Abstract
Background:
Neovascular glaucoma (NVG) is a refractory glaucoma. The management of NVG is very difficult, and it is more difficult when combined with vitreous hemorrhage. The aim of this study was to investigate the effects of ranibizumab plus combined surgery for NVG with vitreous hemorrhage.
Methods:
A total of 26 eyes of 26 NVG patients with vitreous hemorrhage were recruited in this study. The patients aged from 36 to 63 years with a mean age of 51.97 ± 7.60 years. The mean intraocular pressure (IOP) was 46.38 ± 5.75 mmHg (1 mmHg = 0.133 kPa) while being treated with the maximum medical therapy. The mean best-corrected visual acuities converted to logarithm of the minimum angle of resolution (logMAR BCVA) was 2.62 ± 0.43. All the patients underwent intravitreal injection of 0.5 mg (0.05 ml) ranibizumab combined with pars plana vitrectomy (PPV), pars plana lensectomy (PPL) with a preserved anterior capsule, panretinal photocoagulation (PRP), and trabeculectomy (intravitreal ranibizumab [IVR] + PPV + PPL + PRP + trabeculectomy). The IOP and logMAR BCVA were the main outcome measures in this study.
Results:
The follow-up period was 12 months. The mean postoperative IOPs were 26.38 ± 3.75 mmHg, 21.36 ± 3.32 mmHg, 18.57 ± 3.21 mmHg, and 16.68 ± 2.96 mmHg, respectively at 7 days, 1 month, 3 months, and 12 months after PPV + PPL + PRP + trabeculectomy. At the last follow-up, the mean IOP was significantly lower than the preoperative one (t = 6.612, P = 0.001). At 7 days, 1 month, 3 months, and 12 months after PPV + PPL + PRP + trabeculectomy, the mean logMAR BCVA were 1.30 ± 0.36, 1.29 ± 0.37, 1.29 ± 0.39, and 1.26 ± 0.29, respectively. At the last follow-up, the mean logMAR BCVA was significantly improved, and the difference was statistically significant compared with preoperative one (t = 6.133, P = 0.002). The logMAR BCVA improved in 22 eyes (84.62%), and remained stable in 4 eyes (15.38%). The neovascularization in the iris and the angle regressed significantly in all patients 7 days after ranibizumab injection. No serious complications occurred during 12 months of the study.
Conclusions:
IVR + PPV + PPL + PRP + trabeculectomy can control IOP well and improve BCVA without severe complication for NVG patients with vitreous hemorrhage.
Keywords: Neovascular Glaucoma, Panretinal Photocoagulation, Pars Plana Vitrectomy, Ranibizumab, Trabeculectomy
INTRODUCTION
Neovascular glaucoma (NVG) develops secondarily to ocular ischemia.[1,2] Vascular endothelial growth factor (VEGF) from the ischemic retina migrates to the anterior segment and stimulates the neovascularization in the iris and the angle. When neovascular fibrous tissues occupy the trabecular meshwork, aqueous outflow is disturbed, and NVG develops eventually.[3,4] An elevation of intraocular pressure (IOP) due to NVG decreases the ocular perfusion leading to further retinal ischemia, and the ischemia in turn induces more neovascularization.
The management of NVG is very difficult. The conventional treatments such as medication, trabeculectomy, cyclocryotherapy, and cyclophotocoagulation generally have poor success rates in managing NVG.[5,6,7] Patients often suffer from intolerable pain and loss of vision if they cannot be treated timely and effectively.
It is very important to alleviate the ischemia promptly for the treatment of NVG. Panretinal photocoagulation (PRP) is an effective method to resolve the ischemic condition.[8] But the management is particularly difficult in eyes with vitreous hemorrhage and/or coexistent cataract. However, it is possible to resolve these problems by pars plana vitrectomy (PPV). Moreover, PPV combined with pars plana lensectomy (PPL) enables us to apply PRP from the posterior pole to the ora serrata. Mitomycin C (MMC) has been shown to improve the success rate of trabeculectomy in patients with NVG.[6]
Therefore, in the current study, we performed intravitreal ranibizumab (IVR) injection plus PPV, PPL with a preserved anterior capsule, PRP and trabeculectomy with MMC (IVR + PPV + PPL + PRP + trabeculectomy) to treat NVG with vitreous hemorrhage. The surgical procedure may alleviate retinal ischemia and reduce IOP as soon as possible, thus to protect the remaining visual function to the extreme extent.
