INTRODUCTION
Brain edema is a serious clinical event and could cause various neurological symptoms such as dizziness and headache. Drugs frequently used to relieve brain edema include steroid, dehydrant (e.g., mannitol), and diuretics. But the effects of these drugs were limited in patients with severe edema. Bevacizumab has been applied in the treatment of cerebral radiation necrosis.[1] Case studies have reported on the application of bevacizumab in the treatment of severe brain edema.[2] In the present study, we describe significant effects of bevacizumab on severe brain edema in patients with re-irradiation.
MATERIALS AND METHODS
All 10 patients received re-irradiation for brain tumors in the same position. The use of mannitol and hormones could not relieve the severe edema, and the quality of life was poor for these patients. Bevacizumab was administered to treat the severe edema for them. We received written informed patient consent for this procedure. The dosage of bevacizumab was given 5 mg·kg−1·time−1. Magnetic resonance imaging (MRI) was performed before and after the use of bevacizumab.
The extent of edema was measured using the method of Williamson et al.[3] Three maximum edema diameters were measured in the X, Y, and Z directions in T2-weighted MRI images. The volume was estimated using the oblate spheroid formula (π/6 × XYZ).
Equipment: The “moon God” gamma knife manufactured by Shenzhen ET Medical Group of China was used for stereotactic radiosurgery (SRS) of single-fraction irradiation. A CyberKnife manufactured by Accuray in America was used for fractionated stereotactic radiotherapy of 3–5 fractions irradiation. A 23EX medical linear accelerator manufactured by Varian in America was used for intensity-modulated radiotherapy and whole brain radiotherapy.
Statistics: Paired t-test was used to conduct the Karnofsky Performance scale (KPS) score and edema volume between before and after treatment.
RESULTS
From February 2012 to November 2012, 10 patients with severe brain edema were treated with bevacizumab. The patients’ characteristics are shown in Table 1.
Table 1.
Patient's characteristics
| Patient number | Sex | Age (years) | Diagnosis | Primary radiotherapy dose/fraction | Re-irradiation dose/fraction | Re-irradiation site and tumor size (cm) | Radiotherapy interval (weeks) | Began bevacizumab to re-irrediation day 1 (weeks) | Dexamethasone and mannitol before bevacizumab (weeks)* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 70 | Adenoid cystic | 56 Gy/25 f | 25 Gy/5 f | Temporal lobe (4×2.5) | 103 | 25.6 | 7.1 |
| carcinoma | |||||||||
| 2 | Male | 54 | Colon cancer | 40 Gy/20 f | 16 Gy/1 f | Parietal lobe (1.5×1.2) | 6 | 61 | 10.7 |
| Temporal lobe (1.2×1.0) Cerebella (1.0×1.0) | |||||||||
| 3 | Female | 64 | Esophageal | 40 Gy/20 f | 17 Gy/1 f | Occipital lobe (3.0×2) | 26 | 0.6 | 3.4 |
| cancer | |||||||||
| 4 | Female | 48 | Glioblastoma | 63 Gy/35 f | 25/5 f | Frontal lobe (5.0×3.5) | 138 | 18.1 | 11.7 |
| 5 | Male | 30 | Anaplastic | 59.4/33 f | 24/5 f | Frontal horns of lateral | 283.3 | 1.3 | 4.1 |
| astrocytoma | ventricles (5.0×3.0) | ||||||||
| 6 | Male | 62 | Glioblastoma | 60 Gy/30 f | 12 Gy/3 f | Occipital lobe (3.0×1.5) | 13.7 | 4.9 | 6.6 |
| 7 | Male | 51 | Glioblastoma | 60 Gy/30 f | 50.4 Gy/28 f | Frontal lobe (4.5×3.0) | 112 | 4.6 | 1.9 |
| 8 | Male | 55 | SCLC brain | 40 Gy/20 f | 16 Gy/1 f | Parietal lobe (3.0×2.0) | 38.4 | 11.7 | 9.3 |
| metastases | |||||||||
| 9 | Male | 60 | Anaplastic | 60 Gy/30 f | 24 Gy/5 f | Occipital lobe (3.5×3.2) | 25.9 | 65.7 | 12.9 |
| astrocytoma | |||||||||
| 10 | Male | 74 | Glioblastoma | 50.4 Gy/28 f | 10 Gy/1 f | Temporal insula (4.0×3.0) | 7.3 | 3.1 | 2.1 |
*The dose of dexamethasone was 5 mg, 1–2 times/day, and the dose of mannitol was 125 ml, 2–4 times/day. SCLC: Small cell lung cancer.
