Abstract
Background:
The clinical failure after prostatic artery embolization (PAE) with conventional particles was relatively high, in treatment for lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH). We reported the results of PAE with combined polyvinyl alcohol particles 50 μm and 100 μm in size as a primary treatment in 24 patients with severe LUTS secondary to large BPH.
Methods:
From July 2012 to June 2014, we performed PAE in 24 patients (65–85 years, mean 74.5 years) with severe LUTS due to large BPH (≥80 cm3) and refractory to medical therapy. Embolization was performed using combination of 50 μm and 100 μm in particles size. Clinical follow-up was performed using the International Prostate Symptom Score (IPSS), quality of life (QoL), peak urinary flow (Qmax), postvoid residual (PVR) volume, the International Index of Erectile Function (IIEF), prostatic specific antigen (PSA), and prostatic volume measured by magnetic resonance imaging at 1, 3, 6, and every 6-month thereafter. Technical success was defined when PAE was completed in at least one pelvic side. Clinical success was defined as the improvement of both symptoms and QoL. A Student's t-test for paired samples was used.
Results:
PAE was technically successful in 22 patients (92%). Bilateral PAE was performed in 19 (86%) patients and unilateral in 3 (14%) patients. Follow-up data were available for 22 patients observed for mean of 14 months. The clinical improvement at 1, 3, 6, and 12-month was 91%, 91%, 88%, and 83%, respectively. At 6-month follow-up, the mean IPSS, QoL, PVR, and Qmax were from 27 to 8 (P = 0.001), from 4.5 to 2.0 (P = 0.002), from 140.0 ml to 55.0 ml (P = 0.002), and from 6.0 ml/s to 13.0 ml/s (P = 0.001), respectively. The mean prostate volume decreased from 110 cm3 to 67.0 cm3 (mean reduction of 39.1%; P = 0.001). The PSA and IIEF improvements after PAE did not differ from pre-PAE significantly. No major adverse events were noted.
Conclusions:
The combination of 50 μm and 100 μm particles for PAE is a safe and effective treatment method for patients with severe LUTS due to large BPH, which further improves the clinical results of PAE.
Keywords: Angiography, Benign Prostatic Hyperplasia, Embolization, Lower Urinary Tract Symptoms, Prostatic Artery Embolization, Therapeutic
INTRODUCTION
Prostatic arterial embolization (PAE) has been recently proposed as a safe and effective treatment for lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH).[1,2,3] However, the rate of clinical failure after PAE was relatively high. As many as 25% of patients show no improvement significantly in symptoms and peak flow rate. In addition, the average of reduction rate in the prostate volume (PV) after PAE was only 20%.[4]
One component of PAE where the best practice remains to be defined is the choice of embolic agent size. Theoretically, a deeper penetration of the smaller polyvinyl alcohol (PVA) particles into the prostate would lead to greater gland ischemia and PV reduction and hence a greater clinical improvement.[5] In the present study, we report the results of PAE with combined PVA particles 50 μm and 100 μm in size as a primary treatment in 24 patients with severe LUTS due to large BPH (>80 cm3) after failure of medical treatment; all patients were unsuitable for surgery. To the best of our knowledge, this is the first report to describe using the 50-μm particles for PAE.
METHODS
This study was approved by the Ethics Committee of Chinese People's Liberation Army General Hospital, and informed consent was obtained from all patients. All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and with Declaration of Helsinki and its later amendments or comparable ethical standards.
Patients
From July 2012 to June 2014, a total of 24 patients (age range, 65–85 years; mean 74.5 years) diagnosed with severe LUTS due to large BPH (>80 cm3) that was refractory to medical treatment for at least 6-month underwent PAE. Inclusion criteria of PAE were symptomatic LUTS due to BPH, International Prostate Symptom Score (IPSS) ≥12, prostatic specific antigen (PSA) <4 ng/ml, or PSA level between 4 and 10 ng/ml but negative prostate biopsy, PV ≥80 cm3, peak urinary flow (Qmax) <15 ml/s. Exclusion criteria were malignancy, neurogenic bladder dysfunction and/or sphincter decompensation, unregulated coagulation disorders, large bladder diverticula (>5 cm), large bladder stones (>2 cm), chronic renal failure (serum creatinine >1.2 mg/dl), active urinary tract infection, previous surgical treatment for LUTS or BPH, current diagnosis of bladder stones, patients with catheter, or with an episode of acute retention of urine in the last 4 weeks.[6,7,8]
Efficacy variables of IPSS, quality of life (QoL)-related symptoms, International Index of Erectile Function (IIEF), uroflowmetry (peak urinary flow, Qmax; postvoid residual [PVR] volume), PSA and PV were assessed before PAE and at 1, 3, 6, and every 6-month after the procedure. The PV was measured by magnetic resonance imaging (MRI). The MRI protocol for all examinations was the same, including axial and sagittal T2-weighted and noncontrast-enhanced and contrast-enhanced T1-weighted pulse sequences.
