Abstract
Background:
Cirrhosis is a common complication of chronic hepatitis B. It remains unclear if viral and biochemical parameters at baseline affect virological response to entecavir and therefore warrant investigation. In the present study, we aimed to evaluate the efficacy of entecavir therapy by monitoring virological response at the end of the 3rd month of treatment and try to figure out whether baseline factors could help predict it in a cohort of hepatitis B virus (HBV) compensated cirrhosis patients and to determine the cut-off value of a predicting parameter.
Methods:
A total of 91 nucleos(t)ide-naïve patients with HBV induced cirrhosis (compensatory stage) were enrolled in a prospective cohort. HBV DNA and alanine aminotransferase (ALT) were tested at baseline and monitored every 3–6 months after starting therapy.
Results:
Of all 91 patients, the median follow-up time was 12 (9–24) months. Overall, 64 patients (70.3%) achieved virological response in the 3rd month. Univariate analysis showed that the 3rd month virological response can be predicted by baseline HBV DNA levels (P < 0.001, odds ratio [OR]: 2.13, 95% confidence interval [CI]: 1.44–3.15), ALT value (P = 0.023, OR: 1.01, 95% CI: 1.00–1.01) and hepatitis B e antigen (HBeAg) negativity (P = 0.016, OR: 0.30, 95% CI: 0.11–0.80). Multiple regression analysis showed baseline HBV DNA level was the only parameter related to full virological response. Higher baseline HBV DNA strata indicated a higher probability that HBV DNA remains detectable at the 3rd month (P = 0.001). Area under receiver operating characteristic curve for determining the 3rd month virological response by baseline HBV DNA was 77.6% (95% CI: 66.7–85.2%), with a best cut-off value of 5.8 log10.
Conclusions:
Baseline HBV DNA, HBeAg negativity, and ALT were independent factors contributing to virological response at the 3rd month. Further, multiple regression showed that HBV DNA level was the only parameter predicting full virological response as early as the 3rd month, in this cirrhosis cohort.
Keywords: Area Under Receiver Operating Characteristic Curve, Baseline Parameters, Cirrhosis, Entecavir, Hepatitis B viral
INTRODUCTION
An estimate of 240 million people have been chronically infected with hepatitis B virus (HBV) worldwide.[1] Chronic liver injury and ongoing inflammation in chronic hepatitis B (CHB) can lead to cirrhosis. In East Asia, the respective 5-year cumulative incidences of cirrhosis in hepatitis B e antigen (HBeAg) negative and HBeAg positive patients were 13% and 8%, respectively.[2] A cirrhotic liver initially maintains normal liver functions (compensatory stage), but 15% of patients may progress into a decompensated stage in 5-year while 3.7% will develop hepatocellular carcinoma (HCC).[3]
Studies that investigated risk factors for the advancement of CHB to cirrhosis found that prognosis or histological progression[4,5,6] is strongly related to HBeAg positivity, serum HBV DNA, and alanine aminotransferase (ALT) levels.
Further analyses showed that elevated HBV DNA level is a single risk factor for deteriorating liver functions in HBV-related cirrhosis and is strongly related to the advancement of cirrhosis from a compensatory to a decompensated stage and HCC.[4,7] Patients within compensated stage often remain asymptomatic for many years, allowing a normal quality of life. Thus, effective suppression of HBV replication to prevent disease prognosis is critical.
Nucleos(t)ide analogs (NAs) have been proven to be effective at inhibiting HBV replication.[8,9] Entecavir is a new potent NA. Cirrhosis patients who received entecavir treatment had a significantly lower liver-related death rate than the placebo group.[10] Entecavir therapy resulted in more frequent improvement in histology, virological response and biochemical normalization in both HBeAg positive and negative patients compared to lamivudine (LAM).[11,12]
The primary goal of antiviral therapy is to prevent progression of liver injury. An early virological response often offers a positive sign for achieving the therapeutic goal.
