Scedosporium apiospermum (S. apiospermum, sexual form of Pseudallescheria boydii) is a highly invasive and opportunistic pathogen. It can invade various organs of the body, causing lethal infections. S. apiospermum is widely distributed in natural environments, including marshes, wetlands, sewage, putrilage, and salt waters. The incidence of S. apiospermum infection can be especially high in humid temperate and subtropical regions.[1] In recent years, the incidence of S. apiospermum infection has shown an increasing trend in immune-compromised populations, including HIV/AIDS patients, organ transplant recipients, and patients who have received corticosteroids or immunosuppressive drugs for a long period of time. Furthermore, it can occur in individuals with normal immune function under specific conditions such as trauma and near-drowning.[2,3,4,5] Here, we report two patients who had an invasive systemic infection with S. apiospermum after near-drowning associated with contaminated water.
Case 1 is a 36-year-old male. The patient had no previous history of any disease. On March 3, 2008, as a maintenance worker, he was buried in an obviously contaminated sludge water for 10 min during a sewer explosion. He was rushed to our hospital and admitted to the Emergency Intensive Care Unit. At admission, the patient was in mild-coma, had lip cyanosis, and auscultatory coarse breath sounds in both lungs with high-pitched wet crackles. Heart rate was 143 beats/min, and the cardiac rhythm was normal. White blood cell count (WBC) was 17.92 × 109/L and neutrophils was 79.5%. Arterial blood gas analysis showed pH 7.28, PaCO2 27.5 mmHg and PaO2 95.3 mmHg (FiO2 60%). Chest X-ray showed infiltrative changes in both lungs, particularly in the left. The patient was given intra-tracheal intubation and mechanical ventilation. He was later diagnosed with acute respiratory distress syndrome and acute pulmonary edema.
Bronchoscopy performed on day 1 showed hyperemia, edema, and hemorrhage in the bronchial walls which were particularly vivid in the left lung. Bronchoalveolar lavage showed muddy broth-like fluid, and bronchoalveolar lavage fluid (BALF) was cultured for microorganisms. He received imipenem/cilastatin sodium (0.5 g, intravenous [iv], Q6h) to empirical anti-infection regimen. On day 2, caspofungin acetate was added to his empirical anti-fungal infection regimen. On day 4, pulmonary computed tomography (CT) indicated obvious infiltrative changes in both lungs, indicating pulmonary infection [Figure 1a]. Two days later, the patient was smoothly weaned off the ventilator.
Figure 1.
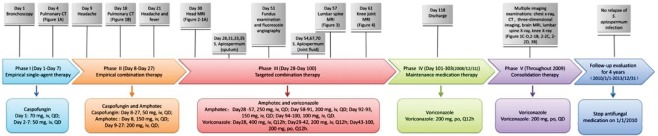
Lung computed tomography findings: (a) Multiple dot- and cloud-like shadows were visible along the bronchovascular bundle. Patchy hyperdense shadows were seen at the posterior segment of the upper lobe of the left lung and the posterior segment of the lower lobes (March 7, 2008). (b) Diffuse round nodules and patchy shadows were seen in both lungs. Most nodules had small cavities with central necrosis, whereas a Halo sign was observed in a small number of nodules (March 21, 2008). (c) Diffuse round nodules and patchy shadows were seen in both lungs. The number of lesions was decreased (May 27, 2008). (d) Diffuse round nodules and patchy shadows were seen in both lungs, but became less obvious or decreased and were gradually absorbed (October 23, 2008).
