Abstract
Background:
This study characterized the cardiac telocyte (TC) population both in vivo and in vitro, and investigated its telomerase activity related to mitosis.
Methods:
Using transmission electron microscopy and a phase contrast microscope, the typical morphological features of cardiac TCs were observed; by targeting the cell surface proteins CD117 and CD34, CD117+CD34+ cardiac TCs were sorted via flow cytometry and validated by immunofluorescence based on the primary cell culture. Then the optimized basal nutrient medium for selected population was examined with the cell counting kit 8. Under this conditioned medium, the process of cell division was captured, and the telomerase activity of CD117+CD34+ cardiac TCs was detected in comparison with bone mesenchymal stem cells (BMSCs), cardiac fibroblasts (CFBs), cardiomyocytes (CMs).
Results:
Cardiac TCs projected characteristic telopodes with thin segments (podomers) in alternation with dilation (podoms). In addition, 64% of the primary cultured cardiac TCs were composed of CD117+CD34+ cardiac TCs; which was verified by immunofluorescence. In a live cell imaging system, CD117+CD34+ cardiac TCs were observed to enter into cell division in a short time, followed by an significant invagination forming across the middle of the cell body. Using a real-time quantitative telomeric-repeat amplification assay, the telomerase concentration in CD117+CD34+ cardiac TCs was obviously lower than in BMSCs and CFBs, and significantly higher than in CMs.
Conclusions:
Cardiac TCs represent a unique cell population and CD117+CD34+ cardiac TCs have relative low telomerase activity that differs from BMSCs, CFBs and CMs and thus they might play an important role in maintaining cardiac homeostasis.
Keywords: Cardiac Telocyte, Cell Division, Telomerase Activity, Telopode
INTRODUCTION
In recent years, investigations have identified a novel population of stromal cells that are called telocytes (TCs) (details at www.telocytes.com).[1] The defining ultrastructural feature of TCs is the presence of special thin, long and uneven caliber (moniliform) prolongations termed telopodes (Tps). To date, TCs have been described in the interstitial compartments of different tissues and organs: Heart,[2,3,4,5,6] respiratory tract,[7,8,9,10] brain,[11] digestive system,[12,13,14,15] skin,[16,17,18] eye,[19] skeletal muscle,[20] digestive glands,[21,22,23,24] urinary tract,[25,26] vasculature[27] and bone marrow[28] etc. Through Tps, TCs interact with other adjacent cells.[29] Collectively, they make up a labyrinthine system[30] that is essential for renewal, regeneration, and repair.[2]
In previous studies, electrophysiological properties were detected for TCs,[5,31,32] the microarray-based gene expression analysis and micro-RNA signature were analyzed[9,33] and some of their genomic features were demonstrated[34] as additionally, the proteomic profile differences between TCs and fibroblasts were reported.[10] However, as a proliferative cell population, telomerase activity related to cell division has not been clear and could provide additional evidence that is different from other cells.
The present study produced morphological evidence for the cultured cardiac TCs that is consistent with the population in situ. CD117+CD34+ cardiac TCs, the predominant subgroup, were sorted and evaluated with highest cell density in high-glucose Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS). Subsequently, the growth of CD117+CD34+ cardiac TCs was continuously observed; Cytokinesis did not occur immediately but occurred a short time after mitosis. Accordingly, a biochemical assay was performed to find that the telomerase activity of the subgroup was relatively low and was less than the cardiac fibroblasts (CFBs). These discoveries provided a new prospective on the dynamics of cardiac TCs in cardiac physiology and pathogenesis.
METHODS
Animals
C57BL/6J mice were obtained from the Animal Research Center of Fudan University, Shanghai, China. Each mouse was euthanized with an overdose of intraperitoneal pentobarbital sodium (Sigma, USA, 100 mg/kg) before target tissues were obtained. The protocol was approved by the committee on the ethics of animal experiments at Fudan University.
Transmission electron microscopy
Hearts were harvested from 6-week-old C57BL/6J male mice and diced into 1 mm3. Then the hearts were fixed in 2.5% glutaraldehyde at a pH of 7.3 for 4 h at 4°C. After a brief rinse with 0.1 mol/lcacodylate buffer, the tissues were fixed with 1% osmium tetroxide in 0.1 mol/l cacodylate buffer at a pH of 7.3 and then dehydrated with ethanol. This was followed by overnight impregnation with propylene oxide and Epon 812. With a LKB-IV ultramicrotome (Leika, Frankfurt, Germany), ultra-thin sections that were 70 nm in thickness were obtained and placed onto a formvar-coated copper grid for uranyl acetate and lead citrate staining. Images were captured with a JEM 1230 transmission electron microscope (Jeol Ltd., Tokyo, Japan) at an acceleration voltage of 80 kV.