New tumor lesions were found in 2 patients more than 60 weeks. Severe brain edema may have been induced by both the tumor and re-irradiation in 2 patients. Dexamethasone and mannitol had been administrated before bevacizumab to patients for long time. The median time of follow-up was 6.5 months (3–18 months) for the survivors. There were 4 patients died include 2 from extracranial disease progression, 1 from intracranial disease progression, and 1 from bleeding of the wound surface in nasal cavity.
Administration and the efficacy of bevacizumab
The median times of bevacizumab treated for each patient was 3 times (range: 2–4 times). The median time between the initial use of bevacizumab and the 1st day (day 1) of the re-irrradiation was 8.3 weeks (0.6–61 weeks). The symptoms and physical signs improved significantly after treatment of bevacizumab. No. 1 patient died before the MRI re-examination. The KPS scoring and edema size were not included in the statistical analysis. MRI re-examination was carried out in the other patients after received bevacizumab. The median KPS scores before and after bevacizumab were 40 (30–60) and 70 (50–80), respectively (paired t-test P = 0.0002). Cerebral MRI indicated significant relief of edema. The median volume of the edema region on T2-weighted images before and after medication was 300.1 ml (116–382.5 ml) and 33.2 ml (7.2–321 ml), respectively (paired t-test P = 0.005). The results are shown in Table 2.
Table 2.
Dosage and effects of bevacizumab and follow-up
| Patient | Weight (kg) | Bevacizumab dosage (mg) | Edema volume before bevacizumab (ml) | Minimum edema volume after bevacizumab (ml) and detection time (weeks) | KPS before bevacizumab* | KPS after bevacizumab* | Bevacizumab administration time (weeks) | Follow-up (months) | State |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 | 400 | 188.9 | – | 40 | – | 0.1, 4 | 1 | Dead |
| 2 | 58 | 300 | 116.0 | 7.2 (4) | 60 | 80 | 1, 3 | 5 | Dead |
| 3 | 42 | 200 | 165.3 | 84.8 (4) | 50 | 70 | 1.6, 3.6, 6 | 11 | Dead |
| 4 | 65 | 300 | 300.1 | 49.9 (9.7) | 40 | 70 | 0.1, 2, 9.9 | 18 | Alive |
| 5 | 75 | 300 | 382.5 | 22.5 (25) | 30 | 60 | 3.1, 6.5, 11, 20.4 | 14 | Dead |
| 6 | 83 | 400 | 379.8 | 32.1 (7.1) | 30 | 70 | 0.1, 2, 1 | 10 | Alive |
| 7 | 78 | 400 | 377.1 | 321.0 (3.9) | 30 | 50 | 0.3, 3.6, 8.2 | 4 | Alive |
| 8 | 70 | 400 | 254.4 | 33.2 (10.1) | 40 | 80 | 3.3, 5.3, 9.1, 11.7 | 9 | Alive |
| 9 | 72 | 400 | 322.5 | 155.5 (6.7) | 20 | 50 | 2.3, 7.7 | 4 | Alive |
| 10 | 62 | 300 | 245.8 | 168.7 (2.9) | 20 | 40 | 0.6, 3 | 3 | Alive |
*Matched for the MRI detect time before and after bevacizumab. MRI: Magnetic resonance imaging; KPS: Karnofsky performance scale.
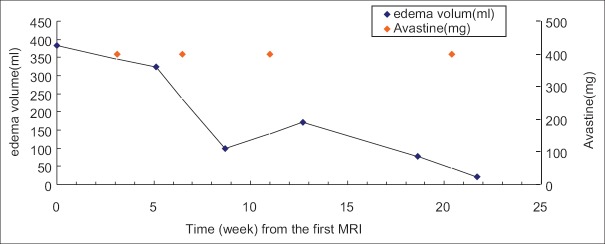
Bevacizumab significantly relieved severe edema induced by SRS using the CyberKnife to treat the recurrent disease for no. 5 patient. The CyberKnife treatment was 0.2 weeks and 19.1 weeks after the check time of the first MRI. Bevacizumab was given at 3.1 weeks, 6.5 weeks, 11 weeks, and 20.4 weeks. The edema volume before the initial use of bevacizumab was 382.5 ml and declined significantly after treatment each times: 323.1 ml (5.1 weeks), 97.8 ml (8.7 weeks), 171.7 ml (12.7 weeks), 79.0 ml (18.6 weeks), and 22.5 ml (21.7 weeks) [Figures 1 and 2].