Although patients with the partial virological response at 3rd month could continue entecavir therapy, the 3rd month (12 weeks) posttreatment is an important time point for antiviral therapy in both surrogate marker and treatment practice. It is at this time point that primary nonresponders to NAs therapy will be identified based on HBV DNA level.[13] Thus, HBV DNA at the 3rd month of treatment reflects if HBV replication is effectively inhibited. Moreover, an early response to antiviral therapy is important. One log10 IU/ml or greater reduction of HBV DNA level from the baseline at the 3rd month could indicate such a response.[14] In addition, the lower the HBV DNA level, the higher the probability of spontaneous seroclearance of HBeAg.[15] Studies[16,17] suggested that early HBV DNA decrease is an important prognostic marker. A negative HBV DNA or a fall of 3 log10 from baseline at the 3rd month projects a 96% probability of negative HBV DNA at week 96 posttreatment.
However, the available data of treating HBV cirrhosis patients with entecavir are limited. Virological and biochemical, or other factors that contributed to virological response at the 3rd month of treatment, have not been investigated.
In the present study, we aimed to evaluate the efficacy of entecavir therapy by monitoring virological response at the end of the 3rd month of treatment and try to figure out whether baseline factors could help predict it in a cohort of HBV compensated cirrhosis patients and to determine the cut-off value of a predicting parameter.
METHODS
Study design and patient inclusion
This was a retrospective cohort study. The data were extracted from the database of a prospective study into which HBV-induced cirrhosis patients were enrolled for antiviral therapy either by entecavir or LAM plus adefovir dipivoxil (ADV) in eight centers located in the mainland of China. The prospective study began in March 2012, and recruitment of subjects is still ongoing. The study was conducted in accordance with the ethics principles of the Declaration of Helsinki and it was approved by the Institutional Ethics Committee.
There are generic and brand name versions of NAs available in China. Selection of NAs was made jointly by patients and their attending physicians, in view of medical insurance coverage, in accordance with the Guideline of Prevention and Treatment for CHB of China. An early virological response was defined as HBV DNA undetectable at the 3rd month. Individuals were all nucleos(t)ide-naïve before recruitment and started therapy at a dose of entecavir at 0.5 mg/d or LAM 100 mg plus ADV 10 mg/d.
Both males and females were included in the study. Eligible participating patients met the following criteria: (1) Patient ages were between 18 and 70 years old with written informed consent (including 18 and 70 at the time of recruitment). (2) CHB-induced cirrhosis was clinically diagnosed by: (1) Liver biopsy, (2) endoscopy of esophageal or gastric varices, excluding noncirrhotic portal hypertension, (3) two of the following (when biopsy and endoscopy were not performed) (a) ultrasonographic evidence or computed tomography/magnetic resonance imaging result that indicated imaging changes in liver morphology, which included nodules in the hepatic parenchyma, serrated change on the liver surface or spleen pachydiameter >4.0 cm or >5 costal region, (b) blood platelet (PLT) <100 × 109/L with no other explanation, (c) albumin (ALB) <35 g/L, INR >1.3, or CHE <5.0 kU/L, (d) liver stiffness measurement (LSM) value >12.4 kPa. (3) HBV DNA levels were more than 1000 U/ml (5 × 103 copies/ml) for HBeAg-positive patients or 100 IU/ml (500 copies/ml) for HBeAg-negative patients.
Exclusion criteria included: (1) Decompensated cirrhosis: Patients with ascites, hepatic encephalopathy, gastrointestinal bleeding, and other complications of cirrhosis (such as spontaneous bacterial peritonitis); (2) allergy to NAs (such as entecavir, ADV, LAM); (3) reported liver disease: Alcoholic liver disease, autoimmune liver disease, heretic liver disease, drug-induced liver disease, nonalcoholic fatty liver disease, and other chronic liver diseases; (4) laboratory tests demonstrating that alpha-fetoprotein was >100 ng/ml or Cr >1.5 × upper limit of normal (ULN); (5) patients with any malignant tumor; (6) any complication of severe heart, lung, kidney, brain, blood diseases or other important organs diseases; (7) complicated to severe mental illness (such as depression, mania, epilepsy, schizophrenia); (8) pregnant and lactating women.