Culture of cardiac telocytes
Hearts were harvested from the 6-week-old C57BL/6J male mice. Tissues were washed twice with phosphate buffered saline (PBS) (Gibco, USA) and minced into approximately 1 mm3 fragments. These pieces were then incubated in HBSS (Gibco) supplemented with 0.25% (w/v) collagenase type II (Sigma, USA) and 0.125% (w/v) trypsin (Sigma) at 37°C for enzymatic dissociation. Released cells were filtered through a cell strainer (40 μm pore size, BD Falcon, USA) and centrifuged at 500 × g for 7 min. The cell pellet was resuspended in DMEM (Sigma) with 10% FBS (Sigma) that was supplemented with 100 IU/ml penicillin G (Gibco) and 0.1 mg/ml streptomycin (Gibco). Then, cells were transferred to a 75 cm2 plastic culture flask (Corning, USA) in a humidified incubator at 37°C with 5% CO2 at a density of 1 × 105 cell/cm2 for 2 h; nonadherent cells were achieved for propagation. The medium was changed every 48 h, and the morphology was examined and captured with a DM IRE2 light microscope (Leica Microsystems, Wetzlar, Germany). After cells had been grown to confluence (80%), these cells were harvested with HBSS (Gibco) that was supplemented with 0.2% trypsin and 0.04% EDTA for further use.
CD117+CD34+ cardiac telocytes sorted by flow cytometry
Dislodged cardiac TCs were incubated with FITC-conjugated CD34 and PE-conjugated CD117 antibodies for 20 min and then sorted with an EPICS ALTRA cell sorter (Beckman Coulter, Fullerton, CA, USA) equipped with a FITC filter (530/30 nm) and a PE filter (585/42 nm). CD117+CD34+ cells were harvested for further use, and their morphology was examined with a DM IRE2 light microscope (Leica Microsystems, Wetzlar, Germany).
Examination of CD117, CD34 and vimentin expression in cardiac telocytes by laser scanning confocal microscope
Cardiac TCs were seeded on a polylysine-coated 1 cm2 glass cover slip and inoculated in culture medium for 48 h at 37°C with 5% CO2, and the culture medium was replenished after 24 h. Cells were then fixed in 4% paraformaldehyde for 15 min, washed twice with PBS and blocked with PBS containing 5% BSA for 30 min. This was followed by primary antibody probing of CD117 (ab5506, Abcam, USA), CD34 (ab8158, Abcam) and vimentin (ab8978, Abcam) at 4°C overnight. Appropriate fluorophore-conjugated secondary antibodies were used to visualize the expression in immunofluorescent cell images that were captured by a TCS SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Cell proliferation assay
CD117+CD34+ cardiac TCs were inoculated on a 96-well plate at a density of 5000 cells/well. To attain the optimal culture condition for these cells, four different culture media were tested:[1] low-glucose DMEM with 10% FBS,[2] low-glucose DMEM with 20% FBS,[3] high-glucose DMEM with 10% FBS and[4] high-glucose DMEM with 20% FBS. All media were supplemented with 100 IU/ml penicillin G and 0.1 mg/ml streptomycin. The proliferation of CD117+CD34+ cardiac TCs in each culture condition at 0.5, 1, 2 and 4 h was examined with the cell counting kit 8 (Sigma Aldrich, USA) recorded at an absorbance of 450 nm with an Infinite M200 microplate reader (TECAN, Männedorf, Switzerland).
Live cell imaging of cardiac telocytes
CD117+CD34+ cardiac TCs were dispersed on a 10 cm2 culture plate at a density of 1 × 104 cells/ml and cultured at 37°C for 24 h. The morphological features of the cardiac TCs were tracked with a Cell-IQ® imaging platform (CM Technologies, Oy, Tampere, Finland) at 20 min interval for 96 h. The video was acquired at a resolution of 1392 × 1040 pixels.
Isolation and culture of bone mesenchymal stem cells
Femurs and tibiae of 6-week-old C57BL/6J male mice were harvested and washed twice with PBS. Bone marrow plugs were extracted by flushing the bone marrow cavities with DMEM. Mononuclear bone marrow cells were then purified by density gradient fractionation at 400 × g for 7 min in 1.073 g/ml perfusate (Pharmacia, Piscataway, NJ, USA). Cells were washed with PBS and inoculated in 10% FBS containing DMEM supplemented with 100 IU/ml penicillin G and 0.1 mg/ml streptomycin and incubated at 37°C with 5% CO2. After 48 h, hematopoietic and other nonadherent cells were washed away by replenishing the culture medium, and the bone mesenchymal stem cells (BMSCs) that remained were allowed to propagate.
