Chicago, IL, USA). All quantitative data were presented as mean ± standard deviation, between-group comparisons were done with unpaired t-test and in-group comparisons adopted repeated measures one-way ANOVA. Median (25–75% percentile) of rank data and nonnormally distributed quantitative variables were also given, between-group comparisons were performed by nonparametric test with Mann–Whitney test. Qualitative variables were expressed as percentages and compared via Chi-square test. A P < 0.05 was considered as statistically significant.
RESULTS
Patients’ characteristics
The characteristics of patients’ age, weight, height, preoperative hemoglobin, postoperative hemoglobin and fasting duration were similar between the two groups. There were no significant differences in the frequency and dosage of epidural rescue, cephalic spread of sensory blockade, the intervals from CSEA induction to delivery and uterine incision to delivery, the total operation time and anesthesia time. In addition, intraoperative blood loss and intraoperative colloid (HES, 130/0.4, Voluven) infusion between two groups were comparable. However, in GDFT group, there were 54% (14/26) patients challenged with a second fluid bolus, and no patients received a third one. The total LR infusion (P < 0.01) and urine output (P < 0.05) in the GDFT group were higher than that in the control group [Table 1].
Table 1.
Parturients’ characteristics
| Parameters | Control group (n = 26) | GDFT group (n = 26) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 32.0 ± 4.8 | 30.0 ± 4.3 | 1.68 | 0.10 |
| Weight (kg) | 86.4 ± 12.9 | 81.8 ± 14.9 | 1.21 | 0.23 |
| Height (cm) | 164.0 ± 4.5 | 162.8 ± 7.8 | 0.66 | 0.52 |
| Preoperative hemoglobin (g/L) | 123.6 ± 13.0 | 119.3 ± 9.8 | 1.35 | 0.18 |
| Fasting duration (h) | 10.6 ± 2.4 | 10.1 ± 2.8 | 0.72 | 0.48 |
| Increased weight during pregnancy (kg) | 17.9 ± 6.7 | 21.6 ± 7.5 | 1.88 | 0.07 |
| Gestational weeks (weeks) | 38 (37–39) | 38 (38–40) | 0.20 | |
| Albumin (g/L) | 32.9 ± 3.6 | 31.4 ± 4.0 | 1.44 | 0.16 |
| Urine protein (g/24 h) | 0.41 (0.11–2.12) | 0.34 (0.16–1.45) | 0.68 | |
| Level of edema | 1.5 (1–2) | 1.5 (1–2) | 0.82 | |
| Duration of hypertension (d) | 8.5 (5.8–14.0) | 7.0 (5.0–8.0) | 0.19 | |
| Maximum SBP (mmHg) | 155.7 ± 13.0 | 154.7 ± 8.5 | 0.34 | 0.73 |
| Maximum DBP (mmHg) | 100.0 ± 10.8 | 102.1 ± 7.2 | 0.83 | 0.41 |
| Severity of HDP | ||||
| Ratio of gestational hypertension (%) | 34.6 | 38.5 | 0.08 | 0.77 |
| Ratio of mild preeclampsia (%) | 42.3 | 38.5 | 0.08 | 0.78 |
| Ratio of severe preeclampsia (%) | 23.1 | 23.0 | 0.01 | 1.00 |
| Level of blockade (dermatome) | 5 (5–5) | 5 (5–5) | 0.30 | |
| Induction to delivery interval (min) | 17.0 ± 2.3 | 18.1 ± 2.0 | 1.83 | 0.07 |
| Uterus incision to delivery interval (min) | 1.8 ± 0.9 | 2.4 ± 1.8 | 1.44 | 0.16 |
| Operation time (min) | 37.6 ± 4.7 | 38.4 ± 5.7 | 0.58 | 0.56 |
| Anesthetic time (min) | 54.4 ± 7.6 | 54.6 ± 7.0 | 0.09 | 0.93 |
| Neonatal birth weight (g) | 3018.0 ± 693.0 | 3092.0 ± 594.4 | 0.41 | 0.68 |
| Intraoperative LR (ml) | 682.3 ± 82.8 | 880.8 ± 215.4 | 4.39 | <0.01 |
| Intraoperative colloid (ml) | 223.1 ± 58.7 | 271.2 ± 141.5 | 1.60 | 0.12 |
| Intraoperative blood loss (ml) | 223.1 ± 58.7 | 271.2 ± 141.5 | 1.60 | 0.12 |
| Intraoperative urine output (ml) | 103.8 ± 37.2 | 132.7 ± 52.8 | 2.28 | 0.03 |
| Postoperative hemoglobin (g/L) | 106.6 ± 9.5 | 104.6 ± 6.4 | 0.89 | 0.38 |
| Frequent of epidural rescue (%) | 46.2 | 53.8 | 0.31 | 0.58 |
| Amount of epidural rescue (ml) | 3 (0–4) | 0 (0–4) | 0.95 |
Values are given as mean value ± SD, median (25–75% percentile), or percentages. SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HDP: Hypertensive disorders of pregnancy; LR: Lactated Ringer’s solution; SD: Standard deviation; GDFT: Goal-directed fluid therapy.