On day 9, he experienced headache, which was spontaneously resolved without specific treatment. He experienced intermittent fever (maximum temperature [Tmax], 39°C], shortness of breath, and cough during this period. A second pulmonary CT taken on day 18 showed disease progression in both lungs [Figure 1b]. On day 21, he suffered from headache and fever with temperature 39°C again, although no nausea or vomiting was noted. The symptoms improved after analgesic treatment. On day 30, the patient complained of headache again with fever of 39.1°C and diuresis, the urine volume was up to 9950 ml/24 h consecutively for about a week. Cephalic region magnetic resonance imaging (MRI) was performed [Figure 2a], from which multiple intracranial abscesses were identified. During treatment, repeated sputum cultures for days 28, 31, 33, and 35, respectively, were confirmed positive for S. apiospermum, but the BALF culture was negative. On day 44, his left eye became swollen and painful, along with blurred vision and photophobia tenderness. Four days later, he developed painful swelling in the right knee, which had normal skin temperature and no obvious local redness. However, knee's range of motion became restricted, and a luxating patella test showed a positive sign. On day 50, he developed lower back pain, with restricted movement. On day 51, the left eye symptoms became more severe, and visual acuity dramatically decreased. Eventually, fungal endophthalmitis was diagnosed by fundus examination and fluorescein angiography. But the patient refused vitreous biopsy or vitrectomy, despite the progressive visual limitations he experienced and evidenced by only having light perception in his left eye. On day 54, his right knee arthrocentesis showed hemorrhagic effusion. Three joint fluid cultures, on days 54, 67, and 70, respectively, showed the presence of S. apiospermum. Lumbar spine MRI was performed on day 57 and showed that there were pathological changes in L4 and L5 vertebrae and L4/L5 and L5/S1 intervertebral disks, indicating vertebral osteomyelitis and intervertebral disk inflammation [Figure 3].
Figure 2.
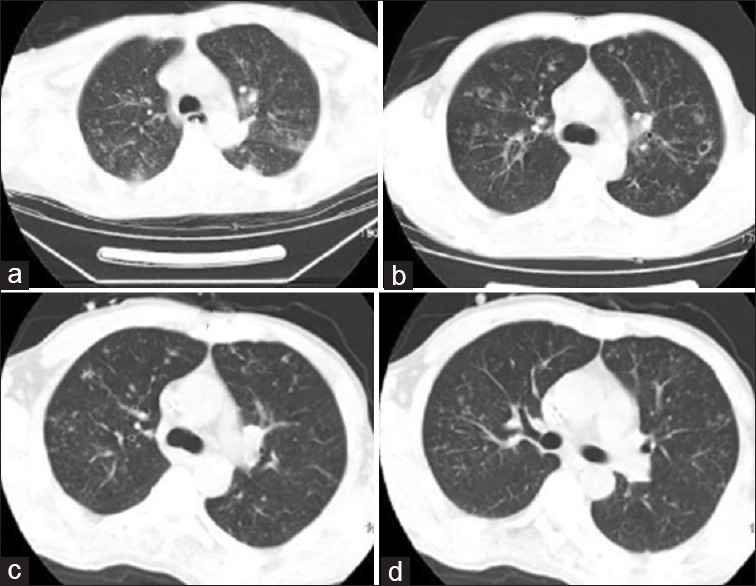
Magnetic resonance imaging findings: (a) Multiple circular hyperdense intracranial lesions of varying sizes (April 1, 2008). (b) These lesions became smaller, showing dotty hyperdense lesions, and some of them disappeared (May 26, 2008).
Figure 3.
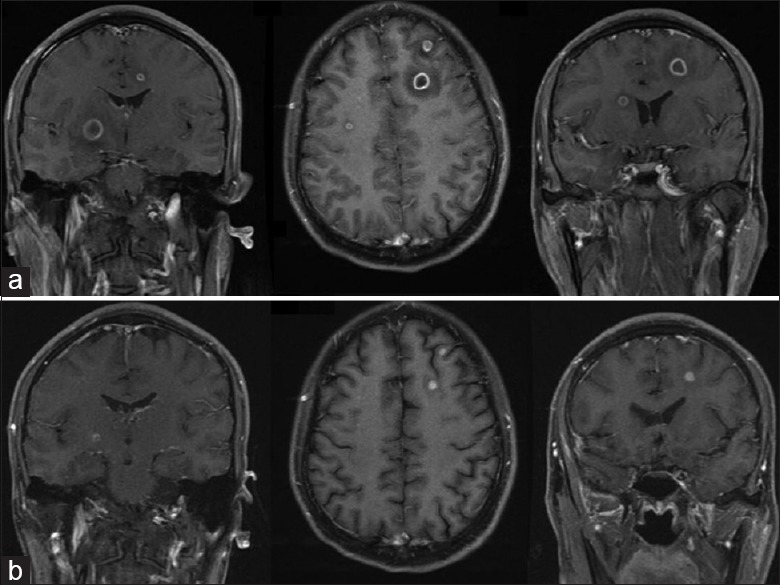
Lumbar spine magnetic resonance imaging: (a) Fat-suppressed T2-weighted imaging (WI), T1-WI, and T2-WI showed pathological changes in L4 and L5 vertebrae and L4/L5 and L5/S1 intervertebral disks, showing vertebral osteomyelitis and diskitis (May 29, 2008). (b) Changes in lumbar vertebrae during the recovery period (October 13, 2008).