Isolation and culture of cardiac fibroblasts and cardiomyocytes
Twenty hearts were excised from 2-day-old C57BL/6J neonatal mice. With the endocardium and epicardium removed under a stereomicroscope, the left ventricular tissues were minced into 1 mm3 cubes and washed twice with PBS, followed by digestion in HBSS containing 0.1 mg/ml trypsin and 0.125 mg/ml collagenase type II for 7 min at 37°C. Then, the supernatant was collected, and the digestion step was repeated 6 times. The collected supernatant was pooled and centrifuged at 400 × g for 7 min. This was followed by an HBSS wash and inoculation in 10% FBS containing DMEM that was supplemented with 100 IU/ml of penicillin G and 0.1 mg/ml streptomycin for 72 h. The less-adherent cardiomyocytes (CMs) present in the supernatant were then transferred to a new culture plate while the CFBs remained attached to the culture plate.
Real-time quantitative telomeric-repeat amplification assay
mRNA was extracted from cardiac TCs, BMSCs, FBs, and CMs with the Trizol reagent (Takara Bio Inc., Shiga, Japan), and then a total of 1 μl of each group was mixed separately with 11.5 μl polymerase chain reaction (PCR) grade water and 12.5 μl of 2 × QTD premix (according to the directions of the Quantitative Telomerase Detection Kit, Allied Biotech, Inc., Germantown, MD, USA). The reaction mix was then added to a 96-well PCR plate and subjected to 20 min of incubation at 25°C, followed by 10 min at 95°C. Thirty-five amplification cycles were then applied, in which each cycle contained 30 s of denaturation at 95°C, a 30 s annealing step at 60°C and a 30 s extension at 72°C. Reactions were performed in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, CA, USA). The amplification level was reflected in the green fluorescence signal associated with the SYBR-bound amplicons; amplicons were analyzed with the SDS software suite (Applied Biosystems). A standard curve of TSR dilutions was generated according to the manufacturer's instructions (Allied Biotech, Inc).
Statistical analysis
Statistical analyses were carried out with the statistical software SPSS v. 20.0 (SPSS, version 20.0, Inc., Chicago, IL, USA). The Pearson χ2 test or χ2 test with continuity correction and Fisher's exact test were used to compare qualitative variables. Quantitative variables were analyzed with Student's t- or Mann–Whitney test. Experimental data were presented as the mean of each condition standard definition, and a P < 0.05 was considered as statistically significant. Receiver operating characteristic curves were used to determine the diagnostic values of the markers.
RESULTS
Characteristic of cardiac telocytes
Telocytes exist in various organs and tissues and are characterized by extremely long and thin Tps, with an alternation of podomers and podoms. Typical morphological features are considered to be essential for their identification by either an electron microscope or a phase contrast microscope. In situ, one cardiac TC in the interstitial space of a mouse heart was visible in parallel with and in close proximity to myocardium bundles, with an oval-shaped cell body and a very long, thin, convoluted and overlapped Tps that formed a three-dimensional labyrinthine network disposition; this is the significant feature that is different from FBs [Figure 1a]. In culture, cardiac TCs featured the characteristic morphology. The moniliform Tps of TCs were obviously observed after passages, with the alternation of thin segments (podomers) and dilated portions (podoms) [Figure 1b]. When cardiac TCs were grown to confluence (80%), these cells were harvested and sorted by flow cytometry, targeting CD34 and CD117 cell membrane protein markers. Additionally, 64% of the cultured cardiac TCs possessed both CD34 and CD117 positive expression [Figure 2a]. Subsequently, the immunophenotype of these selected cardiac TCs was validated via a laser scanning confocal microscope. The result was positive coexpression of CD34 and CD117 in both cell bodies and cell prolongations; specifically, the former was more widely distributed than that of the latter [Figure 2b].
Figure 1.