The selection flow of this study is shown in Figure 1. In this cohort, 563 recruited individuals were diagnosed with HBV compensated cirrhosis and had been treated by entecavir continuously. In 563 patients, 188 had been followed for more than 9 months. Among them, 112 had a consecutive record of every 3 months before HBV DNA first became undetected (follow-up varied at every 3 or 6 month after HBV DNA was undetectable). Five individuals who lacked the baseline HBV DNA or ALT data were excluded. In addition, 3 patients with baseline ALT >10 × ULN were excluded because of the possibility of extrahepatic lithiasis. A total of 13 patients treated with LAM + ADV were excluded. Thus, the total number of patients included in the study was 91, all of whom were treated with entecavir.
Figure 1.
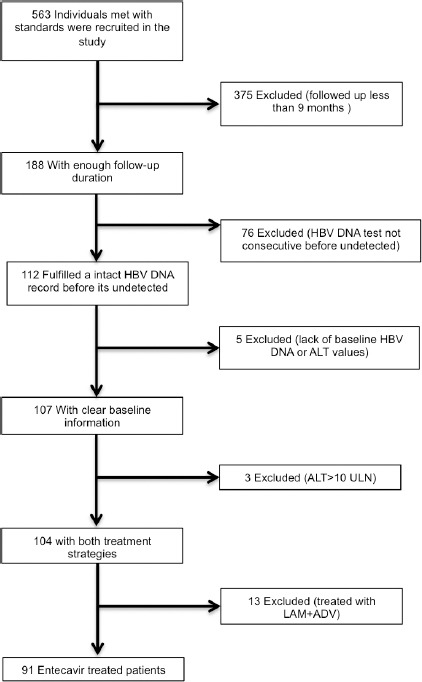
Flow chart of participant selection in the Study.
Laboratory statistics
Alanine aminotransferase and HBV DNA copies were assayed in individual centers. HBV DNA at undetectable levels was defined by HBV DNA ≤500 copies/ml (100 IU/ml). ULN of ALT was defined as 40 U/L. LSM was performed by Fibroscan (Echosens, Paris, France).
Statistical analyses
Statistics with recorded clinical and laboratory data of this study were analyzed using the Statistical Program for Social Sciences (SPSS 20.0 for MAC; SPSS Inc., Chicago, IL, USA). Data were presented as follows: (i) n (%) (ii) mean ± standard deviation (SD) (iii) median (range). Categorical variables were compared by Chi-square testing or Fisher's exact testing as appropriate. Continuous variables were compared by the two-sided Student's t-test or nonparametric test as appropriate. An undetectable HBV DNA level was imputed as 0 for analysis. All statistics were calculated within 95% confidential intervals (95% CI). All reported P values were two-tailed. Statistical significance was taken as P < 0.05.
RESULTS
Baseline characteristics
There were 91 intention-to-treat patients included who were followed for a median 12 (9–24) months. The majority of patients were 40–60 years old with a male preponderance. Among the 91 patients, 54.7% were HBeAg negative, and most of them achieved virological response in the first 3 months. Thirty-eight (41.8%) participants had normal ALT (≤40 U/L). Fifty-two patients had a PLT level <100 × 109/L, 36 of them had HBV DNA undetectable at the 3rd month. Among 25 cases with ALB <35 g/L, 18 experienced early virological response. Among the 64 participants who took LSM with Fibroscan, 12 out of 15 LSM <12.5 kPa individuals experienced early virological response. Among 71 with T-Bil <34 μmol/L individuals, 51 of them had HBV DNA undetectable during the first 3 months [Table 1].
Table 1.