METHODS
Subjects
The study was approved by the Ethical Review Committee of Zhengzhou University and adhered to the provisions of Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from all of the participants involved in the study after a thorough discussion about the potential benefits and risks of IVR + PPV + PPL + PRP + trabeculectomy. A total of 26 eyes of 26 NVG patients with vitreous hemorrhage (11 males, 15 females) were recruited in this study from July 2012 to July 2014. The patients aged from 36 to 63 years with a mean age of 51.97 ± 7.60 years. The etiology of NVG with vitreous hemorrhage included diabetic retinopathy in eight cases, ischemic central retinal vein occlusion in eight cases, ischemic branch retinal vein occlusion in four cases, hypertensive retinopathy in four cases, and ocular ischemia syndrome in two cases. All patients had corneal edema and cataract in different degrees. The mean IOP was 46.38 ± 5.75 mmHg (1 mmHg = 0.133 kPa) while being treated with the maximum medical therapy. The mean best-corrected visual acuities converted to logarithm of the minimum angle of resolution (logMAR BCVA) was 2.62 ± 0.43. All patients underwent IVR + PPV + PPL + PRP + trabeculectomy. Patients who had undergone the following procedure such as previous cyclodestructive procedure, scleral buckle procedure, glaucoma drainage device implantation, or silicone oil surgery were excluded from the study. Only one eye per patient was included in this trial.
Surgical procedures
All procedures were carried out by experienced surgeons. First, all patients were injected intravitreally with ranibizumab 0.5 mg (0.05 ml). Injection procedure was performed as described previously.[9] The standard protocol for intraocular injections included topical anesthesia, disinfection, sterile draping, and lid speculum. Then, 0.05 ml of ranibizumab (10 mg/ml; Novartis, Basel, Switzerland) was delivered using a 27-gauge needle through the inferotemporal quadrant at 3.5–4.0 mm posterior to the limbus. The needle was carefully removed using a sterile cotton applicator. Then the patients received an anti-glaucomatous medication: topical brimonidine and dorzolamide/timolol fixed combination twice a day, and oral acetazolamide (250 mg) three times a day, because intravitreal injections could be associated with the risk of further IOP elevation. Seven days later, when the neovascularization in the iris and the angle regressed significantly, we created a limbus-based conjunctival flap for trabeculectomy. Then a three-port PPV combined with PPL extraction and preserved the anterior lens capsule was performed with a 23-gauge cutter. Triamcinolone acetonide was used to facilitate visualization of the vitreous, and posterior vitreous detachment was induced if it had not yet occurred. Fibrovascular membranes were removed using the total en-bloc excision technique. Then PRP (1600–2000 shots) was performed up to the ora serrata anteriorly. Settings were set as follows: Pulse duration 200 ms; spot size 200–300 μm in the posterior retina and 500 μm in the peripheral retina; power 150 mW, increased by 10–20 mW until a white lesion was attained. Finally, trabeculectomy and iridectomy were performed. The procedure was performed as described previously.[5] After creation of a 4 mm × 4 mm, half-thickness scleral flap, small pieces of surgical sponge soaked in MMC (0.4 mg/ml) were inserted under the conjunctival flap for 5 minutes. The eye was irrigated thoroughly with 200 ml saline. Trabeculectomy was performed with a Kelly Descemet's Membrane Punch (Inami, Tokyo, Japan) followed by peripheral iridectomy. The scleral and conjunctival flaps were sutured with 10–0 nylon sutures. Postoperatively, argon laser suture lysis and needling revision of the filtration bleb were performed as necessary to enhance filtration.
Outcome measures
The main outcome measures in this study were postoperative IOP, logMAR BCVA, and the incidence of complications. All patients underwent a broad ophthalmologic examination at every follow-up visits (baseline, 7 days, 1 month, 3 months, and 12 months after PPV + PPL + PRP + trabeculectomy) including IOP measurement using Goldmann applanation tonometer, logMAR BCVA using Early Treatment Diabetic Retinopathy Study charts at a distance of four meters, gonioscopy, slit-lamp examination, and ophthalmoscopy.
