Abstract
Background:
Pelvic lymph node metastasis (LNM) is an important prognostic factor in cervical cancer. Cervical squamous cell carcinoma accounts for approximately 75–80% of all cervical cancers. Analyses of the effects of the number of positive lymph nodes (LNs), unilateral versus bilateral pelvic LNM and a single group versus multiple groups of pelvic LNM on survival and recurrence of cervical squamous cell carcinoma are still lacking. The study aimed to analyze the effects of the number of positive pelvic LNs and a single group versus multiple groups of pelvic LNM on survival and recurrence.
Methods:
We performed a retrospective review of 296 patients diagnosed with Stage IA–IIB cervical squamous cell carcinoma who received extensive/sub-extensive hysterectomy with pelvic lymphadenectomy/pelvic LN sampling at Peking University People's Hospital from November 2004 to July 2013. Ten clinicopathological variables were evaluated as risk factors for pelvic LNM: Age at diagnosis, gravidity, clinical stage, histological grade, tumor diameter, lymph-vascular space involvement (LVSI), depth of cervical stromal invasion, uterine invasion, parametrial invasion, and neoadjuvant chemotherapy.
Results:
The incidence of pelvic LNM was 20.27% (60/296 cases). Pelvic LNM (P = 0.00) was significantly correlated with recurrence. Pelvic LNM (P = 0.00), the number of positive pelvic LNs (P = 0.04) and a single group versus multiple groups of pelvic LNM (P = 0.03) had a significant influence on survival. Multivariate analysis revealed that LVSI (P = 0.00), depth of cervical stromal invasion (P = 0.00) and parametrial invasion (P = 0.03) were independently associated with pelvic LNM.
Conclusions:
Patients with pelvic LNM had a higher recurrence rate and poor survival outcomes. Furthermore, more than 2 positive pelvic LNs and multiple groups of pelvic LNM appeared to identify patients with worse survival outcomes in node-positive IA-IIB cervical squamous cell carcinoma. LVSI, parametrial invasion, and depth of cervical stromal invasion were identified as independent clinicopathological risk factors for pelvic LNM.
Keywords: Cervical Squamous Cell Carcinoma, Pelvic Lymph Node Metastasis, Prognosis
INTRODUCTION
Cervical cancer is the only gynecological malignancy that is clinically classified according to the International Federation of Gynecology and Obstetrics (FIGO) clinical staging system as opposed to the FIGO surgical pathologic staging system. An evaluation of lymph node (LN) status is not included in the clinical staging system. Local invasion and lymphatic metastasis are well-known as the major diffusion routes of cervical cancer, and lymph node metastasis (LNM) is one of the primary factors that influence survival and prognosis.[1,2] The LNM rate ranges from 15.8% to 25.5% for patients with Stage IB–IIA cervical cancer.[3,4,5] The 5-year overall survival (OS) rate for early cervical cancer is estimated at approximately 80%.[6,7] Once LNM occurs, the overall 5-year survival rate for early stage carcinoma of the uterine cervix is reduced to 53%.[7] Therefore, efforts to improve prognosis after extensive hysterectomy have mainly focused on patients with LNM.
Cervical squamous cell carcinoma accounts for approximately 75–80% of all cervical cancers. Analyses of the effects of the number of positive LNs, unilateral versus bilateral pelvic LNM and a single group versus multiple groups of pelvic LNM on survival and recurrence of cervical squamous cell carcinoma are still lacking. The aim of this study was to analyze the effects of the number of positive pelvic LNs, unilateral versus bilateral pelvic LNM and a single group versus multiple groups of pelvic LNM on survival and recurrence. Moreover, we retrospectively studied the clinicopathological data of 296 women with Stage IA–IIB cervical squamous cell carcinoma to investigate the clinicopathological risk factors for pelvic LNM. The results of this study could provide a basis for prognosis assessment and serve as a guide for individual therapy.
METHODS
Patients
We reviewed clinicopathological data obtained from 296 women with cervical squamous cell carcinoma, FIGO (2009) Stage IA–IIB, who had been treated at Department of Obstetrics and Gynecology, Peking University People's Hospital between November 2004 and July 2013. This study included patients who met the following criteria: Patients with Stage IA–IIB cervical squamous cell carcinoma and patients who underwent extensive/sub-extensive hysterectomy with pelvic lymphadenectomy/pelvic LN sampling. FIGO staging was based on clinical examination and the preoperative results of magnetic resonance imaging and computed tomography. The stage distribution was as follows: 33 (11.2%) patients had Stage IA, 143 (48.3%) patients had Stage IB, 61 (20.6%) patients had Stage IIA, and 59 (19.9%) patients had Stage IIB.