Main baseline characteristics of the study
| Items | HBV DNA undetected | Total | |
|---|---|---|---|
| At month 3 | >month 3 | ||
| Number of patients | 64 | 27 | 91 |
| Age (years) | 50.0 ± 11.2 | 47.9 ± 12.2 | 48.9 ± 11.6 |
| Gender (n (%)) | |||
| Male | 49 (76.6) | 19 (70.4) | 68 (74.7) |
| Alcohol consumption | |||
| history (n (%)) | |||
| No | 50 (78.1) | 24 (88.9) | 74 (81.3) |
| HBV DNA (log10) | 5.2 ± 1.4 | 6.8 ± 1.5 | 5.6 ± 1.6 |
| HBeAg* (n (%)) | |||
| Seronegative | 39 (62.9) | 8 (33.3) | 47 (54.7) |
| ALT (U/L) | 39 (10–281) | 66 (28–388) | 52 (10–388) |
| PLT (×109/L) | 103.6 ± 47.2 | 111.3 ± 66.6 | 92 (9–289) |
| T-Bil (μmol/L) | 19.2 (6.5–130.0) | 19.8 (9.6–174.3) | 19.2 (6.51–74.3) |
| ALB (g/L) | 40.2 ± 6.4 | 40.5 ± 9.4 | 40.3 ± 7.4 |
| PT (s) | 12.3 ± 1.4 | 12.1 ± 1.6 | 12.3 ± 1.4 |
| LSM (kPa) | 16.8 (4.6–72.0) | 11.6 (9.9–16.2) | 16.8 (4.6–72.0) |
Data are expressed as n (%), mean±SD or median (range), as appropriate. *No available data for five patients. HBV: Hepatitis B viral; HBeAg: Hepatitis B e antigen; ALT: Alanine aminotransferase; PLT: Blood platelet; T-Bil: Total bilirubin; ALB: Albumin; PT: Prothrombin time; LSM: Liver stiffness measurement; SD: Standard deviation.
Regression analysis
Baseline factors that may be related to an early undetectable HBV DNA were analyzed by logistic regression [Table 2].
Table 2.
Univariate and multivariate logistic regression analysis for the risk of third month HBV DNA response
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (years) | 0.203 | |||
| Gender | 0.535 | |||
| Alcohol consumption | 0.370 | |||
| HBV DNA (log10) | 2.13 (1.44–3.15) | <0.001* | 1.98 (1.33–2.94) | 0.001* |
| HBeAg | 0.30 (0.11–0.80) | 0.016* | ||
| ALT (U/L) | 1.01 (1.00–1.01) | 0.023* | ||
| PLT (×109) | 0.527 | |||
| T-Bil (μmol/L) | 0.263 | |||
| ALB (g/L) | 0.825 | |||
| PT (s) | 0.616 | |||
| Fibroscan (kPa) | 0.359 | |||
*Statistically significant. OR: Odds ratio; CI: Confidence interval; HBV: Hepatitis B viral; HBeAg: Hepatitis B e antigen; ALT: Alanine aminotransferase; PLT: Blood platelet; T-Bil: Total bilirubin; ALB: Albumin; PT: Prothrombin time.
Baseline HBV DNA level was strongly related to virological response at the 3rd month (P < 0.001, odds ratio [OR]: 2.13, 95% CI: 1.44–3.15). Sixty-four patients (70.3%) had HBV DNA undetected at the 3rd month; 3 of them experienced virological breakthrough. The remaining 27 patients (29.7%) had detectable HBV DNA after the 3rd month; 2 of the 31 patients experienced virological breakthrough after the virological response. The mean ± SD of HBV DNA was lower in early response patients than in late responders.
The ALT baseline value was also associated with early virological response (P = 0.023, OR: 1.01, 95% CI: 1.00–1.01). There were 33 individuals (86.8%) with normal ALT, who had HBV DNA undetected at the 3rd month while 58.5% in patients with abnormal ALT. In addition, 62.9% of the patients in the early virological response group were HBeAg negative while 59.0% patients who were HBeAg positive had HBV DNA undetectable within 3 months (P = 0.016, OR: 0.30, 95% CI: 0.11–0.80).
Age, gender, alcohol consumption, LSM, PLT, T-Bil, ALB, and PT between patients with HBV DNA undetected at the 3rd month and after first 3 months were comparable (P > 0.05).
Baseline ALT and HBV DNA level and HBeAg negativity were further tested by multiple logistic regression. HBV DNA level was the only significant factor that could predict early response (P < 0.001, OR: 1.98, 95% CI: 1.33–2.94).