The surgery was considered to have been successful when IOP ≤21 mmHg was achieved and no serious complications (endophthalmitis, retinal detachment, suprachoroidal hemorrhage, cellulitis, phthisis bulbi, or persistent hypotony [IOP <5 mmHg]) occurred during 12-month follow-up. The success was subdivided into complete and qualified. Complete success was defined as IOP between 6 mmHg and 21 mmHg without any anti-glaucoma medication, and qualified success was defined as IOP between 6 mmHg and 21 mmHg with one medication or more. Failure was defined as IOP >21 mmHg despite the use of maximum-tolerated medications, IOP <5 mmHg after 3 months, the need for additional glaucoma surgery or laser to control IOP, or serious complications as mentioned previously. Using these criteria, we calculated the success rates 3 months and 12 months after IVR + PPV + PPL + PRP + trabeculectomy.
Statistical analysis
Normally distributed data were shown as mean ± standard deviation (SD). Changes in IOP and logMAR BCVA were compared for each follow-up visit with baseline using paired t-test. The main focus was on the 12-month results. P < 0.05 was considered to be statistically significant. Statistical analysis was performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Intraocular pressure
The patients presented with a baseline IOP of 46.38 ± 5.75 mmHg. At 7 days, 1 month, and 3 months after PPV + PPL + PRP + trabeculectomy, the mean IOPs were 26.38 ± 3.75 mmHg, 21.36 ± 3.32 mmHg, and 18.57 ± 3.21 mmHg, respectively. The mean postoperative IOPs were significantly reduced compared with baseline at 7 days (t = 3.563, P = 0.005), 1 month (t = 7.635, P = 0.006), and 3 months (t = 6.597, P = 0.002). At the last follow-up, the mean IOP was 16.68 ± 2.96 mmHg, which was significantly lower than the preoperative one (t = 6.612, P = 0.001) [Figure 1]. An IOP between 6 mmHg and 21 mmHg without any antiglaucoma medication was maintained in 20 of 26 eyes (76.92%) at 3 months and was maintained in 17 of 26 eyes at 12 months (65.38%) after IVR + PPV + PPL + PRP + trabeculectomy. An IOP between 6 mmHg and 21 mmHg with one medication or more was maintained in 5 of 26 eyes (19.23%) at 3 months and was maintained in 5 of 26 eyes at 12 months (19.23%) after IVR + PPV + PPL + PRP + trabeculectomy. The success rates of our procedure were 96.15% at 3-month follow-up and 84.62% at 12-month follow-up.
Figure 1.
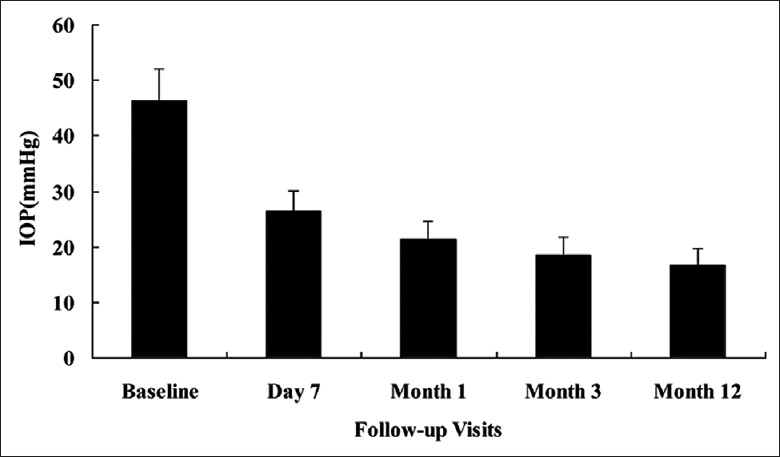
The time course of intraocular pressure (IOP) changes. At baseline, the mean IOP was 46.38 ± 5.75 mmHg. The mean postoperative IOP revealed a significant reduction compared with baseline at 7 days (26.38 ± 3.75 mmHg, P = 0.005), 1 month (21.36 ± 3.32 mmHg, P = 0.006), 3 months (18.57 ± 3.21 mmHg, P = 0.002)), and 12 months (16.68 ± 2.96 mmHg, P = 0.001) after pars plana vitrectomy + pars plana lensectomy + panretinal photocoagulation + trabeculectomy.