Patient demographics are summarized in Table 1. All patients were diagnosed of severe LUTS (IPSS >18 points, QoL score >3, Qmax<12 ml/s) due to BPH with a mean PV of 110 cm3 (82–165 cm3). The patient selection was achieved in a multidisciplinary manner in conjunction with urologists and interventional radiologists. All patients were assessed by a urologist and anesthesiologist as being unsuitable for surgery owing to cardiac (n = 14) and pulmonary (n = 10) disease. Four patients underwent transrectal ultrasound-guided prostate biopsy due to a PSA level >4.0 ng/ml with negative results for malignancy.
Table 1.
Baseline data of the study population (n = 24)
| Variables | Values (mean ± SD) | Range |
|---|---|---|
| Age (years) | 74.5 ± 7.5 | 65–85 |
| IPSS (point) | 27.0 ± 4.5 | 24–35 |
| QoL score | 4.50 ± 1.5 | 4–6 |
| PV (cm3) | 110 ± 25.0 | 82–165 |
| PSA (ng/ml) | 3.80 ± 0.8 | 1.50–5.60 |
| Qmax (ml/s) | 6.00 ± 2.50 | 4.50–8.00 |
| PVR (ml) | 140.0 ± 30.0 | 90–210 |
| IIEF (point) | 20.0 ± 5.5 | 18–24 |
SD: Standard deviation; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; PSA: Prostatic specific antigen; PV: Prostate volume; PVR: Postvoid residual urine; Qmax: Peak urinary flow rate; QoL: Quality of life.
Patients stopped taking all prostatic medications 3 days before embolization and the drugs of acid-suppressing, anti-inflammatory, and antibiotic were given 2 days before the procedure.
Angiography
Procedures were performed under local anesthesia through a unilateral femoral artery puncture approach. It is crucial for successful PAE to reveal the anatomy of prostatic arteries clearly.[9] Initial pelvic angiography was performed to evaluate iliac vessels. Selective angiography of the internal iliac artery was performed using the ipsilateral anterior oblique projection of 35° with caudal-cranial angulation of −10° to identify the arteries supply to the prostate with a 4F catheter. Subselective PA angiography before embolization was performed by injecting 3–5 ml of contrast medium in neutral and ipsilateral anterior oblique projections (35°) with caudal-cranial angulation (−10°) to ensure that the tip of the microcatheter was inside or at the ostium of the prostatic arteries. Cone-beam computed tomography was also performed with a 4–6 s delay after injection of 3–5 ml iodinated contrast agent using the power injector to evaluate for sites of nontarget embolization. If a site of potential nontarget embolization was identified, further selective catheterization was performed before embolization. When spasm occurred, nitroglycerin (200–300 μg) was used intra-arterially.
Embolization
A new embolization technique was used in this study. We started PAE with smaller PVA particles (50 μm, Cook Incorporated, Bloomington, IN, USA) for the distal intra-prostatic embolization, and end with larger (100 μm) for the proximal embolization to complete occlusion and stasis of blood flow to the prostate. Each vial of PVA (1 ml) was diluted in a 40-ml solution of the nonionic contrast medium. The particles were injected slowly under fluoroscopic monitoring. The endpoint of embolization chosen was “near stasis” in the prostatic vessels with interruption of the arterial flow and prostatic gland opacification, without reflux of the particles to undesired arteries. After PAE, angiography of the anterior branch of the internal iliac artery was performed to check for other blood supply to the prostate with the 4F catheter. Embolization was then performed on the contralateral side using the same technique.