Stratification of hepatitis B virus DNA levels and receiver operating characteristic curve
We then stratified patients into six groups using the baseline HBV DNA levels [Figure 2]. We found that patients with elevated HBV DNA at baseline had a higher probability to remain HBV DNA positive at the 3rd month (P < 0.001).
Figure 2.
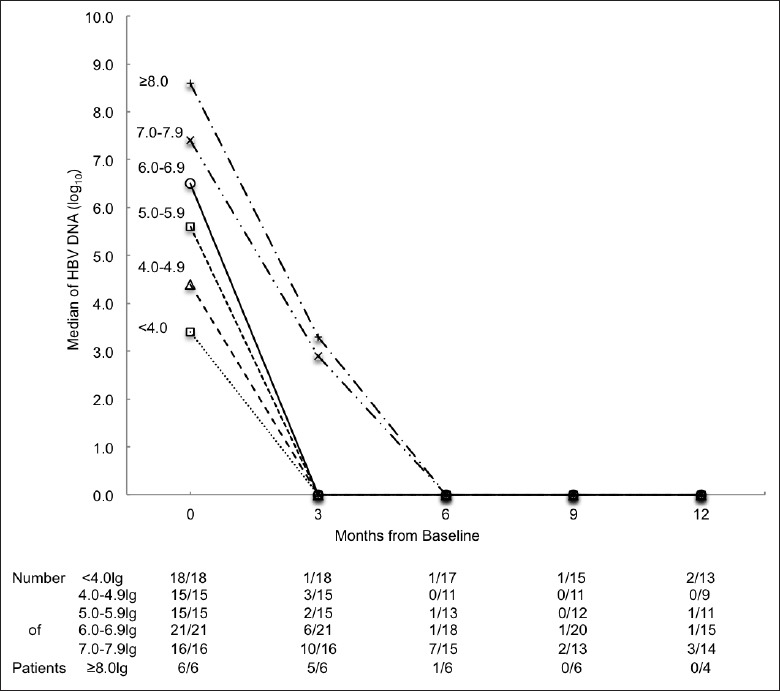
Median of hepatitis B virus (HBV) DNA (log10) of different baseline log HBV DNA strata. Number of patients followed is presented as n/N. The n represents HBV DNA detectable patients. N represents all patients followed at that month.
Median HBV DNA values among patients with baseline serum HBV DNA levels at <7, 7.0–7.9 and ≥8.0 log10 strata were 0.0, 2.9, and 3.3 log10, respectively, at the 3rd month. The higher baseline HBV DNA and the higher median HBV DNA remained at the 3rd month. The median HBV DNA became 0.0 log10 in all groups after the 3rd month. Approximately, 68.2% of all the participants with HBV DNA >7.0 log10, had detectable HBV DNA at the 3rd month, which was significantly higher than 22.2% (5.0–6.9 log10 group) and 12.1% (<5.0 log10 group) (P < 0.01). A 38.1% HBV DNA detectable rate in >7.0 log10 group at month 6 was also significantly higher than 3.6% in <5.0 log10 group (P = 0.003) and 6.5% in 5.0–6.9 log10 group (P = 0.009). There were no significant differences at the 9th and 12th months.
According to the receiver operating characteristic (ROC) curve analysis in this study, the baseline HBV DNA area under the curve for predicting the 3rd month virological response was 77.6% (95% CI: 66.7–88.5%) [Figure 3]. A baseline HBV DNA level at 5.8 log10 showed a best cut-off value with a sensitivity of 85.2% and specificity of 64.1% for predicting the virological response at the 3rd month.
Figure 3.