Best-corrected visual acuities converted to logarithm of the minimum angle of resolution
At baseline, the mean preoperative logMAR BCVA of 26 eyes was 2.62 ± 0.43. At 7 days, 1 month, and 3 months after IVR + PPV + PPL + PRP + trabeculectomy, the mean logMAR BCVA were 1.30 ± 0.36, 1.29 ± 0.37, 1.29 ± 0.39, respectively. During the follow-up, the increase of logMAR BCVA continued on a significantly improved level compared with baseline at 7 days (t = 5.263, P = 0.003), 1 month (t = 8.362, P = 0.002), and 3 months (t = 7.685, P = 0.001) respectively. At the end of the 12-month follow-up, the mean postoperative logMAR BCVA of 26 eyes was significantly improved as 1.26 ± 0.29, and the difference was statistically significant compared with the preoperative one (t = 6.133, P = 0.002) [Figure 2]. The logMAR BCVA improved in 22 eyes (84.62%), and remained stable in four eyes (15.38%).
Figure 2.
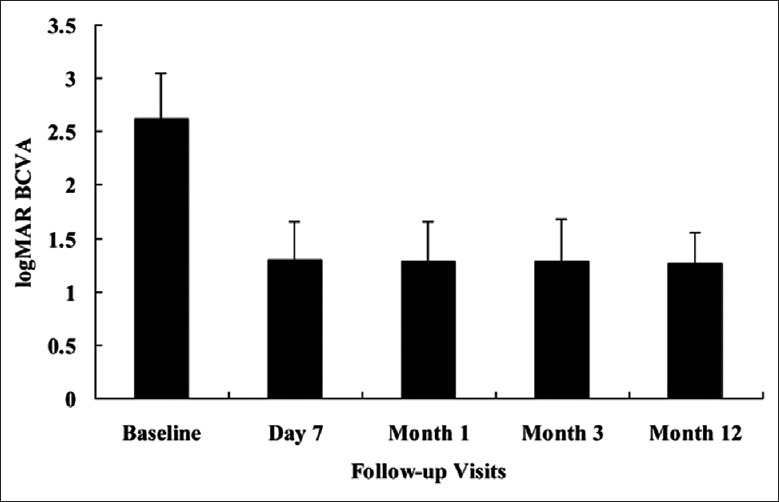
The time course of best-corrected visual acuities converted to logarithm of the minimum angle of resolution (logMAR BCVA) changes. At baseline, the mean logMAR BCVA was 2.62 ± 0.43. The mean postoperative logMAR BCVA revealed a significant improvement compared with baseline at 7 days (1.30 ± 0.36, P = 0.003), 1 month (1.29 ± 0.37, P = 0.002), 3 months (1.29 ± 0.39, P = 0.001), and 12 months (1.26 ± 0.29, P = 0.002) after pars plana vitrectomy + pars plana lensectomy + panretinal photocoagulation + trabeculectomy.
Neovascularization in the iris and the angle
Seven days after the first IVR, the neovascularization in the iris and the angle regressed significantly in all patients. During the complete follow-up visit, the neovascularization in the iris and the angle recurred in three eyes (11.54%) at 6–8 months follow-up and disappeared after reinjection of IVR and further supplemental PRP. The obvious neovascularization in the iris and the angle was not noted in any patients during the following visits.
Complications
The common ocular complications in the early postoperative period were conjunctival hemorrhage and eye pain, fibrin formations in the anterior chamber, choroidal detachment, hypotony, hyphema, vitreous hemorrhage, transient elevation of IOP, and bleb leaks. The most frequently ocular complications were conjunctival hemorrhage and eye pain, which occurred in 10 (38.46%) of the patients. Six cases (23.08%) had fibrin formations in the anterior chamber and were recovery 7 days after topical application of corticosteroids. Five cases (19.23%) had postoperative choroidal detachment and were recovery after topical and systemic application of corticosteroids during the first 5 postoperative days. Four cases (15.38%) had hypotony and were recovery spontaneously 7 days later. Three cases (11.54%) had hyphema and were absorbed completely 7 days later without treatment. Three cases (11.54%) had vitreous hemorrhage and were resolved spontaneously within 14 days without any interventions. The IOP of 3 patients (11.54%) was 26–28 mmHg at 7-day follow-up and dropped to 16–18 mmHg after carteolol eye drops treatment for 7 days. Early bleb leaks occurred in 1 eye (3.85%) and were treated successfully with additional 10–0 nylon sutures.