The patient was finally diagnosed with multiple S. apiospermum infection based on the history of near-drowning associated with contaminated water, lesions in the lungs, central nervous system, eyes, knee joint, lumbar vertebrae, the positive cultures of S. apiospermum of sputum and especially the later onset of knee joint effusion.
Anti-fungal treatment was adjusted based on the symptoms, the level of (1-3)-β-D-glucan (from 20.5 to 170 pg/ml) and fungal culture results. Phase I: Empirical single-agent treatment (from days 2 to 7): Caspofungin only. Phase II: Empirical combination therapy (from days 8 to 27): Caspofungin and amphotericin B cholesteryl sulfate complex for injection (amphotec). Phase III: Targeted combination therapy (from days 28 to 100): Amphotec and voriconazole. Phase IV: Maintenance medication (from day 101 to day 304 [December 31, 2008]) voriconazole. Phase V: Consolidation therapy (from days 305 to day 669 [throughout 2009]): Voriconazole. Total doses of caspofungin, amphotec, voriconazole were 1370 mg, 192,500 mg, and 170,800 mg, respectively.
During this period, he received multiple imaging examinations including plain chest X-ray, chest CT [Figure 1c and d], brain MRI [Figure 2b], plain lumbar spine X-ray, sagittal view [Figure 3b], and knee X-ray, indicating the abatement of the severity of the disease at these sites and the gradual return to normal. Anti-fungal medication was halted on day 670 (January 1, 2010). No relapse of S. apiospermum infection occurred during a 5-year follow-up till December 31, 2014 [Figure 4].
Figure 4.
Case 1 diagnosis and treatment flow chart.
Case 2 is a 29-year-old male. The patient had no previous history of any disease. On September 7, 2010, he accidentally fell into a contaminated biogas digester and submerged about 10 min before rescuing. He regained consciousness about 10 h later. Afterward, he suffered from persistent fever (Tmax, 39.5°C), which did not resolve after symptomatic treatment. Two days later, he started to show signs of hemoptysis (about 200 ml/d). Eight days after the near-drowning, he was transferred to our hospital.
After admission, on day 1, the patient had a fever, his body temperature was 38.8°C and was consciously weak with very minimum awareness of his environment and also had an anemic pale skin appearance. The breath sounds heard in both lungs were coarse, and moist rales were significantly audible. WBC was 21.3 × 109/L, neutrophils was 93.1%, hemoglobin was 93 g/L, and platelet count was 23.5 × 109/L. Arterial blood gas analysis showed pH 7.33, PaCO2 25.4 mmHg and PaO2 53.8 mmHg (FiO2 50%). Lung CT performed on day 1 elucidated multiple cavities and patchy infiltration in both lungs [Figure 5a]. The diagnosis at admission was severe pulmonary infection and acute respiratory distress syndrome. Bronchoscopy visually showed bronchial mucosa congestion and edema. Bronchoalveolar lavage evidenced for muddy blood-like fluid. BALF, sputum, and blood specimens were cultured for pathogens.
Figure 5.