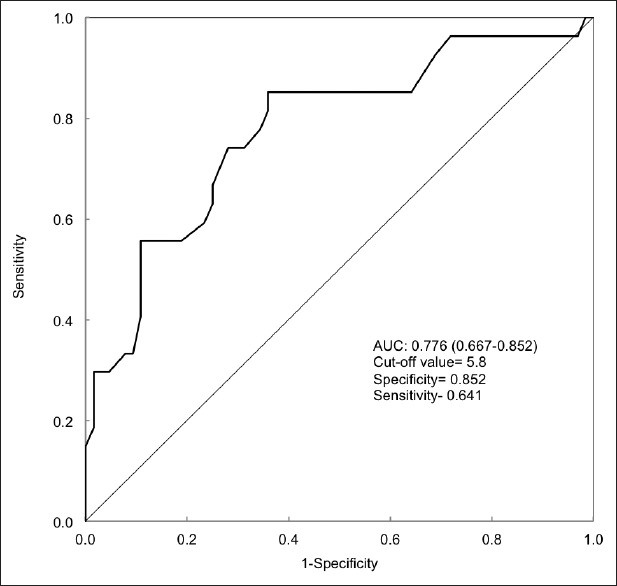
Receiver operating characteristic analysis for 3rd month prediction of hepatitis B virus (HBV) DNA response from baseline. Demonstrate the baseline HBV DNA load predicting 3rd month virologic response.
Finally, we calibrated the accuracy of the cut-off value of predicting the 3rd month HBV DNA virological response [Figure 4]. Median HBV DNA in groups with baseline HBV DNA <5.8 and ≥5.8 log10 were at a respective 0.0 log10 and 2.4 log10 at the 3rd month. About 8.9% of the 45 patients with lower baseline HBV DNA had detectable HBV DNA at the 3rd month, compared to 50.0% in 46 patients with higher baseline HBV DNA (P < 0.001). At the 6th month, 2.6% had sustained detectable HBV DNA in the low baseline HBV DNA group, whereas the level was 23.8% in high baseline HBV DNA patients (P = 0.008). There were no significant differences between two groups in subsequent months.
Figure 4.
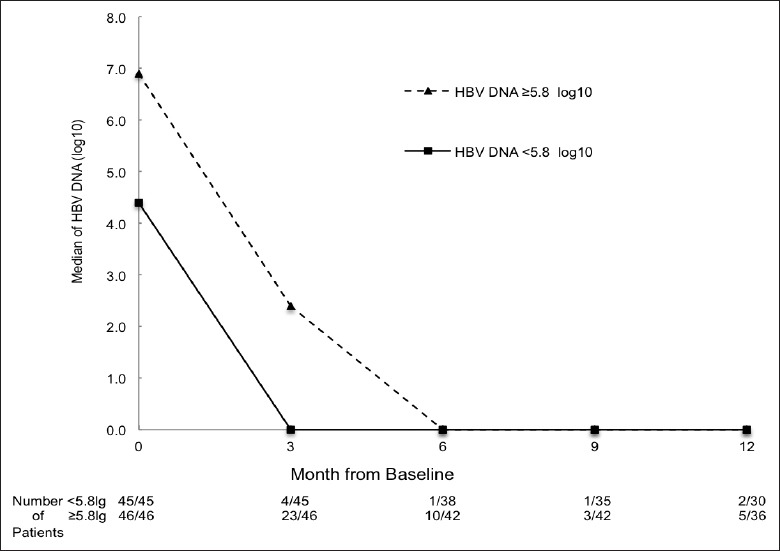
Median of hepatitis B virus (HBV) DNA (log10) by sort of best cut-off value. Number of patients followed is presented as n/N. The n represents HBV DNA detectable patients. N represents all patients followed at that month.
DISCUSSION
In this study, we investigated the relationship between several baseline parameters and virological response to ETV therapy by the 3rd month among patients with HBV induced cirrhosis. There were two notable features in this prospective study: (1) Our cohort is exclusively composed of HBV-related cirrhosis patients who were at a compensatory stage, treatment naive and were selected under strict recruiting and exclusion criteria. (2) We report that the baseline HBV DNA level was the most important factor related to full virologic response at the 3rd month, and we identified a cut-off value of baseline HBV DNA level for predicting antiviral response. To our knowledge, our study is the first one to establish this cut-off value for prediction.