With respect to late postoperative complications, neovascularization in the iris and the angle recurred in 2 eyes (7.69%) at 6-month follow-up and 1 eye (3.85%) at 8-month follow-up, respectively, and disappeared after reinjection of IVR and further supplemental PRP. Epiretinal membrane formation occurred in 2 eyes (7.69%). The late complications of trabeculectomy, such as hypotony maculopathy, late bleb leak, bleb-associated infection, and corneal decompensation, were not found in any of our patients. Severe complications such as endophthalmitis and phthisis bulbi were not observed during the 12-month follow-up.
With regards to systemic complications, 2 patients (7.69%) described nausea 1 day after ranibizumab injection, and were recovery without any interventions. One patient (3.85%) described symptoms of angina pectoris, which was treated symptomatically without further interventions.
DISCUSSION
Neovascular glaucoma is a severe complication of ocular ischemia diseases and has long been recognized as a very difficult case to manage.[10,11] VEGF concentration in NVG patients is elevated in both the aqueous humor and vitreous humor, which shows ischemic severity.[12,13] As VEGF is a key modulator in the angiogenic cascade, its additional inhibition has been recently proposed as a therapeutic option in the treatment of various diseases including NVG.[14,15] Ranibizumab is a fully humanized murine monoclonal antibody fragment, much smaller than the parent molecule and affinity purified to provide stronger binding and inhibition of a number of isoforms of VEGF-A. Blocking VEGF-A can restrain abnormal new blood vessel formation and decrease the resultant vascular leakage. The drug has been intensively studied for intraocular use and approved for the treatment of wet AMD, diabetic macular edema and macular edema following retinal vein occlusion.[16,17,18,19] Studies have shown that IVR injection is a safe and successful alternative that has led to higher surgical success rates.[20,21] Given the remarkable suppression of neovascularization and hemorrhagic complications, it is speculated that preoperative IVR injection may increase the surgical success rate of PPV + PPL + PRP + trabeculectomy for the treatment of NVG with vitreous hemorrhage. Therefore, our patients all received IVR injection before the subsequent combined surgery. In our study, 26 patients were injected intravitreally with 0.5 mg (0.05 ml) ranibizumab. Our studies have shown that 7 days after the first ranibizumab injection, the neovascularization in the iris and the angle regressed significantly in all patients, which may effectively reduce the anterior chamber hemorrhage and vitreous hemorrhage, and may create better conditions for further surgery and thus improve prognosis. The current results corroborated the findings of previous reports.[22,23]
Studies have reported that the anti-VEGF agents caused temporary regression of the iris neovascularization, but the effect dissipated over time.[24,25] So according to the pathogenesis of NVG, we then performed PPV + PPL + PRP + trabeculectomy simultaneously to treat NVG with vitreous hemorrhage. Studies also have reported that incomplete vitrectomy and incomplete retinal photocoagulation to the peripheral pars plana seem to cause extensive fibrin and worsening rubeosis.[10] Vitreous surgery without preservation of the anterior lens capsule may induce acute production of VEGF and inflammatory cytokines because of retinal damage caused by PRP, which results in an acute immediate worsening of the NVG.[8,10] In our procedure, the purpose of PPL with a preserved anterior capsule was to allow visualization for PPV and PRP completely. In addition, the migration of VEGF and inflammatory cytokines from the posterior to the anterior segment may be partly blocked as a result of preservation of the anterior lens capsule. The purpose of PPV was firstly to remove the vitreous hemorrhage in order to improve visual acuity and avoid the development of ghost cell glaucoma or hemolytic glaucoma. Second, PPV can clear VEGF and inflammatory cytokines in the vitreous cavity, thus reduce the formation of neovascularization. Third, PPV may have beneficial effects upon retinal ischemia by allowing circulation in the vitreous cavity of fluid oxygenated by unaffected retina or other sites in the eye such as the ciliary body. However, removal of the vitreous may allow angiogenic factors into the anterior chamber increasing the risk of NVG; therefore, PRP may be required. Studies have reported that PRP can effectively improve the retinal ischemic condition, thus may prevent the development of NVG.[26,27] The purpose of PRP in our study was to try to counteract retinal ischemia and prevent the production and migration of VEGF and inflammatory cytokines from the posterior to the anterior segment. In addition, PRP can effectively prevent retinal detachment. Some authors applied PRP to the ciliary body to decrease the aqueous humor yield, but the incidence rate of severe hypotony or phthisis bulbi was high.[10] We did not observe hypotony and phthisis bulbi in the current cases because we did not apply PRP to the ciliary body. The purpose of trabeculectomy was to timely relief the transient elevation of IOP after PRP so as to avoid causing further damage to the optic nerve by fluctuations of IOP. It can help protect the remaining visual function of the NVG patients. MMC modulates wound healing by blunting the proliferative phase of fibroblast and endothelial cell growth and replication. The effects of MMC on fibroblasts have been shown to be potent and durable.[28] MMC inhibits fibroblast proliferation and has been shown to increase the success rate of filtering surgery. Trabeculectomy with MMC may play a long-term control of IOP.