Postprocedural management
The patients stayed in the hospital for 5–7 days for observation because of all were elderly. The patients were monitored for adverse effects. Appropriate hydration was administered 2–3 days after PAE. The drugs of acid-suppressing, anti-inflammatory, and antibiotic were continuously given for 7 days following PAE. After undergoing successful PAE, all prostatic medications were stopped during the entire follow-up period if there was consistent clinical improvement.
Outcome measures
Technical success was defined as unilateral or bilateral prostatic arterial catheterization and embolization were performed successfully. Clinical success, as suggested by other authors,[2,6,7] was defined as improving symptoms (IPSS reduction at least 25% of the total score and lower than 18 points) after PAE and improving of QoL (reduction of QoL of at least 1 point or ≤3 points), with increase of Qmax by at least 2.5 ml/s and Qmax of at least 7 ml/s, and other invasive therapies were not required after the procedure. Clinical failure after PAE was considered when one of the following criteria was met: IPSS ≥20 and/or reduction <25%; QoL ≥4 or reduction <1; Qmax improvement <2.5 ml/s; additional treatments required (i.e., need for surgery as a result of persisting severe LUTS).
Postembolization symptoms and complications were registered and classified according to the quality improvement guidelines for percutaneous transcatheter embolization.[10] Complications were considered as minor if they could be addressed by ambulatory medical treatment, and major if they resulted in prolonged hospitalization, hospital readmission, or required surgery.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD) and range. Categorical data are presented as count and percentage. A Student's t-test for paired samples was used when appropriate. P ≤ 0.05 was considered as statistically significant. Statistical analysis was performed using SPSS 16.0 software for Windows (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Prostatic artery embolization was technically successful in 22 patients (92%). Embolization was impossible in two patients (13%) owing to severe tortuosity and atherosclerotic changes of the iliac arteries and the prostatic medication was resumed. Bilateral PAE was performed in 19 (86%) patients [Figure 1] while the remaining 3 (14%) patients underwent unilateral PAE due to severe atherosclerotic stenosis of a unilateral PA [Figure 2].
Figures 1.
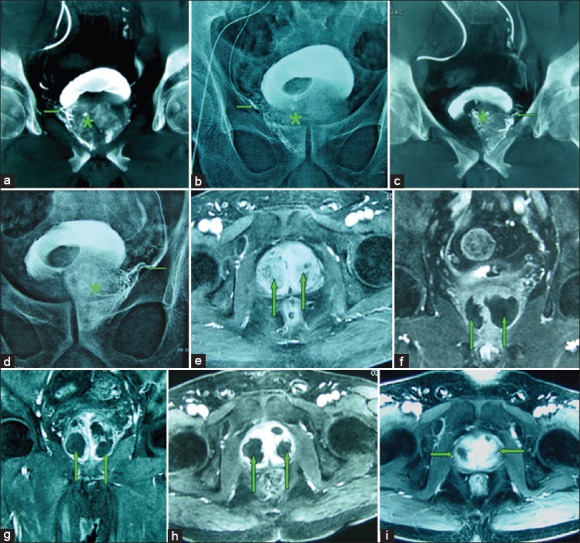
(a-i) Images from an 82-year-old man with significant lower urinary tract symptoms due to large benign prostatic hyperplasia (BPH) (100 cm3) underwent bilateral prostatic artery embolization (PAE). (a) Cone-beam computed tomography (CT) image with coronal view after super-selective catheterization of the right prostatic artery (→) demonstrates contrast medium staining in the right prostate lobe (*). (b) Image obtained at the end of embolization shows complete embolized of the right prostatic artery (→) and the right prostatic lobe opacification (*). (c) Cone-beam CT image with coronal view after super-selective catheterization of the left prostatic artery (→) demonstrates contrast medium staining in the left prostate lobe (*). (d) Image obtained at the end of embolization shows complete embolized of the left prostatic artery (→) and the left prostatic lobe opacification (*). (e) Axial contrast-enhanced T1-weighted magnetic resonance image (MRI) obtained before PAE shows a large BPH (straight arrows). (f) Coronal contrast-enhanced T1-weighted MRI obtained at 1-month after PAE shows significantly infarct areas (85%) on the both side of the prostate (straight arrows). (g) Axial contrast-enhanced T1-weighted MRI obtained at 3-month after PAE shows significantly infarct areas on the both side of the prostate (straight arrows), with the volume reduction of 32%. (h) Axial contrast-enhanced T1-weighted MRI obtained at 6-month after PAE shows significantly infarct areas on the both side of the prostate (straight arrows), with the volume reduction of 45%. (i) Axial contrast-enhanced T1-weighted MRI obtained at 12-month after PAE shows the prostate volume reduction of 46%; this patient experienced marked clinical improvement during 18-month follow-up, with International Prostate Symptom Score improvement of 80%.