Figure 1.
Edema volume and bevacizumab treatment time of no. 5 patient.
Figure 2.
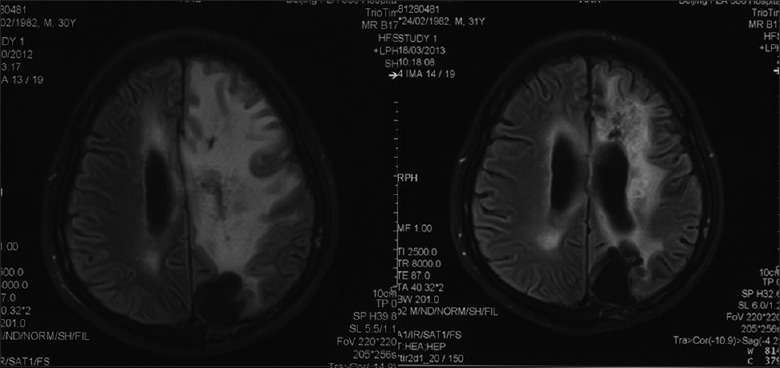
T2-weighted magnetic resonance imaging of no. 5 patient before bevacizumab treatment (left) and 25 weeks later (right).
Major toxicity
No. 1 patient dead from bleeding of the maxillary sinus wound. The effect of bevacizumab on edema was significant for the 1st time in this patient. Bevacizumab was administered 28 days later. The drug caused sudden large bleeding from the maxillary sinus wound the next morning and the patient died from asphyxia.
Cerebral infarction: Two patient with brain metastasis presented with slight to medium cerebral infarction. No. 2 patient had Colon cancer and brain metastasis and received bevacizumab on July 6 and 20, 2012. A region of cerebral infarction measuring 2 cm × 4 cm appeared at the internal capsule on the left on July 29, 2012. Muscular strength of the right limb declined to Level 3 and then recovered to Level 4 1 month later. No. 3 patient received bevacizumab on July 6 and 30, 2012. On October 14, 2012, the patient lose consciousness and in a coma for 3 days. The brain MRI indicated a region of cerebral infarction measuring 3 cm × 3 cm in the right temporal lobe. After 1-week of symptomatic treatment, the patient recovered completely.
Hypertension and hyperthermia. No. 7 patient received bevacizumab 10 times for treating gliomas before re-irradiation. After each time, He experienced low fever with a maximum temperature of 37.5°C. The symptoms disappeared spontaneously after 3 days each time. The patient had no history of hypertension and did not develop hypertension during the treatment. After re-irradiation, bevacizumab was administrated once more for severe edema and caused blood pressure increasing to 150–160 mmHg/100–110 mmHg. Blood pressure decreased spontaneously to 115 mmHg/85 mmHg 4 weeks later without using antihypertensive agents. Bevacizumab treated for edema again 4.6 weeks later, and blood pressure did not increase.
DISCUSSION
Wang et al.[1] reported the significant efficacy of bevacizumab in the treatment of cerebral necrosis. The KPS of patients improved, and the dosage of steroid was decreased, possibly because of the relieving effect of bevacizumab on brain edema. Williams et al.[2] reported that bevacizumab had significant efficacy for treating brain edema after SRS in 1 patient. Our study included 10 patients who suffered severe brain edema after re-irradiation without the effect of steroid and mannitol. The treatment of bevacizumab quickly relieved the patients’ symptoms, and improvements of the living status were significant on the 2nd day in our study. The treatment of bevacizumab allowed us reducing the dosage of hormone and mannitol for patient with severe brain edema[4] and improving their life quality.
For no. 1 patient, the efficacy lasted for 3 weeks after the first treatment of bevacizumab until the symptoms worsened. No. 4 patient received 2 times treatment of bevacizumab within 4 weeks, and the effect keep for 3–4 months until the neurological symptoms associated with cerebral edema began worsen. For no. 5 patient, bevacizumab was given 4 times in 25 weeks, and the brain edema became very slight for a long time. The dose of bevacizumab in this paper was about 5 mg/kg with a relatively long interval.
The severe brain edema of 2/10 patients in our study may be caused by tumor and irradiation. Bevacizumab may be had an effect on both factors. Re-irradiation to the same site is the higher risk factor than irradiation for the 1st time that induced severe brain edema. Other factors that induced severe brain edema include irradiation dose, tumor size, tumor site and individual difference, etc. Anyway, bevacizumab could relief the brain edema significant in sorts of patients.