The increased body weight during pregnancy, neonatal birth weight, gestational age, maximum SBP and DBP, level of albumin and urine protein in 24 h compared well between the two groups. The duration of hypertension, the severity of HDP, and the level of edema were also not dissimilar between the two groups [Table 1].
Maternal outcomes
Following twice fluid challenge tests, the SBP, MBP, CO and SV in the GDFT group were significantly higher, and the HR was lower than that in the control group [Table 2 and Figure 3]. The intraoperative SpO2 value was maintained above 98% in all patients. In each group, compared with the baseline values, the timepoint of placental expulsion induced most noticeable changes in all hemodynamic parameters. In GDFT group, both fluid challenge tests produced significant increases in CO and SV (P < 0.05); meanwhile, no such changes occurred in the control group [Table 2 and Figure 3]. In general, the drops in SBP and MBP prior to delivery were more noticeable in the control group [Table 2 and Figure 3].
Table 2.
Intraoperative maternal hemodynamic changes in the both groups
| Parameter | Control group (n = 26) | GDFT group (n = 26) | P |
|---|---|---|---|
| SBP (mmHg) | |||
| T1 | 133.30 ± 15.77 | 134.60 ± 16.39 | 0.78 |
| T2 | 123.20 ± 17.89* | 129.00 ± 10.49 | 0.16 |
| T3 | 125.20 ± 17.67 | 126.20 ± 13.47* | 0.81 |
| T4 | 122.50 ± 15.68* | 129.40 ± 9.17 | 0.06 |
| T5 | 121.60 ± 15.95* | 130.40 ± 14.93 | 0.04 |
| T6 | 123.10 ± 18.46* | 130.30 ± 15.11 | 0.13 |
| T7 | 113.20 ± 22.60* | 116.90 ± 18.42* | 0.51 |
| T8 | 119.60 ± 14.07* | 120.80 ± 16.79* | 0.78 |
| MBP (mmHg) | |||
| T1 | 94.92 ± 15.05 | 94.27 ± 11.65 | 0.86 |
| T2 | 89.31 ± 11.80 | 92.38 ± 9.81 | 0.31 |
| T3 | 96.27 ± 15.07 | 90.46 ± 12.79 | 0.14 |
| T4 | 91.12 ± 15.26 | 98.12 ± 9.43 | 0.05 |
| T5 | 87.27 ± 15.06 | 94.88 ± 14.38 | 0.07 |
| T6 | 84.27 ± 14.10* | 93.65 ± 13.71 | 0.02 |
| T7 | 78.04 ± 14.76* | 83.00 ± 14.54* | 0.23 |
| T8 | 82.69 ± 10.68* | 83.46 ± 14.57* | 0.83 |
| HR (beat/min) | |||
| T1 | 88.12 ± 12.94 | 83.92 ± 12.61 | 0.24 |
| T2 | 90.00 ± 12.14 | 85.96 ± 8.73 | 0.18 |
| T3 | 93.73 ± 15.45 | 80.12 ± 10.74 | <0.01 |
| T4 | 85.81 ± 11.74 | 78.12 ± 11.12* | 0.02 |
| T5 | 84.81 ± 12.39 | 78.23 ± 11.22* | 0.05 |
| T6 | 88.23 ± 18.20 | 83.23 ± 12.78 | 0.26 |
| T7 | 98.12 ± 15.39* | 84.42 ± 12.89 | <0.01 |
| T8 | 81.15 ± 10.88 | 82.00 ± 12.97 | 0.80 |
| CO (L/min) | |||
| T1 | 8.79 ± 2.81 | 7.81 ± 1.92 | 0.15 |
| T2 | 8.83 ± 2.46 | 8.39 ± 2.01 | 0.48 |
| T3 | 8.08 ± 1.84 | 9.49 ± 1.92* | 0.01 |
| T4 | 8.00 ± 1.89 | 9.54 ± 1.94* | 0.01 |
| T5 | 8.48 ± 2.31 | 8.82 ± 1.90 | 0.56 |
| T6 | 9.17 ± 2.99 | 9.06 ± 1.98* | 0.88 |
| T7 | 10.91 ± 3.58* | 10.34 ± 3.20* | 0.55 |
| T8 | 9.42 ± 3.97 | 8.79 ± 2.41 | 0.49 |
| SV (ml/beat) | |||
| T1 | 96.38 ± 26.65 | 94.35 ± 20.11 | 0.76 |
| T2 | 100.90 ± 24.63 | 96.92 ± 20.10 | 0.52 |
| T3 | 87.08 ± 14.22 | 116.40 ± 28.05* | <0.01 |
| T4 | 93.42 ± 16.79 | 124.00 ± 28.89* | <0.01 |
| T5 | 99.92 ± 29.98 | 112.50 ± 26.29* | 0.11 |
| T6 | 104.30 ± 33.93 | 107.00 ± 18.11 | 0.73 |
| T7 | 113.30 ± 35.52* | 118.00 ± 27.88* | 0.59 |
| T8 | 113.70 ± 37.37* | 108.30 ± 25.59 | 0.55 |
Values are given as mean value +/- SD. *P<0.05 versus the baseline (T1) while P values in the table indicate the statistical significance between the both groups. SBP: Systolic blood pressure; MBP: Mean blood pressure; HR: Heart rate; CO: Cardiac output; SV: Stroke volume; T1: Baseline; T2: Following coloading with LR of 10 ml/kg; T3: 4 min following LR coloading (following first fluid challenge test); T4: 8 min following LR coloading (following second fluid challenge test); T5: Stable blockade level obtained (thoracic level 5–3); T6: Immediate after delivery; T7: Placental expulsion; T8: Discharge from operating room; SD: Standard deviation; GDFT: Goal-directed fluid therapy; LR: Lactated Ringer’s solution.