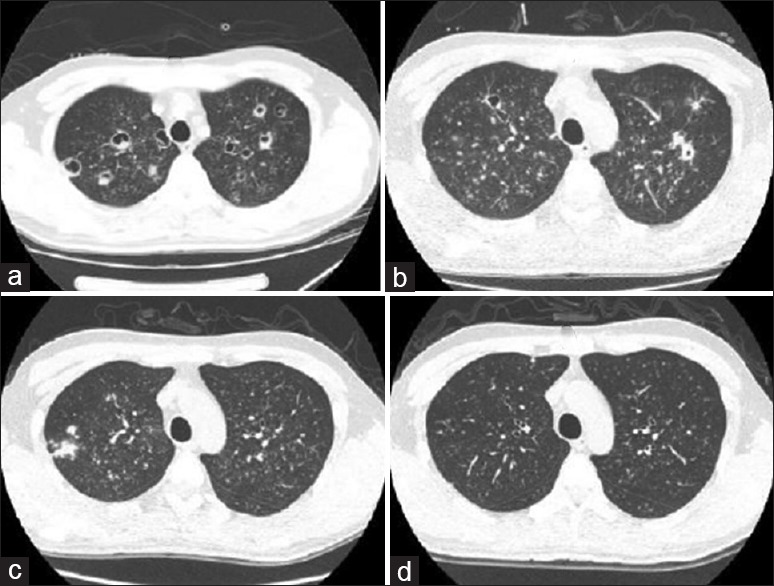
Chest computed tomography: (a) Various bronchial vascular bundles were found in both lungs. They were scattered with multiple cavities. Diffuse cloud-like and fuzzy dot-like hyperdense shadows were found in both lungs, suggesting the presence of fungal infection (September 15, 2010). (b) Various bronchial vascular bundles were found in both lungs. They were scattered with multiple cavities. Diffuse cloud-like and fuzzy dot-like hypertense shadows were found in both lungs, which were somehow absorbed when compared with those in a (September 23, 2010). (c) Various bronchial vascular bundles were found in both lungs. Diffuse cloud-like and fuzzy dot-like hyperdense shadows were found in both lungs, which were absorbed when compared with b (October 25, 2010). (d) Various bronchial vascular bundles were found in both lungs. They were scattered with multiple nodular and hyperdense shadows. Compared with c, the conditions were markedly improved.
He was treated with empirical antibiotics (imipenem/cilastatin sodium, 1.0 g, iv, Q8h) and empirical anti-fungal agent (voriconazole, 400 mg, iv, Q12h on day 1, followed by 200 mg, iv, Q12h). On day 7, sputum and BALF cultures were positive for S. apiospermum; and the level of (1-3)-β-D-glucan was 45 pg/ml. He was treated with Voriconazole, 200 mg iv, Q12h, for 14 consecutive days, which was then switched to 200 mg, oral, b.i.d. On day 14, hemoptysis abated to less than 100 ml/d until day 21 when hemoptysis gradually decreased to less than 10 ml/d, and he was successfully weaned off the ventilator. However, his right eye developed a blurred vision on day 38. Eye ultrasound performed on the next day showed that there were multiple patchy hypertrophic light bands inside the corpus vitreum of both eyes, particularly evidenced in the right. He was diagnosed with fungal infection in the right eye. Triage treatment with an anti-fungal drug was provided and he was confirmed with S. apiospermum infection based on the history of near-drowning, infiltrative changes in both lungs, and positive sputum and BALF cultures for S. apiospermum after admission on day 7.
Maintenance therapy was provided after his discharge (voriconazole, 200 mg, oral, b.i.d., which was switched to 200 mg, oral, q.d. after day 107). On day 184, he complained of having lower back pain, with progressive restricted movement and decreased range of motion in the spine. Spinal plain X-ray, sagittal view performed showed localized vertebral anomalies in thoracic vertebrae 11 and 12, and obvious narrowing of the disk spaces. He received thoracic spinal surgery on day 190 in another hospital, and fungal infection of the thoracic vertebrae was pathologically confirmed. After surgery, he continued voriconazole treatment (200 mg, q.d.) until day 473 (December 31, 2011). During this period, chest CTs showed that the thoracic spine gradually returned to normal [Figure 5b-5d]. Lower back pain symptoms disappeared, and his spinal movement and range of motion returned to nearly normal. Unfortunately, he had only mild perception in his right eye. Voriconazole treatment was then stopped, and the total doses were 116 200 mg. Up to December 31, 2014, no relapse of S. apiospermum infection was observed during a 3 years of follow-up [Figure 6].