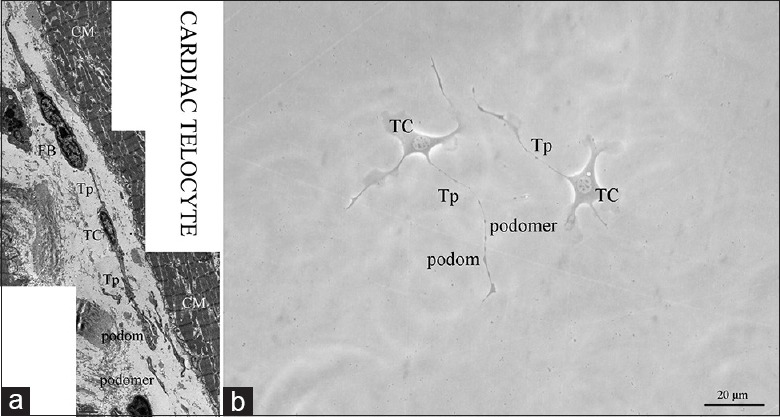
The morphology of cardiac TCs in situ and in culture. (a) Transmission electron microscope showed that Tps, characteristic cellular prolongations of cardiac TCs, were special long, predominantly narrow (usually about 100 nm) and moniliform forms, accommodating mitochondria, endoplasmic reticulum and vesicles in podoms (usually <1 μm width). And in the merged two-dimensional image, the Tps were observed to be discontinuous, suggesting their three-dimensional distribution in heart tissue; (b) Under a phase contrast microscope, cardiac TCs featured long, thin Tps with typical moniliform podoms and podomers. TC: Telocyte; Tp: Telopode; CM: Cardiomyocyte; FB: Fibroblast; Bar: 20 μm.
Figure 2.
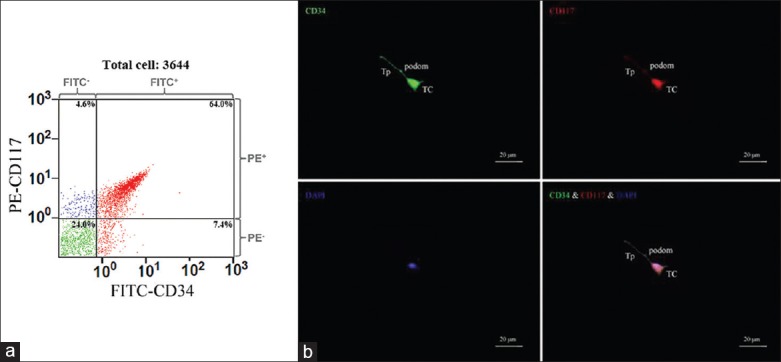
Flow cytometry analysis and immunofluorescence of CD117+CD34+ cardiac TCs. (a) As shown by fluorescenceactivated cell sorting, 64% of the cultured cardiac TCs possessed both CD34+ and CD117+; (b) Laser scanning confocal microscope validated that sorted cultured cells co-expressed markers of cardiac TCs, CD34 and CD117. TC: Telocyte; Tp: Telopode; Bar: 20 μm.
Optimizational medium for CD117+CD34+ cardiac telocytes
Different cell lines show differences in metabolism and nutritional requirements that dictate the media optimization method. For the simple generation of cell mass, cell growth rate and viability are paramount in the quest for obtaining the highest possible cumulative viable cell density. Hence, cell culture media must be able to support maximal cell growth and sustain cell viability by improving cell densities. Four culture conditions were evaluated for the ability to propagate CD117+CD34+ cardiac TCs. Cell numbers were measured at 1, 2, and 4 h in culture. The results showed that high-glucose DMEM with 10% FBS was the most effective medium for stimulating proliferation (P < 0.05) [Figure 3].
Figure 3.
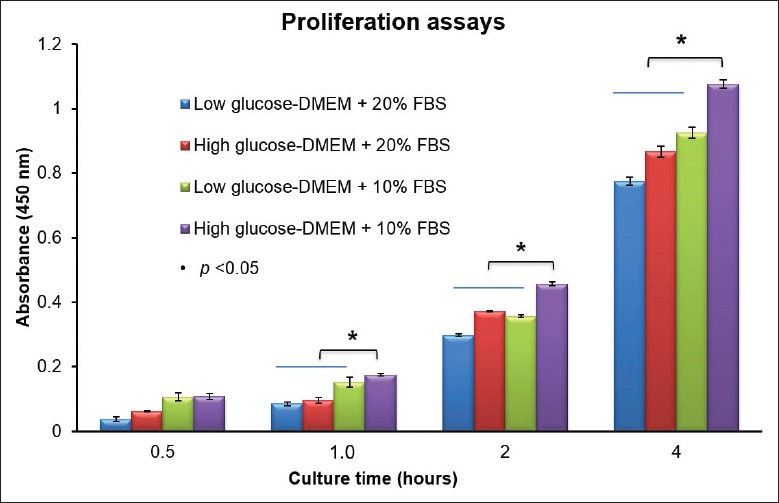
Optimization of culture medium for CD117+CD34+ cardiac TCs. Four culture conditions were evaluated for the ability to propagate CD117+CD34+ cardiac TCs. Cell numbers were measured at 1, 2, and 4 h in culture. The results showed that high-glucose DMEM with 10% FBS was the most effective at stimulating proliferation (P < 0.05). TC: Telocyte; DMEM: Dulbecco's Modified Eagle's Medium; FBS: Fetal bovine serum.