Figure 3.
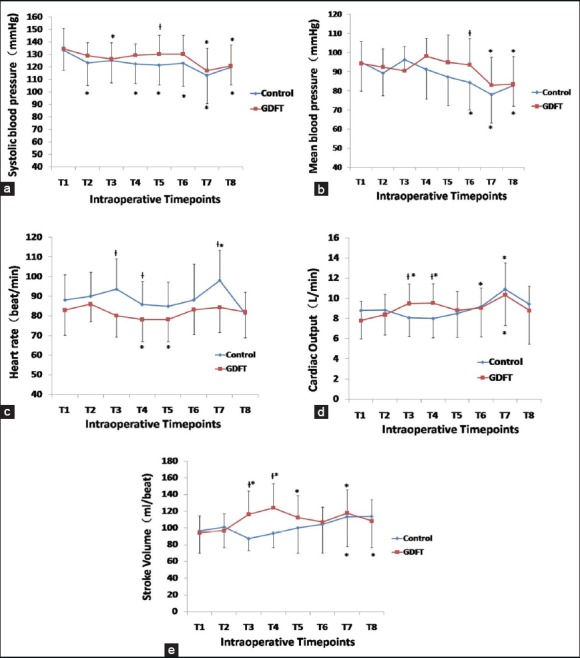
Mean differences in hemodynamic variables between the two groups. (a) Systolic blood pressure; (b) Mean blood pressure; (c) Heart rate; (d) Cardiac output; (e) Stroke volume were monitored at the following time points in both groups. T1: Baseline; T2: Following coloading with LR of 10 ml/kg; T3: 4 min following LR coloading (following first fluid challenge test); T4: 8 min following LR coloading (following second fluid challenge test); T5: Stable blockade level obtained (thoracic level 5–3); T6: Immediate after delivery; T7: Placental expulsion; T8: Discharge from operating room. Diamond with blue line and square with red line indicate the control group and the GDFT group at that time, respectively. Data were expressed as mean ± standard deviation; *Significantly different at P < 0.05 compared with the baseline (T1) in each group, †Significantly different at P < 0.05 compared between the two groups. GDFT: Goal-directed fluid therapy; LR: Lactated Ringer's solution.
In the control group, the incidence of hypotension prior to delivery was significantly higher than that in the GDFT group (61.5% vs. 19.2%, P < 0.01). The doses of phenylephrine injection before fetal delivery were subsequently higher in the control group (P < 0.01). The incidences of nausea and vomiting, headache and the totaled use of ephedrine were comparable between two groups [Table 3]. No parturient in either group experienced any other events, e.g., seizure, pulmonary edema, cardiac failure or malignant arrhythmia.
Table 3.
Maternal adverse events prior to fetal delivery
| Parameter | Control group (n = 26) | GDFT group (n = 26) | Statistics | P |
|---|---|---|---|---|
| Phenylepherine | 25 (0–50) | 0 (0–0) | <0.01 | |
| dose (μg) | ||||
| Ephedrine dose (mg) | 0 (0–0) | 0 (0–0) | 0.17 | |
| Incidence of | 61.5 | 19.2 | 9.67 | <0.01 |
| hypotension (%) | ||||
| Incidence of nausea | 0 | 7.6 | 2.08 | 0.15 |
| and vomiting (%) | ||||
| Incidence of | 0 | 3.8 | 1.02 | 0.31 |
| headache (%) |
Values are given as median (25–75% percentile) or percentages. GDFT: Goal-directed fluid therapy.