Figure 2.
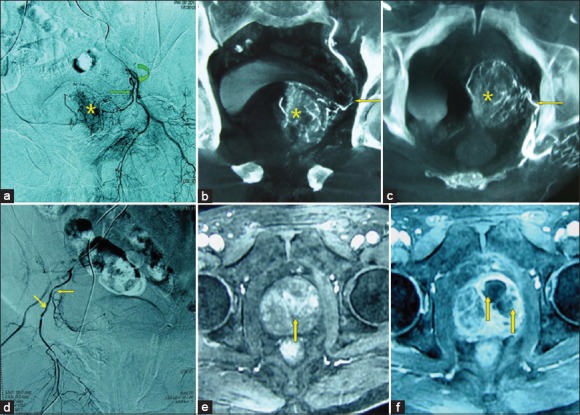
(a-f) Images from a 78-year-old man with significant lower urinary tract symptoms due to benign prostatic hyperplasia (BPH) (140 cm3) underwent unilateral prostatic artery embolization (PAE). (a) DSA of the anterior division of the left internal iliac artery with ipsilateral oblique view demonstrates the left prostatic artery (straight arrow) arising from the left internal pudendal artery (curved arrow) and contrast staining in the left prostate lobe (*). (b) Cone-beam computed tomography (CT) image with coronal view after super-selective catheterization of the left prostatic artery (←) demonstrates the left prostate lobe arteries and contrast staining (*). (c) Cone-beam CT image with axial view after super-selective catheterization of the left prostatic artery (←) demonstrates the left prostate lobe arteries and contrast staining (*). (d) DSA of the anterior division of the right internal iliac artery with ipsilateral oblique view demonstrates the right prostatic artery (←) arising from the right internal pudendal artery (↘) with severe stenosis at the ostium. Super-selective catheterization of the right prostatic artery was failed, and only the left prostatic artery was embolized. (e) Axial contrast-enhanced T1-weighted magnetic resonance image (MRI) obtained before PAE shows a large BPH (straight arrows). (f) Axial contrast-enhanced T1-weighted MRI obtained at 1-month after PAE shows infarct areas predominantly on the left side of the prostate (straight arrows); the patient experienced significantly clinical improvement during 12-month follow-up, with a prostatic volume reduction of 31%, International Prostate Symptom Score improvement of 60%.
Follow-up data were available for 22 patients, who were observed for a mean of 14 months (range: 4–26 months). The clinical improvement at 1, 3, 6, and 12-month was 91% (20 of 22 patients), 91% (20 of 22 patients), 88% (15 of 17 patients), and 83% (10 of 12 patients), respectively. Clinical failure was observed in 2 (9%) patients with only unilateral PAE, and these two patients resumed conservative treatments. The PSA values in the two patients were increased by 4.0 times and 7.5 times, respectively, relative to their mean baseline values at 24 h after embolization. The PV reduction rate at 3-month follow-up in the two patients was 12% and 15%, respectively.
The follow-up data of the 20 patients with clinical success are summarized in Table 2. Significantly infarcts (mean: 60%, range: 50–85%) were seen in all 20 patients with clinical success as measured by MRI, predominantly in the prostatic central zone; the infarcts were reduced progressively in size, and sustained after 6 months [Figure 1e-1i]. At 6-month follow-up, the mean IPSS score decreased from 27.0 ± 4.5 to 8.0 ± 3.5 (P = 0.001), mean QoL score decreased from 4.50 ± 1.5 to 2.0 ± 1.0 (P = 0.002), mean Qmax increased from 6.00 ± 2.50 ml/s to 13.0 ± 3.5 ml/s (P = 0.001), mean PVR decreased from 140.0 ± 30.0 ml to 55.0 ± 15.0 ml (P = 0.002), and mean PV decreased from 110 ± 25.0 cm3 to 67.0 ± 25.0 cm3 (mean reduction of 39%; P = 0.001). Twelve patients were followed more than 12 months, and these changes were sustained throughout the observation period. The IIEF improvements after PAE did not differ from pre-PAE significantly.
Table 2.