At the 12-month follow-up, the mean IOP was 16.68 ± 2.96 mmHg which was significantly lower than the preoperative one. An IOP between 6 mmHg and 21 mmHg without any antiglaucoma medication was maintained in 17 eyes (65.38%). An IOP between 6 mmHg and 21 mmHg with one medication or more was maintained in 5 eyes at 12-month (19.23%). The success rate of our procedure was 84.62% follow-up. Kinoshita et al[10] performed PPV combined with PPL with a preserved anterior capsule, PRP throughout the pars plana, and silicon oil (SO) tamponade (PPV + PPL + PRP + SO tamponade) for NVG. Their success rates (IOP ≤21 mmHg and sustained light perception) were 92.3% after 3 months and 69.2% after 1 year. The current procedure differs from that of Kinoshita et al in that we choose trabeculectomy instead of SO tamponade. Because trabeculectomy can reduce the transient elevated IOP induced by PRP as soon as possible, so as to avoid causing further damage to the optic nerve by fluctuations of IOP and protect the remaining visual function of the NVG patients. However, SO tamponade may lead to the elevation of IOP, which probably result in irreversible loss of vision. Marey et al[29] reported the effect of preoperative IVB injection before trabeculectomy for NVG. The results showed that the mean preoperative IOP dropped from 41.45 ± 5.89 mmHg to 19.3 ± 5.5 mmHg and 17.75 ± 3.74 mmHg at 6 months and 12 months postoperatively, respectively. The major difference between our study and theirs was that our patient's condition was more serious because of vitreous hemorrhage, we performed more such as PPV, PPL with a preserved anterior capsule and PRP to treat NVG with vitreous hemorrhage. The aim of our current procedure was to perform PPV combined with PPL with a preserved anterior capsule as early as possible, thus applying PRP as soon as possible, so as to prevent the formation of neovascularization, reduce elevated IOP and protect the remaining visual function to the extreme extent.
At the 12-month follow-up, the mean postoperative logMAR BCVA of 26 eyes was significantly improved as 1.26 ± 0.29, and the difference was statistically significant compared with baseline. The logMAR BCVA improved in 22 eyes (84.62%), and remained stable in four eyes (15.38%). Our results were similar to the results of Kolomeyer et al.[30] They performed combined PPV and Baerveldt tube insertion procedure in eyes with NVG. Their results showed that logMAR visual acuities at 18-, 24-, 36-, and 48-month follow-up time points were significantly better than preoperative vision (P < 0.05). But in their study, 14 eyes (16%) had a final visual acuity of no light perception. The major difference between our technique and theirs was that PRP was applied in our surgical technique. Since NVG develops secondarily to ocular ischemia and neovascularization. A prompt application of PRP to alleviate the effects of ischemia is very important for the treatment of NVG. PRP as soon as possible may solve the fundamental problem of retinal ischemia, reduce the possibility of recurrence of neovascularization, so as to preserve the remaining visual function to the extreme extent in patients with NVG with vitreous hemorrhage. IVR + PPV + PPL + PRP + trabeculectomy have effectively prevented the patients with visual loss, so as to improve the quality of life of patients in our studies. There were no cases of serious ocular or systemic complications occurred during 12-month of the study.