Neonatal outcomes
The Apgar scores at 1 and 5 min following delivery were similar between both groups [Table 4]. However, In addition, the incidence of PuvCO2 more than 46 mmHg in umbilical vein was significantly elevated (53.8% vs. 23.1%, P < 0.05), and the incidence of PuvO2 ≤21 mmHg was remarkably elevated in the control than in the GDFT group (34.6% vs. 7.8%, P < 0.05). Although without reaching statistical significance, a similar pattern change was found in umbilical arterial samples [Table 5].
Table 4.
Neonatal outcomes after delivery
| Parameter | Control group (n = 26) | GDFT group (n = 26) | Statistics | P |
|---|---|---|---|---|
| Apgar at 1 min | 10 (10–10) | 10 (10–10) | 0.90 | |
| Apgar at 5 min | 10 (10–10) | 10 (10–10) | 1.00 | |
| Character of | 0 (0–0) | 0 (0–0) | 0.71 | |
| amniotic fluid | ||||
| Umbilical ABG | ||||
| pH | 7.29 ± 0.07 | 7.32 ± 0.04 | 2.05 | 0.04 |
| PCO2 (mmHg) | 53.07 ± 8.75 | 48.93 ± 7.81 | 1.80 | 0.08 |
| PO2 (mmHg) | 19.89 ± 11.07 | 20.09 ± 7.60 | 0.07 | 0.94 |
| SO2 (%) | 27.57 ± 18.44 | 33.79 ± 15.95 | 1.09 | 0.28 |
| Base deficit | −1.6 (−2.4–−0.6) | −0.7 (−2.4–1.0) | 0.52 | |
| (mmol/L) | ||||
| Lactic acid | 2.25 ± 2.11 | 1.82 ± 0.63 | 1.01 | 0.32 |
| (mmol/L) | ||||
| Umbilical VBG | ||||
| pH | 7.33 ± 0.07 | 7.36 ± 0.02 | 2.08 | 0.04 |
| PCO2 (mmHg) | 46.49 ± 7.59 | 43.84 ± 4.81 | 1.50 | 0.14 |
| PO2 (mmHg) | 23.07 ± 6.41 | 26.93 ± 6.01 | 2.24 | 0.03 |
| SO2 (%) | 46.53 ± 21.91 | 54.17 ± 20.07 | 1.31 | 0.20 |
| Base deficit | −1.4 (−2.6–−0.5) | −1.0 (−1.9–0) | 0.15 | |
| (mmol/L) | ||||
| Lactic acid | 2.04 ± 1.97 | 1.84 ± 0.50 | 0.50 | 0.62 |
| (mmol/L) |
Values are given as mean value ±SD or median (25–75% percentile). ABG: Arterial blood gas; VBG: Venous blood gas; PCO2: Partial pressure of carbon dioxide; PO2: Partial pressure of oxygen; SO2: Oxygen saturation; SD: Standard deviation; GDFT: Goal-directed fluid therapy.
Table 5.
Neonatal adverse events after delivery
| Incidence of events (%) | Control group (n = 26) | GDFT group (n = 26) | Statistics | P |
|---|---|---|---|---|
| Umbilical VBG | ||||
| pH <7.28 | 7.6 | 0 | 2.08 | 0.15 |
| PCO2 >46 mmHg | 53.8 | 23.1 | 5.20 | 0.02 |
| PO2 ≤21 mmHg | 34.6 | 7.8 | 5.65 | 0.02 |
| Umbilical ABG | ||||
| pH <7.20 | 7.6 | 0 | 2.08 | 0.15 |
| PCO2 >55 mmHg | 30.8 | 19.2 | 0.92 | 0.34 |
| PO2 ≤15 mmHg | 46.2 | 26.9 | 2.07 | 0.15 |
| Character of | 7.6 | 3.8 | 0.28 | 0.60 |
| amniotic fluid=2 | ||||
| Apgar <7 | 11.5 | 0 | 3.18 | 0.07 |
Values are given as percentages. VBG: Venous blood gas; ABG: Arterial blood gas; PCO2: Partial pressure of carbon dioxide; PO2: Partial pressure of oxygen; GDFT: Goal-directed fluid therapy.