Clinical values over time of response variables after PAE with clinical success
| Variables | 1-month (n = 20) | 3-month (n = 20) | 6-month (n = 15) | 12-month (n = 10) |
|---|---|---|---|---|
| IPSS (point) | 12.0 ± 6.0 (4–16) | 7.0 ± 4.0 (4–14) | 8.0 ± 3.5 (4–12) | 7.5 ± 4.5 (5–12) |
| QoL score | 2.5 ± 1.0 (0–3) | 2.0 ± 1.0 (1–3) | 2.0 ± 1.5 (1–3) | 2.0 ± 1.0 (0–3) |
| PV (cm3) | 100.0 ± 25 (75–145) | 68.0 ± 20.0 (55–100) | 67.0 ± 25.0 (50–95) | 69.0 ± 20.0 (55–97) |
| Qmax (ml/s) | 12.0 ± 4.5 (10–17) | 13.0 ± 2.5 (9–18) | 13.0 ± 3.5 (9–19) | 12.0 ± 3.0 (9–17) |
| PVR (ml) | 70.0 ± 20.0 (20–80) | 60.0 ± 15.0 (10–50) | 55.0 ± 15.0 (5–50) | 40.0 ± 10.0 (10–60) |
| IIEF (point) | 18.0 ± 6.0 (16–24) | 19.0 ± 4.0 (17–24) | 18.0 ± 5.0 (18–26) | 17.0 ± 6.0 (16–24) |
Values are mean ± SD (range). SD: Standard deviation; PAE: Prostatic artery embolization; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; PSA: Prostatic specific antigen; PV: Prostate volume; PVR: Postvoid residual urine; Qmax: Peak urinary flow rate; QoL: Quality of life.
Serum total PSA values before and after PAE are summarized in Table 3. At 24 h after embolization, the mean serum total PSA increased from 3.8 ± 0.8 ng/ml to 95.0 ± 45.0 ng/ml (mean, 25 times relative to the mean baseline values; P < 0.001). By 1 week after embolization, mean PSA dropped to 38.0 ± 10.0 ng/ml (mean 10 times; P < 0.001). By 1 month after embolization, mean PSA dropped to the baseline values and then was sustained over time.
Table 3.
Total serum PSA values before and after PAE (n = 20)
| Periods | Serum PSA (ng/ml) | t | P* | |
|---|---|---|---|---|
| Mean ± SD | Range | |||
| Pre-PAE | 3.1 ± 1.6 | 0.9–5.9 | – | – |
| 24 h | 83.9 ± 51.7 | 16.6–153.0 | −7.130 | 0.000 |
| 1-week | 30.0 ± 20.1 | 5.0–60.5 | −6.385 | 0.000 |
| 1-month | 3.1 ± 1.0 | 1.4–4.6 | 0.104 | 0.918 |
| 3-month | 3.6 ± 1.4 | 1.2–5.9 | −1.925 | 0.069 |
| 6-month | 3.1 ± 1.0 | 1.0–4.25 | 0.121 | 0.905 |
| 12-month | 3.2 ± 0.8 | 1.6–4.1 | −0.260 | 0.798 |
*P versus pre-PAE. PSA: Prostatic specific antigen; SD: Standard deviation; PAE: Prostatic artery embolization.
Mean procedural time was 115 min (range: 80–185 min) with a mean fluoroscopy time of 36 min (range: 18–60 min). The patients stayed in the hospital for 5–7 days for observation. No major adverse events were noted in this series. During the procedure, all patients did not feel any pain. As minor complications, transient hematuria occurred in 3 (14%) patients, transient hemospermia occurred in 2 (9%) patients, and transient rectal bleeding occurred in 3 (14%) patients. About 36% (8/22) of patients experienced a burning sensation in the urethra and irritative voiding at 3–5 days after PAE. All these minor complications disappeared during the first 1-week. Seven patients (32%) had acute urinary retention at 1–3 days after PAE and a temporary bladder catheter was placed, for relief, for 3–7 days and the patients were able to void spontaneously before discharge. There were no incidences of ejaculatory disorders postprocedure.