Cell division and telomerase activity of CD117+CD34+ cardiac telocytes
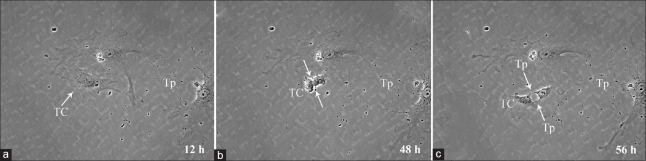
Cell division is the process that cells undergo to divide, with two types depending on the purpose: Meiosis and mitosis. In both types of cell division, the nucleus splits and DNA are replicated. Mitosis produces daughter cells that have all of the genetic material of the parent cell, as well as cytoplasm and the cell membrane. Twelve hours into culture, adherent CD117+CD34+ cardiac TCs began to divide [Figure 4a]. Forty-eight hours into culture, CD117+CD34+ cardiac TCs entered into cell division with an obvious invagination forming across the middle of the cell body [Figure 4b]. Fifty-six hours into culture, CD117+CD34+ cardiac TCs expressed characteristic morphological forms, Tps, as the daughter cells migrated in opposite directions [Figure 4c]. Based on this phenomenon, cytokinesis, the process of dividing the cytoplasm and the cell membrane, may follow a short time after mitosis, but not immediately. Subsequently, the activity of telomerase in CD117+CD34+ cardiac TCs was examined using a real-time quantitative telomeric-repeat amplification assay. BMSCs, CFBs, and CMs were included in the analyses as references. The telomerase concentration in CD117+CD34+ cardiac TCs was 2.5- and 1.5-time lower than in BMSCs and CFBs, respectively (P < 0.05), and was significantly higher than in CMs (P < 0.05) [Figure 5].
Figure 4.
The cell division of CD117+CD34+ cardiac TCs monitored by live cell imaging system. (a) 12 h into culture, adherent CD117+CD34+ cardiac TCs began to divide. (b) 48 h into culture, CD117+CD34+ cardiac TCs entered into cell division with an invagination forming across the middle of the cell body. (c) Fifty-six hours into culture, CD117+CD34+ cardiac TCs expressed characteristic morphological forms, Tps, as the daughter cells migrated in opposite directions. TC: Telocyte; Tp: Telopode.
Figure 5.
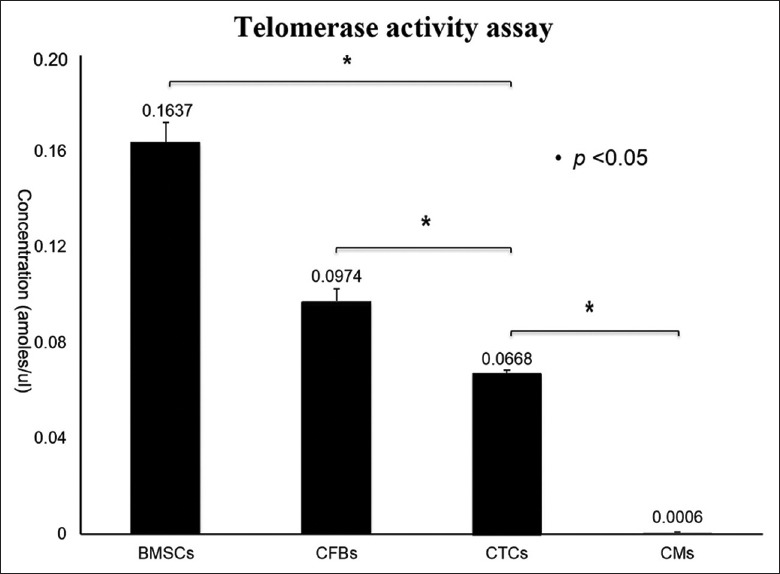
Comparing the telomerase expression activity between CD117+CD34+ cardiac TCs and, FBs, CMs. The activity of telomerase in CD117+CD34+ cardiac TCs was examined using a real-time quantitative telomeric-repeat amplification assay. BMSCs, CFBs, and CMs were included in the analyses as references. The telomerase concentration in CD117+CD34+ cardiac TCs was 2.5- and 1.5-time lower than in BMSCs and CFBs, respectively (P < 0.05); and was significantly higher than in CMs (P < 0.05). CTCs: Cardiac telocytes, BMSCs: Bone mesenchymal stem cells, CFBs: Cardiac fibroblasts, CMs: Cardiomyocytes.