In conclusion, although NVG with vitreous hemorrhage remains very hard to treat, there may be a chance to control the IOP and preserve the remaining visual function. Our findings showed that IVR + PPV + PPL + PRP + trabeculectomy can control IOP well and improve BCVA without severe complications for NVG with vitreous hemorrhage. But its long-term safety and effectiveness still need further observation.
Footnotes
Edited by: Xin Chen
Source of Support: This study was supported by grants from the Projects of Henan Health Department (No. 201304007) and Henan Science and Technology Department (No. 142102310110).
Conflict of Interest: None declared.
REFERENCES
- 1.An TS, Kwon SI. Neovascular glaucoma due to branch retinal vein occlusion combined with branch retinal artery occlusion. Korean J Ophthalmol. 2013;27:64–7. doi: 10.3341/kjo.2013.27.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakabayashi Y, Usui Y, Okunuki Y, Ueda S, Kimura K, Muramatsu D, et al. Intraocular VEGF level as a risk factor for postoperative complications after vitrectomy for proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:6403–10. doi: 10.1167/iovs.12-10367. [DOI] [PubMed] [Google Scholar]
- 3.Noma H, Mimura T, Yasuda K, Shimura M. Vascular endothelial growth factor and its soluble receptors-1 and -2 in iris neovascularization and neovascular glaucoma. Ophthalmologica. 2014;232:102–9. doi: 10.1159/000360303. [DOI] [PubMed] [Google Scholar]
- 4.Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 2014;28:510–20. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito Y, Higashide T, Takeda H, Ohkubo S, Sugiyama K. Beneficial effects of preoperative intravitreal bevacizumab on trabeculectomy outcomes in neovascular glaucoma. Acta Ophthalmol. 2010;88:96–102. doi: 10.1111/j.1755-3768.2009.01648.x. [DOI] [PubMed] [Google Scholar]
- 6.Elmekawey H, Khafagy A. Intracameral ranibizumab and subsequent mitomycin C augmented trabeculectomy in neovascular glaucoma. J Glaucoma. 2014;23:437–40. doi: 10.1097/IJG.0b013e3182946398. [DOI] [PubMed] [Google Scholar]
- 7.Fong AW, Lee GA, O’Rourke P, Thomas R. Management of neovascular glaucoma with transscleral cyclophotocoagulation with diode laser alone versus combination transscleral cyclophotocoagulation with diode laser and intravitreal bevacizumab. Clin Experiment Ophthalmol. 2011;39:318–23. doi: 10.1111/j.1442-9071.2010.02449.x. [DOI] [PubMed] [Google Scholar]
- 8.Ajvazi H, Goranci I, Lutaj P. Management of neovascular glaucoma. Oftalmologia. 2013;57:39–43. [PubMed] [Google Scholar]
- 9.Li Z, Zhou M, Wang W, Huang W, Chen S, Li X, et al. A prospective comparative study on neovascular glaucoma and non-neovascular refractory glaucoma following Ahmed glaucoma valve implantation. Chin Med J. 2014;127:1417–22. [PubMed] [Google Scholar]
- 10.Kinoshita N, Ota A, Toyoda F, Yamagami H, Kakehashi A. Surgical results of pars plana vitrectomy combined with pars plana lensectomy with anterior capsule preservation, endophotocoagulation, and silicon oil tamponade for neovascular glaucoma. Clin Ophthalmol. 2011;5:1777–81. doi: 10.2147/OPTH.S26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lüke J, Nassar K, Lüke M, Grisanti S. Ranibizumab as adjuvant in the treatment of rubeosis iridis and neovascular glaucoma – Results from a prospective interventional case series. Graefes Arch Clin Exp Ophthalmol. 2013;251:2403–13. doi: 10.1007/s00417-013-2428-y. [DOI] [PubMed] [Google Scholar]