DISCUSSION
Hypertensive disorder of pregnancy, which affects 3–8% of all pregnancies, remains one of the leading causes of maternal mortality and morbidity.[24] Cesarean delivery was used to deliver baby in HDP parturient with a range from 11% to 57% and providing anesthesia is a great challenge due to the accompanied uncontrolled hypertension, reduced intravascular volume and multi-organ dysfunction. The choice of anesthesia for lower segment cesarean delivery in HDP parturient remains controversial owing to the considerably varied clinical spectrum of HDP.[4] The known risks of general anesthesia include difficult intubation, aspiration, significant hemodynamic changes during intubation, impaired intervillous blood supply and drug interactions between anesthetics and antihypertensive medication.[25] Regional anesthesia is contraindicated in the presence of coagulopathy and raised intracranial pressure. The central neuraxial block is related to severe hypotension, high motor blockade and convulsion occurring during manipulation.[26] In addition, the sensitivity to vasopressors is enhanced in HDP parturient.[27] Despite these potential risks associated with central neuraxial blocks anesthesia, it provides relative stable hemodynamics, decreased catecholamine concentrations,[25] improved uteroplacental and maternal peripheral perfusion[28] and higher Apgar scores at 1 min,[28,29] thus CSEA is the preferred choice for HDP patients. Similar to a previous report,[4] CSEA was safely provided in fifty-two stable HDP parturient without any major complications in our study.
Hypertensive disorder of pregnancy, an underlying multi-organ disorder, is unique to pregnancy. The perioperative fluid management presents challenges to anesthetists. The pregnancy induced maternal plasma volume expansion is attenuated in HDP parturient. In general, a deficit of 600–800 ml/m2 has been reported when compared with normal pregnancy.[7] This deficit is considered secondary to vascular constriction induced hypertension, which is associated with impaired organ perfusion, especially impaired uteroplacental perfusion.[30] Rapid fluid infusion may improve maternal and fetal organ perfusion, but it also results in a significant increase in alveolar-arterial oxygen difference and shunt fraction, indicating maternal interstitial pulmonary edema.[31] In addition, the risk of pulmonary edema is elevated in HDP parturient due to the increased capillary permeability, reduced colloid oncotic pressure gradient and impaired left ventricular function.[6,32] Consequently, adequate volume expansion may benefit HDP parturient while excessive volume expansion will increase risks of developing pulmonary edema.[33]
The main goal of the perioperative fluid management is to improve the maternal organ perfusion, placental blood supply, and fetal oxygen delivery.[6] Improvement of maternal organ perfusion can be achieved by well-controlled BP and adequate volume expansion. Adequate fluid administration can further compensate the preoperative dehydration and CSEA induced acute decrease in cardiac preload to prevent hypotension occurring.[34] However, the exact volume of fluid expansion is difficult to predict and varies according to different infusion timing, patient status, and anesthetic regimens. Goal-directed fluid therapy, providing individualized fluid management according to the individual demographics and medical status, may be a solution for such patients.[35,36] GDFT therapy based on dynamic parameters has been proven to improve postoperative outcomes and reduce postoperative complications in low to high risk patients under mechanical ventilation.[12,13,37] Our study applied LiDCOrapid system to guide GDFT under spontaneous breath. The validity and usefulness of LiDCOrapid system have been proven in healthy patients,[14] even in parturient with severe preeclampsia.[38] LiDCOrapid system can continuously assess SV based on noninvasive pulse contour analysis, which provides a reliable hemodynamic trend.[14] Previous studies have indicated that GDFT strategies via LiDCO supplied fluid responsiveness can optimize oxygen delivery and reduce total cardiovascular complications, including arrhythmias and acute pulmonary edema in high-risk patients.[39,40] Even in a parturient with acute myocardial infarction, LiDCO system guided a close control of hemodynamic parameters, which improved maternal outcome and newborn well-being.[41] Compared with BP, CO is a better predictor of adequate maternal organ and uteroplacental perfusion,[11] which is the basis of CO optimization by GDFT. In our study, GDFT strategies via LiDCO supplied fluid responsiveness were used to optimize venous return and CO to reduce the incidences of maternal hypotension and subsequent neonatal adverse events. Our data also verified the advantages of CO optimization. The previous GDFT studies in parturient employed invasive monitors[38] were performed under mechanical ventilation[41] or during the postpartum period,[38] while our study employed a noninvasive system to perform intraoperative GDFT management in parturient under spontaneous breath. The protocol is feasible in daily obstetric fluid management.
To date, the most suitable intravascular loading regimen is still controversial. There is no evidence to support the benefits of HES over crystalloid during GDFT therapy.[42] Coloading volumes between 500 and 1000 ml of crystalloid (Ringer's lactate or normal saline) or colloid can equally reduce the incidence of hypotension.[43] However, considering the cost-effectiveness, long-term intravascular stay to develop overload following delivery, potential impacts on hemostasis and renal function of colloid, crystalloid fluid was preferred in our study. The loading volume varies according to different intrathecal doses, similar to previous experience,[4,10] our study employed LR solution of 10 ml/kg as coloading regimen, but the advantage of crystalloid fluid warrants further study.