Toxicity
Authors reported that bevacizumab could cause various degrees of bleeding. Yoshida et al.[5] reported the use of bevacizumab combined with chemotherapy treating 26 patients with unresectable or metastatic colorectal cancer 1 of whom experienced grade 3 cerebral hemorrhage. Nagane et al.[6] reported bevacizumab treating 31 cases of recurrent malignant gliomas and only 1 case of asymptomatic cerebral hemorrhage with unnecessary of treatment. In our study, 1 patient with adenoid cystic carcinoma of the sinus experienced fatal hemorrhage from the wound of nasal cavity after the administration of bevacizumab 2nd times indicating that bevacizumab should be used with caution in all patients and banned for patient with wound.
There is a clear correlation between cerebral infarction and bevacizumab although the conclusion is based on individual cases.[7] However, the large-sample-based data analyses by Campbell et al.[8,9] indicated that the incidence of ischemic stroke was not significantly different between the bevacizumab treating group and control group. Fraum et al.[10] analyzed 8 cases of ischemic stroke and concluded that the possible risk factors include hypertension, deep vein thrombosis, pulmonary embolism, arrhythmia, and dyslipidemia. In our study, no. 2 patient was diagnosed with diabetes while no. 3 patient without.
Other toxicity of bevacizumab
A meta-analysis by Amit et al.[11] reported that the adverse reactions associated with bevacizumab also included proteinuria (risk ratio [RR] 7.08, P < 0.00001) and hypertension (RR 4.96, P < 0.00001). One patient in our study experienced hypertension, and none developed proteinuria.
In summary, the treatment of bevacizumab is effective on severe brain edema induced by re-irradiation. Bevacizumab is safe for patients whom without wounds. Our research suggests a new clinical indication of bevacizumab as treating for severe brain edema should be taken into account.
Footnotes
Edited by: Yi Cui
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wang Y, Pan L, Sheng X, Mao Y, Yao Y, Wang E, et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res. 2012;17:25. doi: 10.1186/2047-783X-17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams BJ, Park DM, Sheehan JP. Bevacizumab used for the treatment of severe, refractory perilesional edema due to an arteriovenous malformation treated with stereotactic radiosurgery. J Neurosurg. 2012;116:972–7. doi: 10.3171/2012.1.JNS111627. [DOI] [PubMed] [Google Scholar]
- 3.Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: A pilot study. Stereotact Funct Neurosurg. 2008;86:359–66. doi: 10.1159/000163557. [DOI] [PubMed] [Google Scholar]
- 4.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida M, Goto M, Kii T, Nishitani H, Kawabe S, Kuwakado S, et al. Retrospective study as first-line chemotherapy combined anti-VEGF antibody with fluoropyrimidine for frail patients with unresectable or metastatic colorectal cancer. Digestion. 2013;87:59–64. doi: 10.1159/000343943. [DOI] [PubMed] [Google Scholar]
- 6.Nagane M, Nishikawa R, Narita Y, Kobayashi H, Takano S, Shinoura N, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;42:887–95. doi: 10.1093/jjco/hys121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besozzi G, Ferrara A, Epifani E, Intini D, Apruzzese M, Provenzano A, et al. Acute stroke after intravitreal bevacizumab to treat choroidal neovascularization due to angioid streaks in pseudoxanthoma elasticum: A severe systemic adverse event after an off-label procedure. Int Ophthalmol. 2013;33:181–3. doi: 10.1007/s10792-012-9647-9. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RJ, Bell CM, Paterson JM, Bronskill SE, Moineddin R, Whitehead M, et al. Stroke rates after introduction of vascular endothelial growth factor inhibitors for macular degeneration: A time series analysis. Ophthalmology. 2012;119:1604–8. doi: 10.1016/j.ophtha.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Campbell RJ, Gill SS, Bronskill SE, Paterson JM, Whitehead M, Bell CM. Adverse events with intravitreal injection of vascular endothelial growth factor inhibitors: Nested case-control study. BMJ. 2012;345:e4203. doi: 10.1136/bmj.e4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraum TJ, Kreisl TN, Sul J, Fine HA, Iwamoto FM. Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. J Neurooncol. 2011;105:281–9. doi: 10.1007/s11060-011-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amit L, Ben-Aharon I, Vidal L, Leibovici L, Stemmer S. The impact of Bevacizumab (Avastin) on survival in metastatic solid tumors – A meta-analysis and systematic review. PLoS One. 2013;8:e51780. doi: 10.1371/journal.pone.0051780. [DOI] [PMC free article] [PubMed] [Google Scholar]