Figure 6.
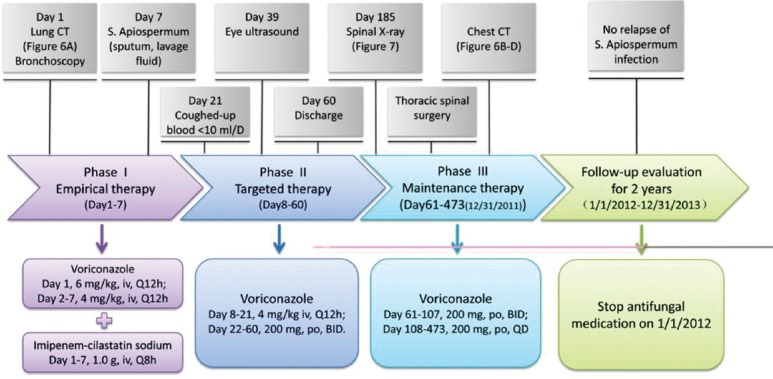
Case 2 diagnosis and treatment flow chart.
Our two patients with S. apiospermum infection after contaminated water near-drowning were characterized by severe clinical conditions and prolonged disease course. The treatment success was due to positive empirical antifungal therapy. Voriconazole was chosen for definitive treatment after confirming the S. apiospermum infection by tissue culture. The voriconazole treatment lasted for a longer time than expected including iv and oral administrations in Case 1 for 21 months and Case 2 for 16 months, respectively. The two patients had pulmonary infiltration as the initial symptom. However, the initial culture of the airway secretions yielded no result of specific infection. Knee-joint fluid cultures confirmed the presence of S. apiospermum in Case 1. Based on our experience with Case 1, Case 2 was timely diagnosed by BALF culture and repeated sputum cultures, which showed positive results for S. apiospermum. Case 1 patient experienced pulmonary infection, intracranial abscesses, eye infection, spine infection, and knee joint infection whereas in Case 2, the patient developed lung infection, eye infection, and thoracic spine infection. In Case 1, the patient received relatively complex treatment, which included empirical monotherapy (caspofungin), empirical combination therapy (caspofungin plus amphotec), targeted combination therapy (voriconazole [minimum inhibitory concentration-90 (MIC-90) of 0.25 μg/ml] plus amphotec [MIC-90 of 1 μg/ml]), maintenance and consolidation therapy (voriconazole). The treatment course lasted for up to 22 months, and Voriconazole for 21 months. In Case 2, however, the patient was considered to have S. apiospermum infection upon admission, and his treatment was rapidly shifted from empirical antifungal treatment to a 1-week targeted therapy against S. apiospermum during which period, voriconazole (MIC-90 of 0.25 μg/ml) was promptly administered. Nevertheless, the treatment course extended to 16 months. Furthermore, both patients were almost blinded in one of their eyes, and the patient in Case 2 had to receive thoracic spine surgery.
Previous studies have shown that S. apiospermum can invade nearly all the tissues and organs of the human body with unique features in each. When it invades the bones and joints, it can cause osteomyelitis, diskitis, and arthritis, which are manifested as joint swelling, pain, and restricted movement. Disease progression can be slow and last for up to several months.[6] The two patients developed knee osteoarthritis and thoracic vertebral inflammation several months later after disease onset. Ocular and corneal infections with S. apiospermum can cause pain, decreased vision, and even death in severe cases.[7,8] Both patients were almost blinded in one eye owing to ocular infection. Literatures focused on lung infections are most common. Similar to many other fungal infections, lung infections with S. apiospermum can be manifested as fungal balls, allergic bronchitis, and/or invasive pulmonary fungal disease. Invasive infection of the lungs by S. apiospermum can be characterized by extensive cavities, fibrosis, and nodules, which are clinically manifested as fever, cough, sputum production, and even massive hemoptysis and respiratory failure.[9] Both cases of ours showed similar manifestations. For patients with central nervous system infections (including intracranial abscess and meningitis), the symptoms are highly dependent on the site of infection. Brain abscesses can cause neurological signs and symptoms corresponding to the affected sites, whereas patients with meningitis often have neck rigidity and projectile vomiting. The initial symptom in Case 1's patient was headache, accompanied by diabetes insipidus, and then intracranial infection was confirmed. After active antifungal therapy, the symptoms of diabetes insipidus mitigated due to the elimination of intracranial abscesses.