Our univariate analysis found that patients with lower HBV DNA or negative HBeAg at baseline tended to have a higher probability to achieve a full virologic response at the 3rd month. Our results are in line with previous studies.[13,18,19] However, multiple regression analysis only confirmed the baseline HBV DNA level as a sole factor related to the virological response. This highlights the importance of the baseline HBV DNA level in achieving early full virological response, reflecting the fact that a shorter treatment course is sufficient to decrease HBV DNA to undetectable levels.
We also evaluated the best cut-off value that can be used to predict the virological response at the 3rd month. We found that a <5.8 log10 baseline HBV DNA cut-off represented significantly higher likelihood to achieve virological response at the 3rd month. This cut-off was lower than that calculated from CHB patients.[20] This can be explained by the fact that the median HBV DNA level is lower in HBV-related cirrhosis patients than CHB patients. There was no significant difference in the HBV DNA undetectable rate between groups that either had more or fewer HBV DNA copies than 5.8 log10 at baseline after 6 months.
In this study, we also investigated the relationship between baseline ALT value and the 3rd month full virological response. Patients with ALT ≤1 × UNL had a higher probability of achieving a full virological response within the first three months than patients with ALT >1 × UNL in this cohort. Once a chronic HBV-induced patient experienced HBeAg seroconversion, as did more than half of our cohort, serum HBV DNA level was significantly reduced. The patients who were HBeAg negative, accompanied by a reduction in HBV DNA and normal ALT, were referred to as inactive carriers. Our results showed that patients with normal ALT had lower HBV DNA at baseline and a lower baseline HBV DNA level was favored for achieving an early full virological response in our cohort as discussed above.[7,21,22]
It appears that our results indicating that a baseline normal ALT is a favorable factor for early loss of detectable HBV DNA contradicted the previous reports, which showed an elevated ALT. For instance, a value >5 × UNL at baseline was more likely to have HBeAg seroconversion and better virological response in treated CHB patients.[23,24,25] However, the real difference is due to the varied cohorts. Our cohort exclusively consisted of cirrhosis patients, half of which were inactive carriers. The published cohorts primarily consisted of CHB patients. CHB patients can be variable and may be at two different phases: One phase having high HBV DNA but accompanied only by slightly elevated ALT, and the other phase having a medium HBV DNA level accompanied by significantly elevated ALT. It takes a shorter time course to promote a change to undetectable from the medium HBV DNA than from a high HBV DNA level. The principle was the same: A relatively lower baseline HBV DNA promoted an earlier virological response.
The relationship between LSM and HBV DNA kinetics in Chinese HBV-induced compensated cirrhosis remains to be established. Our study showed that patients with different LSM baseline levels showed no significant difference in early virological response.
Our data showed that the baseline HBV DNA level was the most effective factor related to an early full virological response to ETV therapy. The lower the HBV DNA at baseline, the earlier the virological response was in our cohort.
One limitation of our study was although the enrolled patients were clear clinical diagnosed by strict criteria and every routine 3-month follow-up, the total cases were still limited and relatively short follow-up, as it was launched in March 2012 and patient recruitment is currently ongoing. In addition, our strict criteria of recruiting and exclusion of patients may impede the extrapolation of results to a broader range of HBV induced cirrhosis patients, especially to those who had combined autoimmune liver disease, severe kidney or cardiovascular diseases, and human immunodeficiency virus infection.
Footnotes
Edited by: Li-Shao Guo
Source of Support: This work was supported by grants from the Key Project from Beijing Municipal Science and Technology Commission (No. D121100003912003), Program of National Science and Technology Major Project (No. 2013ZX10002004), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201308).
Conflict of Interest: None declared.