DISCUSSION
Previous transmission electron microscope studies showed that TCs exhibited unique morphological features such as Tps, composed of podomers (usually approximately 100 nm) in alternation with podoms (usually <1-μm width) that accommodated the mitochondria and endoplasmic reticulum. Tps extended away from the relatively small cell body and integrated the three-dimensional interstitial environment where peripheral interstitial cells, nerve fibers, capillaries and resident stem cells existed.[12,29,30,35] The present results suggested that in situ cardiac TCs were relatively rich in mitochondria in both cell body and Tps, which correlated with previous findings.[10] Additionally, the characteristic appearance of Tps either in vivo or in vitro was consistent with the criteria reported previously.[2]
Numerous studies have described the cellular immunophenotype of the TC population with a list of membrane molecular markers such as CD117, CD34, PDGFRa, PDGFRb.[13,20,36,37,38] CD117 and CD34 are the predominant cell membrane markers of cardiac TCs, although they are not the special markers. Combined with both positive expression and typical morphology, a diagnosis for cardiac TCs is recommended. In the current study, 64% of the cultured primary cardiac TCs, the main subgroup, possessed both CD34 and CD117 positive expressions. Focusing on the CD117+CD34+ cardiac TCs would help researchers continue to move the study forward and accurately provide biological specializations of the population.
Media can be categorized by the degree of complexity of their added ingredients, such as animal serum and protein growth factors. Serum-containing media require nutrients and growth factors supplied by animal serum for supporting cell growth. Medium optimization focuses on reducing percentages of serum present or achieving maximum cell density. Finding a good cell culture medium is very important because it affects overall process performance. Our results showed that high-glucose DMEM with 10% FBS was the most effective at stimulating proliferation of CD117+CD34+ cardiac TCs. The selected medium met applicable quality standards and regulatory requirements. Under this conditioned medium, various biochemical assays were performed to elucidate the special population.
Cell proliferation involves a series of highly regulated events that lead to DNA replication and cell division. In cells, the levels of telomerase components are growth-regulated. Previous studies indicated that mouse telomerase RNA component levels correlate well with levels of histone H4, a proliferation marker,[39] and the levels of the telomerase RNA component in human are tightly correlated with the proliferation marker MIB-1 in ependymal tumors.[40] Hence, telomerase concentration could explain the difference in proliferation among various cell lines. To date, the differences in expression of cardiac TCs from other cells have yet to be fully delineated. Published data demonstrated that the microarray-based gene expression analysis and micro-RNA signature were analyzed[9,33] and some of their genomic features were demonstrated.[34] Additionally, the proteomic profile differences between TCs and fibroblasts were reported.[10] To provide additional evidence of cardiac TCs as a unique cell population, dynamic live cell imaging related to cell division was recorded; in addition, the telomerase activity associated with mitosis was analyzed compared to BMSCs with a high concentration, CFBs with a common concentration and CMs with an almost “zero” concentration. In our study, the telomerase concentration in CD117+CD34+ cardiac TCs was 1.5 times lower than in CFBs, showing a difference of statistical significance (P < 0.05). The obtained data agreed with the observed phenomenon; cytokinesis of CD117+CD34+ cardiac TCs occurred not immediately, but a short time after mitosis. Therefore, this study enabled us to suggest the involvement of cardiac TCs in the modulation of stress-induced reactions.
In conclusion, our work evaluated the optimized basic medium for CD117+CD34+ cardiac TCs. In addition, we also demonstrated the mitosis phenomenon of the population and its relatively low telomerase activity.
Footnotes
Edited by: Li-Shao Guo
Source of Support: This study was supported by a grant from the Young Scientists Fund of the National Natural Science Foundation of China (No. 81300232).
Conflict of Interest: None declared.