Compared with Apgar scores, umbilical blood gas values, especially umbilical arterial pH are more sensitive indicators of perinatal outcomes.[44,45] In our study, although the median Apgar score at 1 and 5 min were similar, the incidences of hypercapnia and hypoxemia were significantly different between groups. Fetal acidosis (pH <7.20) occurred in two cases (7.6%) and Apgar score at 1 min <7 occurred in three cases (11.5%) in the control group, indicating unfavorable outcomes of the unborn.[46] In HDP parturient, uteroplacental blood supply is impaired with potential risks of placental abruption;[8] in addition, the blood flow is pressure dependent and hence prolonged severe maternal hypotension can threaten uteroplacental perfusion and result in fetal distress or neonatal acidosis.[47] Following spinal anesthesia, the venous return and CO were optimized via LiDCOrapid guided fluid management in the GDFT group. Those strategies significantly decreased the incidences of maternal hypotension and uteroplacental hypo-perfusion to minimize the risks of developing neonatal hypoxia and hypercapnia.[48] On the other side, the GDFT strategies employed in our study also reduced the total dosages of vasopressors administered. Although phenylephrine is related with a decreased risk of fetal acidosis and a higher BE value than ephedrine;[49] the indirect effect of phenylephrine results in norepinephrine release.[50] Thus, as verified by our data, the excessive use of phenylephrine without sufficient fluid loading in HDP parturient may further induce vasoconstriction of the uteroplacental circulation with increased oxygen extraction and impaired oxygen delivery,[51] and eventually cause the fetal acid-base imbalance.
There are some limitations of our study. Colloid preload and coload, which are effective strategies to prevent spinal anesthesia-induced hypotension, were not employed; so it can be questionable whether the findings of our study can be extrapolated to the patients with colloid preload and coload.
In summary, our data suggested that dynamic responsiveness guided fluid therapy with the LiDCOrapid system can reduce the incidence of maternal hypotension and the requirements of vasopressors, and subsequently decrease neonatal adverse events. The GDFT protocol reported in this study [Figure 2] may provide benefits to stable late-onset HDP parturient and the newborns during cesarean delivery under CSEA.
ACKNOWLEDGMENTS
We thank Dr. Qi Zhou and Dr. Xiaoying Han for their help during sampling of umbilical blood.
Footnotes
Edited by: Yi Cui
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Dennis AT. Management of pre-eclampsia: Issues for anaesthetists. Anaesthesia. 2012;67:1009–20. doi: 10.1111/j.1365-2044.2012.07195.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferrazzani S, Luciano R, Garofalo S, D’Andrea V, De Carolis S, De Carolis MP, et al. Neonatal outcome in hypertensive disorders of pregnancy. Early Hum Dev. 2011;87:445–9. doi: 10.1016/j.earlhumdev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Barra S, Cachulo Mdo C, Providência R, Leitão-Marques A. Hypertension in pregnancy: The current state of the art. Rev Port Cardiol. 2012;31:425–32. doi: 10.1016/j.repc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Kumar N, Jain A, Chakraborty M. Spinal anesthesia for lower segment Cesarean section in patients with stable eclampsia. J Clin Anesth. 2011;23:202–6. doi: 10.1016/j.jclinane.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: An endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 6.Engelhardt T, MacLennan FM. Fluid management in pre-eclampsia. Int J Obstet Anesth. 1999;8:253–9. doi: 10.1016/s0959-289x(99)80106-x. [DOI] [PubMed] [Google Scholar]
- 7.Hays PM, Cruikshank DP, Dunn LJ. Plasma volume determination in normal and preeclamptic pregnancies. Am J Obstet Gynecol. 1985;151:958–66. doi: 10.1016/0002-9378(85)90675-1. [DOI] [PubMed] [Google Scholar]
- 8.Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br Med Bull. 2003;67:161–76. doi: 10.1093/bmb/ldg005. [DOI] [PubMed] [Google Scholar]
- 9.Mercier FJ, Bonnet MP, De la Dorie A, Moufouki M, Banu F, Hanaf A, et al. Spinal anaesthesia for caesarean section: Fluid loading, vasopressors and hypotension. Ann Fr Anesth Reanim. 2007;26:688–93. doi: 10.1016/j.annfar.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Langesaeter E, Rosseland LA, Stubhaug A. Continuous invasive blood pressure and cardiac output monitoring during cesarean delivery: A randomized, double-blind comparison of low-dose versus high-dose spinal anesthesia with intravenous phenylephrine or placebo infusion. Anesthesiology. 2008;109:856–63. doi: 10.1097/ALN.0b013e31818a401f. [DOI] [PubMed] [Google Scholar]