DISCUSSION
A new embolization protocol was used in this study. We started embolization with smaller-sized PVA particles (50-μm) to first block these smaller intra-prostate vessels, and end with relatively larger (100-μm) for the proximal embolization. This technique has produced greater PV reduction (mean 39%) than previously described methods (18–30%).[1,2,3,4] The infarction of the prostate was reported in only 50–70% of the patients with a mean infarction rate of 30–50% after PAE using 100–300-μm particle size.[5] Using our protocol, the infarct areas >50% were observed in all clinical success patients as measured by MRI. In addition, the serum total PSA values increased significantly at 24 h after embolization were observed, with a mean 25 times relative to the mean baseline values. These also suggested that greater prostate ischemia occurred after PAE.
The rationale for PAE is that prostate ischemia leads to PV reduction and hence clinical improvement.[5,11] Therefore, it is logical to assume that PV reduction could be predicted by the extent of infarction after PAE. However, Pisco et al.[12] reported that they did not observe a clear relationship between reduction in prostatic volume and clinical outcome when clinical failure was seen in some patients with a significant PV reduction (>15%). Nearly half of their patients do not exhibit ischemic changes on MRI after PAE. Thus, they stated that clinical success could not be predicted on the basis of ischemic changes and PV reduction. In our initial experience with PAE, only those patients with postembolization MRI findings of significant prostatic infarction had prolonged control of their symptoms.
The optimal embolic agent size remains to be determined. It is reasonable to assume that smaller-size particles may induce greater ischemia with a more distal penetration into the prostate,[5,13] and hence lead to a better clinical outcome. Embolization with larger particles (i.e., ≥200 μm), as previously reported results, may not an optimal size for PAE because of early proximal occlusion. BPH develops primarily in the peri-urethral region of the prostate, therefore embolization of this part is important for improvement of LUTS.[14]100 μm PVA particles have been reported safely for PAE without untargeted embolization.[5,12] Anatomically, the prostatic part of the urethra is supplied by a branch of the prostatic artery with a diameter of 40–60 μm.[15] Based on these data, particles with 50 μm in size may penetrate into the peri-urethral region of the prostate, with a better result than that of particles ≥100 μm in size. Nevertheless, injury of the urethral wall should be concerned using the small size particles. In the present study, there were no major complications from PAE in any patient treated, and all minor complications could be addressed with conservative care. This showed that the PAE with PVA particles 50-μm are as safe as larger ones (e.g., 100–200 μm).
The long-term outcome of these embolized prostates is still unknown. Revascularization after PAE may play a role in recurrent symptoms of LUTS and prostate re-growth. Carnevale et al.[16] reported the initial two patients are presenting signs of de novo prostate growth as measured through MRI after maintained clinical benefit for 4 years. Pisco et al.[12] observed revascularization of the prostatic arteries after PAE in their patients with second PAE procedure. Theoretically, block these intra-prostate smaller vessels with small sized particles may prevent the revascularization through the anastomoses.
There are some limitations to the present study. Firstly, the small number of patients treated at a single center with limited follow-up. Continued follow-up is ongoing, and longer follow-up in our patients will bring additional information in the future. Secondly, the present study included only in large volume BPH patients with unsuitable for surgery. Further analyses are necessary to establish the role of PAE in patients who are candidates for surgery, or the PV <80 cm3. Thirdly, only PVA particles were used for our procedures. Further investigation concerning different type of embolic agents is necessary. Although the results are promising, more studies are needed, especially multicentre randomized controlled trials.
In conclusion, our preliminary experience suggested that the combination of 50 μm and 100 μm particles for PAE led to greater ischemia and infarction. This technique allows better distribution of embolic material in the intra-prostatic arteries and reduces the risk of revascularization through the anastomoses.
ACKNOWLEDGMENTS
We thank Dr. Xin Ma, from the Department of Urology, Chinese People's Liberation Army General Hospital, for his consultations.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: This work was supported by grants from the National Scientific Foundation Committee of China (No. 81471769), the Central Health Research Project (No. 2013BJ09), and Chinese People's Liberation Army Scientific Foundation of the Twelve-Five Programme (No. BWS11J028).
Conflict of Interest: None declared.