REFERENCES
- 1.Hinescu ME, Gherghiceanu M, Mandache E, Ciontea SM, Popescu LM. Interstitial Cajal-like cells (ICLC) in atrial myocardium: Ultrastructural and immunohistochemical characterization. J Cell Mol Med. 2006;10:243–57. doi: 10.1111/j.1582-4934.2006.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gherghiceanu M, Popescu LM. Cardiac telocytes – Their junctions and functional implications. Cell Tissue Res. 2012;348:265–79. doi: 10.1007/s00441-012-1333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao B, Chen S, Liu J, Yuan Z, Qi X, Qin J, et al. Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat. J Cell Mol Med. 2013;17:123–33. doi: 10.1111/j.1582-4934.2012.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bani D, Nistri S. New insights into the morphogenic role of stromal cells and their relevance for regenerative medicine. Lessons from the heart. J Cell Mol Med. 2014;18:363–70. doi: 10.1111/jcmm.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng J, Shim W, Lu J, Lim SY, Ong BH, Lim TS, et al. Electrophysiology of human cardiac atrial and ventricular telocytes. J Cell Mol Med. 2014;18:355–62. doi: 10.1111/jcmm.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Liao Z, Chen S, Yuan Z, Yilin C, Lee KK, et al. Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats. J Cell Mol Med. 2014;18:780–9. doi: 10.1111/jcmm.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y, Bai C, Wang X. Potential significance of telocytes in the pathogenesis of lung diseases. Expert Rev Respir Med. 2012;6:45–9. doi: 10.1586/ers.11.91. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Bai C, Wang X. Telocyte morphologies and potential roles in diseases. J Cell Physiol. 2012;227:2311–7. doi: 10.1002/jcp.23022. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Zhang M, Qian M, Wang L, Cismasiu VB, Bai C, et al. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J Cell Mol Med. 2013;17:567–77. doi: 10.1111/jcmm.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Cretoiu D, Yan G, Cretoiu SM, Popescu LM, Fang H, et al. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J Cell Mol Med. 2014;18:1035–59. doi: 10.1111/jcmm.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popescu BO, Gherghiceanu M, Kostin S, Ceafalan L, Popescu LM. Telocytes in meninges and choroid plexus. Neurosci Lett. 2012;516:265–9. doi: 10.1016/j.neulet.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Cretoiu D, Cretoiu SM, Simionescu AA, Popescu LM. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol. 2012;27:1067–78. doi: 10.14670/HH-27.1067. [DOI] [PubMed] [Google Scholar]
- 13.Vannucchi MG, Traini C, Manetti M, Ibba-Manneschi L, Faussone-Pellegrini MS. Telocytes express PDGFRa in the human gastrointestinal tract. J Cell Mol Med. 2013;17:1099–108. doi: 10.1111/jcmm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milia AF, Ruffo M, Manetti M, Rosa I, Conte D, Fazi M, et al. Telocytes in Crohn's disease. J Cell Mol Med. 2013;17:1525–36. doi: 10.1111/jcmm.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Zheng Y, Manole CG, Wang X, Wang Q. Telocytes in human oesophagus. J Cell Mol Med. 2013;17:1506–12. doi: 10.1111/jcmm.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceafalan L, Gherghiceanu M, Popescu LM, Simionescu O. Telocytes in human skin – Are they involved in skin regeneration? J Cell Mol Med. 2012;16:1405–20. doi: 10.1111/j.1582-4934.2012.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rusu MC, Mirancea N, Manoiu VS, Vâlcu M, Nicolescu MI, Paduraru D. Skin telocytes. Ann Anat. 2012;194:359–67. doi: 10.1016/j.aanat.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Manetti M, Guiducci S, Ruffo M, Rosa I, Faussone-Pellegrini MS, Matucci-Cerinic M, et al. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med. 2013;17:482–96. doi: 10.1111/jcmm.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luesma MJ, Gherghiceanu M, Popescu LM. Telocytes and stem cells in limbus and uvea of mouse eye. J Cell Mol Med. 2013;17:1016–24. doi: 10.1111/jcmm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suciu LC, Popescu BO, Kostin S, Popescu LM. Platelet-derived growth factor receptor-ß-positive telocytes in skeletal muscle interstitium. J Cell Mol Med. 2012;16:701–7. doi: 10.1111/j.1582-4934.2011.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolescu MI, Bucur A, Dinca O, Rusu MC, Popescu LM. Telocytes in parotid glands. Anat Rec (Hoboken) 2012;295:378–85. doi: 10.1002/ar.21540. [DOI] [PubMed] [Google Scholar]
- 22.Nicolescu MI, Popescu LM. Telocytes in the interstitium of human exocrine pancreas: Ultrastructural evidence. Pancreas. 2012;41:949–56. doi: 10.1097/MPA.0b013e31823fbded. [DOI] [PubMed] [Google Scholar]