- 11.Robson SC, Boys RJ, Rodeck C, Morgan B. Maternal and fetal haemodynamic effects of spinal and extradural anaesthesia for elective caesarean section. Br J Anaesth. 1992;68:54–9. doi: 10.1093/bja/68.1.54. [DOI] [PubMed] [Google Scholar]
- 12.Ramsingh DS, Sanghvi C, Gamboa J, Cannesson M, Applegate RL., 2nd Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: A randomized controlled trial. J Clin Monit Comput. 2013;27:249–57. doi: 10.1007/s10877-012-9422-5. [DOI] [PubMed] [Google Scholar]
- 13.Scheeren TW, Wiesenack C, Gerlach H, Marx G. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: A prospective randomized multicentre study. J Clin Monit Comput. 2013;27:225–33. doi: 10.1007/s10877-013-9461-6. [DOI] [PubMed] [Google Scholar]
- 14.Bliacheriene F, Carmona MJ, Barretti Cde F, Haddad CM, Mouchalwat ES, Bortolotto MR, et al. Use of a minimally invasive uncalibrated cardiac output monitor in patients undergoing cesarean section under spinal anesthesia: Report of four cases. Rev Bras Anestesiol. 2011;61:610–8. doi: 10.1016/S0034-7094(11)70072-1. 334. [DOI] [PubMed] [Google Scholar]
- 15.Missant C, Rex S, Wouters PF. Accuracy of cardiac output measurements with pulse contour analysis (PulseCO) and Doppler echocardiography during off-pump coronary artery bypass grafting. Eur J Anaesthesiol. 2008;25:243–8. doi: 10.1017/S0265021507002979. [DOI] [PubMed] [Google Scholar]
- 16.Marquez J, McCurry K, Severyn DA, Pinsky MR. Ability of pulse power, esophageal Doppler, and arterial pulse pressure to estimate rapid changes in stroke volume in humans. Crit Care Med. 2008;36:3001–7. doi: 10.1097/CCM.0b013e31818b31f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montenij LJ, de Waal EE, Buhre WF. Arterial waveform analysis in anesthesia and critical care. Curr Opin Anaesthesiol. 2011;24:651–6. doi: 10.1097/ACO.0b013e32834cd2d9. [DOI] [PubMed] [Google Scholar]
- 18.Pal GK, Shyma P, Habeebullah S, Pal P, Nanda N, Shyjus P. Vagal withdrawal and sympathetic overactivity contribute to the genesis of early-onset pregnancy-induced hypertension. Int J Hypertens. 2011;2011:361417. doi: 10.4061/2011/361417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mythen MG, Swart M, Acheson N, Crawford R, Jones K, Kuper M, et al. Perioperative fluid management: Consensus statement from the enhanced recovery partnership. Perioper Med (Lond) 2012;1:2. doi: 10.1186/2047-0525-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prakash S, Pramanik V, Chellani H, Salhan S, Gogia AR. Maternal and neonatal effects of bolus administration of ephedrine and phenylephrine during spinal anaesthesia for caesarean delivery: A randomised study. Int J Obstet Anesth. 2010;19:24–30. doi: 10.1016/j.ijoa.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Ghaffari S, Malaki M, Rezaeifar A, Abdollahi Fakhim S. Effect of peripheral edema on oscillometric blood pressure measurement. J Cardiovasc Thorac Res. 2014;6:217–21. doi: 10.15171/jcvtr.2014.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorp JA, Dildy GA, Yeomans ER, Meyer BA, Parisi VM. Umbilical cord blood gas analysis at delivery. Am J Obstet Gynecol. 1996;175:517–22. doi: 10.1053/ob.1996.v175.a74401. [DOI] [PubMed] [Google Scholar]
- 23.Gregg AR, Weiner CP. “Normal” umbilical arterial and venous acid-base and blood gas values. Clin Obstet Gynecol. 1993;36:24–32. doi: 10.1097/00003081-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–6. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 25.Ramanathan J, Coleman P, Sibai B. Anesthetic modification of hemodynamic and neuroendocrine stress responses to cesarean delivery in women with severe preeclampsia. Anesth Analg. 1991;73:772–9. doi: 10.1213/00000539-199112000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds F. Epidural analgesia in obstetrics. BMJ. 1989;299:751–2. doi: 10.1136/bmj.299.6702.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talledo OE. O: Renin-angiotensin system in normal and toxemic pregnancies. IV. Inactivation of angiotensin in toxemic pregnancy. Am J Obstet Gynecol. 1968;101:254–6. doi: 10.1016/0002-9378(68)90195-6. [DOI] [PubMed] [Google Scholar]
- 28.Okafor UV, Efetie ER, Igwe W, Okezie O. Anaesthetic management of patients with pre-eclampsia/eclampsia and perinatal outcome. J Matern Fetal Neonatal Med. 2009;22:688–92. doi: 10.1080/14767050902994473. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay S, Das A, Pahari S. Fetomaternal outcome in severe preeclamptic women undergoing emergency cesarean section under either general or spinal anesthesia. J Pregnancy. 2014;2014:325098. doi: 10.1155/2014/325098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol (1985) 1995;79:15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Belfort M, Akovic K, Anthony J, Saade G, Kirshon B, Moise K., Jr The effect of acute volume expansion and vasodilatation with verapamil on uterine and umbilical artery Doppler indices in severe preeclampsia. J Clin Ultrasound. 1994;22:317–25. doi: 10.1002/jcu.1870220506. [DOI] [PubMed] [Google Scholar]