REFERENCES
- 1.Fact sheets of Hepatitis B. [Accessed May 27, 2015]. at Available from: http://www.who.int/mediacentre/factsheets/fs204/en/
- 2.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Xu B, Hu DC, Rosenberg DM, Jiang QW, Lin XM, Lu JL, et al. Chronic hepatitis B: A long-term retrospective cohort study of disease progression in Shanghai, China. J Gastroenterol Hepatol. 2003;18:1345–52. doi: 10.1046/j.1440-1746.2003.03187.x. [DOI] [PubMed] [Google Scholar]
- 4.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–86. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Zoutendijk R, Reijnders JG, Zoulim F, Brown A, Mutimer DJ, Deterding K, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62:760–5. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]
- 6.Alam S, Ahmad N, Mustafa G, Shrestha A, Alam AK, Khan M. Evaluation of normal or minimally elevated alanine transaminase, age and DNA level in predicting liver histological changes in chronic hepatitis B. Liver Int. 2011;31:824–30. doi: 10.1111/j.1478-3231.2011.02491.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Society of Hepatology and Chinese Society of Infectious Disease, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version) Chin J Hepatol. 2011;19:13–24. doi: 10.3760/cma.j.issn.1007-3418.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–47. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 11.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 12.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–20. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Pawlotsky JM, Dusheiko G, Hatzakis A, Lau D, Lau G, Liang TJ, et al. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: Recommendations for a standardized approach. Gastroenterology. 2008;134:405–15. doi: 10.1053/j.gastro.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang HI, Hung HL, Lee MH, Liu J, Jen CL, Su J, et al. Incidence and determinants of spontaneous seroclearance of hepatitis B e antigen and DNA in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10:527–34.e1. doi: 10.1016/j.cgh.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Hass HG, Bock T, Nehls O, Kaiser S. Rapid HBV DNA decrease (week 12) is an important prognostic factor for first-line treatment with adefovir dipivoxil for chronic hepatitis B. J Gastroenterol. 2009;44:871–7. doi: 10.1007/s00535-009-0078-y. [DOI] [PubMed] [Google Scholar]
- 17.Lü W, Yang HH, Fan YM, Li T, Zhang LF, Mui C, et al. Serum HBV DNA level at week 12 is superior to viral response at week 24 in predicting long-term treatment outcome of telbivudine for chronic hepatitis B patients. Chin Med J. 2013;126:2333–6. [PubMed] [Google Scholar]
- 18.Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: Risk scores integrating host and virus profiles. Hepatology. 2013;58:546–54. doi: 10.1002/hep.26385. [DOI] [PubMed] [Google Scholar]
- 19.Hou JL, Jia JD, Wei L, Zhao W, Wang YM, Cheng M, et al. Efficacy and safety of entecavir treatment in a heterogeneous CHB population from a ‘real-world’ clinical practice setting in China. J Viral Hepat. 2013;20:811–20. doi: 10.1111/jvh.12115. [DOI] [PubMed] [Google Scholar]
- 20.Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naïve chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106:1264–71. doi: 10.1038/ajg.2011.45. [DOI] [PubMed] [Google Scholar]
- 21.Liaw YF. Hepatitis B virus replication and liver disease progression: The impact of antiviral therapy. Antivir Ther. 2006;11:669–79. [PubMed] [Google Scholar]
- 22.Zhang QQ, An X, Liu YH, Li SY, Zhong Q, Wang J, et al. Long-term nucleos(t)ide analogues therapy for adults with chronic hepatitis B reduces the risk of long-term complications: A meta-analysis. Virol J. 2011;8:72. doi: 10.1186/1743-422X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien RN, Lin CH, Liaw YF. The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol. 2003;38:322–7. doi: 10.1016/s0168-8278(02)00419-1. [DOI] [PubMed] [Google Scholar]
- 24.Reijnders JG, Leemans WF, Hansen BE, Pas SD, de Man RA, Schutten M, et al. On-treatment monitoring of adefovir therapy in chronic hepatitis B: Virologic response can be assessed at 24 weeks. J Viral Hepat. 2009;16:113–20. doi: 10.1111/j.1365-2893.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang CC, Tseng KC, Peng CY, Hsieh TY, Lin CL, Su TH, et al. Viral load and alanine aminotransferase correlate with serologic response in chronic hepatitis B patients treated with entecavir. J Gastroenterol Hepatol. 2013;28:46–50. doi: 10.1111/j.1440-1746.2012.07269.x. [DOI] [PubMed] [Google Scholar]