REFERENCES
- 1.de Assis AM, Moreira AM, de Paula Rodrigues VC, Yoshinaga EM, Antunes AA, Harward SH, et al. Prostatic artery embolization for treatment of benign prostatic hyperplasia in patients with prostates >90 g: A prospective single-center study. J Vasc Interv Radiol. 2015;26:87–93. doi: 10.1016/j.jvir.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Bagla S, Martin CP, van Breda A, Sheridan MJ, Sterling KM, Papadouris D, et al. Early results from a United States trial of prostatic artery embolization in the treatment of benign prostatic hyperplasia. J Vasc Interv Radiol. 2014;25:47–52. doi: 10.1016/j.jvir.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Carnevale FC, da Motta-Leal-Filho JM, Antunes AA, Baroni RH, Marcelino AS, Cerri LM, et al. Quality of life and clinical symptom improvement support prostatic artery embolization for patients with acute urinary retention caused by benign prostatic hyperplasia. J Vasc Interv Radiol. 2013;24:535–42. doi: 10.1016/j.jvir.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 4.McWilliams JP, Kuo MD, Rose SC, Bagla S, Caplin DM, Cohen EI, et al. Society of Interventional Radiology position statement: Prostate artery embolization for treatment of benign disease of the prostate. J Vasc Interv Radiol. 2014;25:1349–51. doi: 10.1016/j.jvir.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Bilhim T, Pisco J, Campos Pinheiro L, Rio Tinto H, Fernandes L, Pereira JA, et al. Does polyvinyl alcohol particle size change the outcome of prostatic arterial embolization for benign prostatic hyperplasia? Results from a single-center randomized prospective study. J Vasc Interv Radiol. 2013;24:1595–602.e1. doi: 10.1016/j.jvir.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Kurbatov D, Russo GI, Lepetukhin A, Dubsky S, Sitkin I, Morgia G, et al. Prostatic artery embolization for prostate volume greater than 80 cm3: Results from a single-center prospective study. Urology. 2014;84:400–4. doi: 10.1016/j.urology.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Golzarian J, Antunes AA, Bilhim T, Carnevale FC, Konety B, McVary KT, et al. Prostatic artery embolization to treat lower urinary tract symptoms related to benign prostatic hyperplasia and bleeding in patients with prostate cancer: Proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2014;25:665–74. doi: 10.1016/j.jvir.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–40. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Monaco R, Garategui L, Kizilevsky N, Peralta O, Rodriguez P, Palacios-Jaraquemada J. Human cadaveric specimen study of the prostatic arterial anatomy: Implications for arterial embolization. J Vasc Interv Radiol. 2014;25:315–22. doi: 10.1016/j.jvir.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Angle JF, Siddiqi NH, Wallace MJ, Kundu S, Stokes L, Wojak JC, et al. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2010;21:1479–86. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Sun F, Sánchez FM, Crisóstomo V, Díaz-Güemes I, López-Sánchez C, Usón J, et al. Transarterial prostatic embolization: Initial experience in a canine model. AJR Am J Roentgenol. 2011;197:495–501. doi: 10.2214/AJR.10.5947. [DOI] [PubMed] [Google Scholar]
- 12.Pisco JM, Rio Tinto H, Campos Pinheiro L, Bilhim T, Duarte M, Fernandes L, et al. Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: Results of short- and mid-term follow-up. Eur Radiol. 2013;23:2561–72. doi: 10.1007/s00330-012-2714-9. [DOI] [PubMed] [Google Scholar]
- 13.Brook OR, Faintuch S, Brook A, Goldberg SN, Rofsky NM, Lenkinski RE. Embolization therapy for benign prostatic hyperplasia: Influence of embolization particle size on gland perfusion. J Magn Reson Imaging. 2013;38:380–7. doi: 10.1002/jmri.23981. [DOI] [PubMed] [Google Scholar]
- 14.Carnevale FC, Antunes AA, da Motta Leal Filho JM, de Oliveira Cerri LM, Baroni RH, Marcelino AS, et al. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: Preliminary results in two patients. Cardiovasc Intervent Radiol. 2010;33:355–61. doi: 10.1007/s00270-009-9727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanov M. Extraglandular and intraglandular vascularization of canine prostate. Microsc Res Tech. 2004;63:188–97. doi: 10.1002/jemt.20028. [DOI] [PubMed] [Google Scholar]
- 16.Carnevale FC, da Motta-Leal-Filho JM, Antunes AA, Baroni RH, Freire GC, Cerri LM, et al. Midterm follow-up after prostate embolization in two patients with benign prostatic hyperplasia. Cardiovasc Intervent Radiol. 2011;34:1330–3. doi: 10.1007/s00270-011-0136-8. [DOI] [PubMed] [Google Scholar]