- 32.Sibai BM, Mabie WC. Hemodynamics of preeclampsia. Clin Perinatol. 1991;18:727–47. [PubMed] [Google Scholar]
- 33.Mercier FJ. Cesarean delivery fluid management. Curr Opin Anaesthesiol. 2012;25:286–91. doi: 10.1097/ACO.0b013e3283530dab. [DOI] [PubMed] [Google Scholar]
- 34.McDonald S, Fernando R, Ashpole K, Columb M. Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective cesarean delivery: A randomized controlled trial. Anesth Analg. 2011;113:803–10. doi: 10.1213/ANE.0b013e31822c0f08. [DOI] [PubMed] [Google Scholar]
- 35.Benes J, Giglio M, Brienza N, Michard F. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: A meta-analysis of randomized controlled trials. Crit Care. 2014;18:584. doi: 10.1186/s13054-014-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reydellet L, Blasco V, Mercier MF, Antonini F, Nafati C, Harti-Souab K, et al. Impact of a goal-directed therapy protocol on postoperative fluid balance in patients undergoing liver transplantation: A retrospective study. Ann Fr Anesth Reanim. 2014;33:e47–54. doi: 10.1016/j.annfar.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Haas S, Eichhorn V, Hasbach T, Trepte C, Kutup A, Goetz AE, et al. Goal-directed fluid therapy using stroke volume variation does not result in pulmonary fluid overload in thoracic surgery requiring one-lung ventilation. Crit Care Res Pract. 2012;2012:687018. doi: 10.1155/2012/687018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyer RA, Piercy JL, Reed AR, Strathie GW, Lombard CJ, Anthony JA, et al. Comparison between pulse waveform analysis and thermodilution cardiac output determination in patients with severe pre-eclampsia. Br J Anaesth. 2011;106:77–81. doi: 10.1093/bja/aeq292. [DOI] [PubMed] [Google Scholar]
- 39.Lobo SM, Ronchi LS, Oliveira NE, Brandão PG, Froes A, Cunrath GS, et al. Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Crit Care. 2011;15:R226. doi: 10.1186/cc10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, et al. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: A meta-analysis. Br J Anaesth. 2014;112:648–59. doi: 10.1093/bja/aet466. [DOI] [PubMed] [Google Scholar]
- 41.Frassanito L, Vagnoni S, Zanfini BA, Catarci S, Maggiore S, Draisci G. General anesthesia for caesarean delivery in a pregnant woman affected by acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2012;16:1123–6. [PubMed] [Google Scholar]
- 42.Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112:281–9. doi: 10.1093/bja/aet307. [DOI] [PubMed] [Google Scholar]
- 43.Loubert C. Fluid and vasopressor management for Cesarean delivery under spinal anesthesia: Continuing professional development. Can J Anaesth. 2012;59:604–19. doi: 10.1007/s12630-012-9705-9. [DOI] [PubMed] [Google Scholar]
- 44.Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: Analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824–31. doi: 10.1111/j.1471-0528.2012.03335.x. [DOI] [PubMed] [Google Scholar]
- 45.Georgieva A, Moulden M, Redman CW. Umbilical cord gases in relation to the neonatal condition: The EveREst plot. Eur J Obstet Gynecol Reprod Biol. 2013;168:155–60. doi: 10.1016/j.ejogrb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: Systematic review and meta-analysis. BMJ. 2010;340:c1471. doi: 10.1136/bmj.c1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper DW. Caesarean delivery vasopressor management. Curr Opin Anaesthesiol. 2012;25:300–8. doi: 10.1097/ACO.0b013e3283530d62. [DOI] [PubMed] [Google Scholar]
- 48.Fahey J, King TL. Intrauterine asphyxia: Clinical implications for providers of intrapartum care. J Midwifery Womens Health. 2005;50:498–506. doi: 10.1016/j.jmwh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Veeser M, Hofmann T, Roth R, Klöhr S, Rossaint R, Heesen M. Vasopressors for the management of hypotension after spinal anesthesia for elective caesarean section. Systematic review and cumulative meta-analysis. Acta Anaesthesiol Scand. 2012;56:810–6. doi: 10.1111/j.1399-6576.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 50.Santha E, Lendvai B, Gerevich Z. Low temperature prevents potentiation of norepinephrine release by phenylephrine. Neurochem Int. 2001;38:237–42. doi: 10.1016/s0197-0186(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 51.Ngan Kee WD, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–12. doi: 10.1097/ALN.0b013e3181b160a3. [DOI] [PubMed] [Google Scholar]