- 23.Matyja A, Gil K, Pasternak A, Sztefko K, Gajda M, Tomaszewski KA, et al. Telocytes: New insight into the pathogenesis of gallstone disease. J Cell Mol Med. 2013;17:734–42. doi: 10.1111/jcmm.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosco C, Díaz E, Gutiérrez R, González J, Pérez J. Ganglionar nervous cells and telocytes in the pancreas of Octodon degus: Extra and intrapancreatic ganglionar cells and telocytes in the degus. Auton Neurosci. 2013;177:224–30. doi: 10.1016/j.autneu.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Gevaert T, De Vos R, Van Der Aa F, Joniau S, van den Oord J, Roskams T, et al. Identification of telocytes in the upper lamina propria of the human urinary tract. J Cell Mol Med. 2012;16:2085–93. doi: 10.1111/j.1582-4934.2011.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi G, Lin M, Xu M, Manole CG, Wang X, Zhu T. Telocytes in the human kidney cortex. J Cell Mol Med. 2012;16:3116–22. doi: 10.1111/j.1582-4934.2012.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Lu S, Liu H, Ge J, Zhang H. Scanning electron microscope evidence of telocytes in vasculature. J Cell Mol Med. 2014;18:1486–9. doi: 10.1111/jcmm.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Zhang H, Yang L, Lu S, Ge J. Telocytes in mice bone marrow: Electron microscope evidence. J Cell Mol Med. 2014;18:975–8. doi: 10.1111/jcmm.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu S, Li H, Zhang H, Ge J. Research update on the association between telocytes distribution with blood vessels on various tissues and the biological properties of telocytes. Chin J Cardiovasc Dis. 2014;42:352–6. [PubMed] [Google Scholar]
- 30.Cretoiu SM, Cretoiu D, Popescu LM. Human myometrium - The ultrastructural 3D network of telocytes. J Cell Mol Med. 2012;16:2844–9. doi: 10.1111/j.1582-4934.2012.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum ST, Svalø J, Nielsen K, Larsen T, Jørgensen JC, Bouchelouche P. Immunolocalization and expression of small-conductance calcium-activated potassium channels in human myometrium. J Cell Mol Med. 2012;16:3001–8. doi: 10.1111/j.1582-4934.2012.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cretoiu SM, Cretoiu D, Marin A, Radu BM, Popescu LM. Telocytes: Ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction. 2013;145:357–70. doi: 10.1530/REP-12-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cismasiu VB, Radu E, Popescu LM. miR-193 expression differentiates telocytes from other stromal cells. J Cell Mol Med. 2011;15:1071–4. doi: 10.1111/j.1582-4934.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Zheng M, Zhang M, Qian M, Zheng Y, Li M, et al. Differences in the expression of chromosome 1 genes between lung telocytes and other cells: Mesenchymal stem cells, fibroblasts, alveolar type II cells, airway epithelial cells and lymphocytes. J Cell Mol Med. 2014;18:801–10. doi: 10.1111/jcmm.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Díaz-Flores L, Gutiérrez R, Sáez FJ, Díaz-Flores L, Jr, Madrid JF. Telocytes in neuromuscular spindles. J Cell Mol Med. 2013;17:457–65. doi: 10.1111/jcmm.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao J, Wang F, Liu Z, Yang C. Telocytes in liver: Electron microscopic and immunofluorescent evidence. J Cell Mol Med. 2013;17:1537–42. doi: 10.1111/jcmm.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mou Y, Wang Y, Li J, Lü S, Duan C, Du Z, et al. Immunohistochemical characterization and functional identification of mammary gland telocytes in the self-assembly of reconstituted breast cancer tissue in vitro . J Cell Mol Med. 2013;17:65–75. doi: 10.1111/j.1582-4934.2012.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campeanu RA, Radu BM, Cretoiu SM, Banciu DD, Banciu A, Cretoiu D, et al. Near-infrared low-level laser stimulation of telocytes from human myometrium. Lasers Med Sci. 2014;29:1867–74. doi: 10.1007/s10103-014-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Wijnen AJ, Wright KL, Lian JB, Stein JL, Stein GS. Human H4 histone gene transcription requires the proliferation-specific nuclear factor HiNF-D. Auxiliary roles for HiNF-C (Sp1-like) and HiNF-A (high mobility group-like) J Biol Chem. 1989;264:15034–42. [PubMed] [Google Scholar]
- 40.Rushing EJ, Yashima K, Brown DF, White CL, 3rd, Shay JW, Risser RC, et al. Expression of telomerase RNA component correlates with the MIB-1 proliferation index in ependymomas. J Neuropathol Exp Neurol. 1997;56:1142–6. doi: 10.1097/00005072-199710000-00008. [DOI] [PubMed] [Google Scholar]