Probable pathophysiological mechanisms of S. apiospermum infection after a near-drowning include a local spread from sites near the brain, such as the paranasal sinuses or cribriform plate, and a hematogenous spread from the lungs. The patients in both cases developed aspiration pneumonia after a contaminated water near-drowning. S. apiospermum in sewage can rapidly enter the lungs via the respiratory tract. After growth and reproduction in the lungs, S. apiospermum invades multiple tissues and organs, successively causing invasive pneumonia, intracranial abscesses, eye infection, knee joint infection, vertebral osteomyelitis, and diskitis.
Both patients received antifungal treatment lasting for over 1-year because no conventional therapy is yet recommended.
It's difficultly to diagnose Scedosporium infection because there is the lack of specific symptoms and signs of fungal infection. Katragkou et al.[3] reported that the median “time to diagnosis of Scedosporium infection” was 28 days. This could be attributed to the low sensitivity of routine culture methods. Our patients were diagnosis on day 54 and day 7, respectively. After diagnosis, we chose voriconazole anti-Scedosporium according to the drug sensitive, but the treatment periods were still longer and the total doses were very large, 170,800 mg in Case 1 and 116,200 mg in Case 2. As reported in the literature, although there was evident in vitro sensitivity, the clinical outcome was still inefficient, especially when complicated with dissemination as seen in these cases. Even though we chose combined anti-Scedosporium therapy (Case 1), the two cases still needed a longer time of treatment.
Footnotes
Edited by: Xiu-Yuan Hao
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.de Hoog GS, Marvin-Sikkema FD, Lahpoor GA, Gottschall JC, Prins RA, Guého E. Ecology and physiology of the emerging opportunistic fungi Pseudallescheria boydii and Scedosporium prolificans. Mycoses. 1994;37:71–8. doi: 10.1111/j.1439-0507.1994.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 3.Katragkou A, Dotis J, Kotsiou M, Tamiolaki M, Roilides E. Scedosporium apiospermum infection after near-drowning. Mycoses. 2007;50:412–21. doi: 10.1111/j.1439-0507.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Utsumi Y, Suzuki N, Nakajima Y, Murata O, Sasaki N, et al. Multiple Scedosporium apiospermum abscesses in a woman survivor of a tsunami in northeastern Japan: A case report. J Med Case Rep. 2011;5:526. doi: 10.1186/1752-1947-5-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakadate T, Nakamura Y, Yamauchii K, Endo S. Two cases of severe pneumonia after the 2011 Great East Japan Earthquake. Western Pac Surveill Response J. 2012;3:67–70. doi: 10.5365/WPSAR.2012.3.2.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung LH, Norwood LA. Osteomyelitis due to Pseudallescheria boydii. South Med J. 1993;86:231–4. doi: 10.1097/00007611-199302000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Yoon S, Kim S, Lee KA, Kim H. A case of Scedosporium apiospermum keratitis confirmed by a molecular genetic method. Korean J Lab Med. 2008;28:307–11. doi: 10.3343/kjlm.2008.28.4.307. [DOI] [PubMed] [Google Scholar]
- 8.Hernández Prats C, Llinares Tello F, Burgos San José A, Selva Otaolaurruchi J, Ordovás Baines JP. Voriconazole in fungal keratitis caused by Scedosporium apiospermum. Ann Pharmacother. 2004;38:414–7. doi: 10.1345/aph.1D128. [DOI] [PubMed] [Google Scholar]
- 9.Severo LC, Oliveira Fde M, Irion K. Respiratory tract intracavitary colonization due to Scedosporium apiospermum: Report of four cases. Rev Inst Med Trop Sao Paulo. 2004;46:43–6. doi: 10.1590/s0036-46652004000100009. [DOI] [PubMed] [Google Scholar]