- 12.Kuzmin A, Lipatov D, Chistyakov T, Smirnova O, Arbuzova M, Ilin A, et al. Vascular endothelial growth factor in anterior chamber liquid patients with diabetic retinopathy, cataract and neovascular glaucoma. Ophthalmol Ther. 2013;2:41–51. doi: 10.1007/s40123-013-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altintas AG, Arifoglu HB, Tutar E, Koklu G, Ozcan PY. Effect on anterior chamber bevacizumab injection combined with seton implantation in treatment of rubeosis iridis in neovascular glaucoma. Cutan Ocul Toxicol. 2012;31:124–7. doi: 10.3109/15569527.2011.621917. [DOI] [PubMed] [Google Scholar]
- 14.Li XJ, Zhang JS. Intravitreal bevacizumab injection for chronic central serous chorioretinopathy. Chin Med J. 2010;123:2145–7. [PubMed] [Google Scholar]
- 15.Waisbourd M, Shemesh G, Kurtz S, Rachmiel R, Moisseiev E, Zayit-Soudri S, et al. Topical bevacizumab for neovascular glaucoma: A pilot study. Pharmacology. 2014;93:108–12. doi: 10.1159/000358600. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 17.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124–33.e1. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Zhou MW, Wang W, Huang WB, Chen SD, Li XY, Gao XB, et al. Adjunctive with versus without intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in the treatment of neovascular glaucoma. Chin Med J. 2013;126:1412–7. [PubMed] [Google Scholar]
- 21.Nourinia R, Ahmadieh H, Shahheidari MH, Zandi S, Nakao S, Hafezi-Moghadam A. Intravitreal fasudil combined with bevacizumab for treatment of refractory diabetic macular edema; a pilot study. J Ophthalmic Vis Res. 2013;8:337–40. [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T, Inatani M, Takihara Y, Awai-Kasaoka N, Ogata-Iwao M, Tanihara H. Prognostic risk factors for failure of trabeculectomy with mitomycin C after vitrectomy. Jpn J Ophthalmol. 2012;56:464–9. doi: 10.1007/s10384-012-0171-2. [DOI] [PubMed] [Google Scholar]
- 23.Tu Y, Fay C, Guo S, Zarbin MA, Marcus E, Bhagat N. Ranibizumab in patients with dense cataract and proliferative diabetic retinopathy with rubeosis. Oman J Ophthalmol. 2012;5:161–5. doi: 10.4103/0974-620X.106099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park UC, Shin JY, McCarthy LC, Kim SJ, Park JH, Chung H, et al. Pharmacogenetic associations with long-term response to anti-vascular endothelial growth factor treatment in neovascular AMD patients. Mol Vis. 2014;20:1680–94. [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy AK, Cabrera M, Yeh S, Davis JL, Albini TA. Optical coherence tomography-guided ranibizumab injection for cystoid macular edema in well-controlled uveitis: Twelve-month outcomes. Retina. 2014;34:2431–8. doi: 10.1097/IAE.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 26.Tatsumi T, Yamamoto S, Uehara J, Sugawara T, Baba T, Inoue M, et al. Panretinal photocoagulation with simultaneous cryoretinopexy or intravitreal bevacizumab for neovascular glaucoma. Graefes Arch Clin Exp Ophthalmol. 2013;251:1355–60. doi: 10.1007/s00417-012-2236-9. [DOI] [PubMed] [Google Scholar]
- 27.Wittström E, Holmberg H, Hvarfner C, Andréasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. Eur J Ophthalmol. 2012;22:563–74. doi: 10.5301/ejo.5000089. [DOI] [PubMed] [Google Scholar]
- 28.Matlach J, Panidou E, Grehn F, Klink T. Large-area versus small-area application of mitomycin C during trabeculectomy. Eur J Ophthalmol. 2013;23:670–7. doi: 10.5301/ejo.5000287. [DOI] [PubMed] [Google Scholar]
- 29.Marey HM, Ellakwa AF. Intravitreal bevacizumab with or without mitomycin C trabeculectomy in the treatment of neovascular glaucoma. Clin Ophthalmol. 2011;5:841–45. doi: 10.2147/OPTH.S21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolomeyer AM, Seery CW, Emami-Naeimi P, Zarbin MA, Fechtner RD, Bhagat N. Combined pars plana vitrectomy and pars plana Baerveldt tube placement in eyes with neovascular glaucoma. Retina. 2015;35:17–28. doi: 10.1097/IAE.0000000000000235. [DOI] [PubMed] [Google Scholar]