Clinicopathological variables
The following 10 clinicopathological variables were evaluated as risk factors for pelvic LNM: Age at diagnosis, gravidity, clinical stage, histological grade, tumor diameter, lymph-vascular space involvement (LVSI), depth of cervical stromal invasion, uterine invasion, parametrial invasion, and neoadjuvant chemotherapy (NACT). Patients were divided into 2 age groups: ≤35 years of age and >35 years of age. Two gravidity groupings were used in this analysis: ≤2 and >2. Histological grade was divided into two groups: G1 carcinomas, which are well-differentiated tumors, and G2-G3 carcinomas, which are moderately and poorly differentiated tumors. The largest dimension was recorded as tumor diameter by the pathologist. The pathological slides of each patient were reviewed by two gynecologic pathologists to confirm LVSI, depth of cervical stromal invasion, uterine invasion, and parametrial invasion. The 109 women with IA–IIB tumors received NACT before surgery. The NACT scheme included concurrent cisplatin-containing chemotherapy for one to three cycles to reduce the tumor size and clinical stage. In the cases that underwent NACT, the histological grade was diagnosed by preoperative pathological examination of biopsied tissues. Moreover, the number of positive pelvic LNs was divided into two groups: ≤2 and >2. In general, patients with postoperative pathologic findings of LVSI, pelvic LNM or parametrial invasion were advised to undergo postoperative adjuvant irradiation and/or concurrent cisplatin-containing chemotherapy for four cycles. The follow-up period was calculated from the date of surgery to the date of last follow-up (July 1, 2014) or the time of death. Recurrence was defined as disease found at any time after surgery. Survival time was calculated from the date of surgery.
Statistical analysis
SPSS version 16.0 (International Business Machines Corporation, New York, USA) was used for statistical analyses. The OS was calculated using the life-table method and the Kaplan-Meier method. Differences were analyzed using the log-rank test. The relationship between clinicopathological variables and pelvic LNM was examined by univariate analysis using the χ2 test, continuity correction test, and Fisher's exact test. The independent effects of the clinicopathological variables on pelvic LNM were determined by multiple logistic regression analysis. A P < 0.05 was defined as statistically significance.
RESULTS
Baseline characteristics
All 296 patients were followed up for 5–112 months, and the mean follow-up period was 48 months. The mean age was 45 years old (range 25–74 years old). The mean number of pelvic LNs removed was 27 (range 10–55), and the mean number of positive pelvic LNs was 3 (range 1–31). Sixty patients (20.27%) had positive pelvic LNs.
Pelvic lymph node metastasis and recurrence
Pelvic LNM was significantly correlated with recurrence (P = 0.00). However, the number of positive pelvic LNs, unilateral/bilateral pelvic LNM and single group/multiple groups of pelvic LNM all had no significant influence on recurrence (P > 0.05) [Table 1].
Table 1.
The relationship between pelvic LNM and recurrence
| Characteristics | Cases, n | Nonrecurrence, n (%) | Recurrence, n (%) | P |
|---|---|---|---|---|
| Pelvic LNM (n = 296) | 0.00 | |||
| Negative | 236 | 213 (90.25) | 23 (9.75) | |
| Positive | 60 | 42 (70.00) | 18 (30.00) | |
| Number of positive pelvic LNs (n = 60) | 0.15 | |||
| ≤2 | 47 | 35 (74.47) | 12 (25.53) | |
| >2 | 13 | 7 (53.85) | 6 (46.15) | |
| Unilateral/bilateral pelvic LNM (n = 60) | 0.25 | |||
| Unilateral pelvic LNM | 36 | 28 (77.78) | 8 (22.22) | |
| Bilateral pelvic LNM | 24 | 15 (62.50) | 9 (37.50) | |
| Single group/multiple groups of pelvic LNM (n = 41) | 0.15 | |||
| Single group | 28 | 23 (82.14) | 5 (17.86) | |
| Multiple groups | 13 | 8 (61.54) | 5 (38.46) |
LNM: Lymph node metastasis; LNs: Lymph nodes.
Pelvic lymph node metastasis and survival
Pelvic LNM (P = 0.00), number of positive pelvic LNs (P = 0.04) and single group/multiple groups of pelvic LNM (P = 0.03) significantly influenced survival. However, unilateral/bilateral pelvic LNM had no significant effect on survival (P > 0.05) [Table 2]. The OS of total 296 patients was 87% [Figure 1]. The OS of pelvic LN-negative group and pelvic LN-positive group was 91%, 67%, respectively [Figure 2]. The OS of the number of positive pelvic LNs ≤2 group and the number of positive pelvic LNs >2 group was 76%, 35%, respectively [Figure 3]. The OS of single group of pelvic LNM and multiple groups of pelvic LNM was 78%, 44%, respectively [Figure 4].
Table 2.
The relationship between pelvic LNM and survival
| Characteristics | Cases, n | Surviving, n (%) | OS, % | P |
|---|---|---|---|---|
| Pelvic LNM (n = 296) | 0.00 | |||
| Negative | 236 | 219 (92.80) | 91 | |
| Positive | 60 | 46 (76.67) | 67 | |
| Number of positive pelvic LNs (n = 60) | 0.04 | |||
| ≤2 | 47 | 39 (82.98) | 76 | |
| >2 | 13 | 7 (53.85) | 35 | |
| Unilateral/bilateral pelvic LNM (n = 60) | 0.45 | |||
| Unilateral pelvic LNM | 36 | 29 (80.56) | 73 | |
| Bilateral pelvic LNM | 24 | 17 (70.83) | 60 | |
| Single group/multiple groups of pelvic LNM (n = 41) | 0.03 | |||
| Single group | 28 | 24 (85.71) | 78 | |
| Multiple groups | 13 | 8 (61.54) | 44 |
LNM: Lymph node metastasis; LNs: Lymph nodes; OS: Overall survival.
Figure 1.
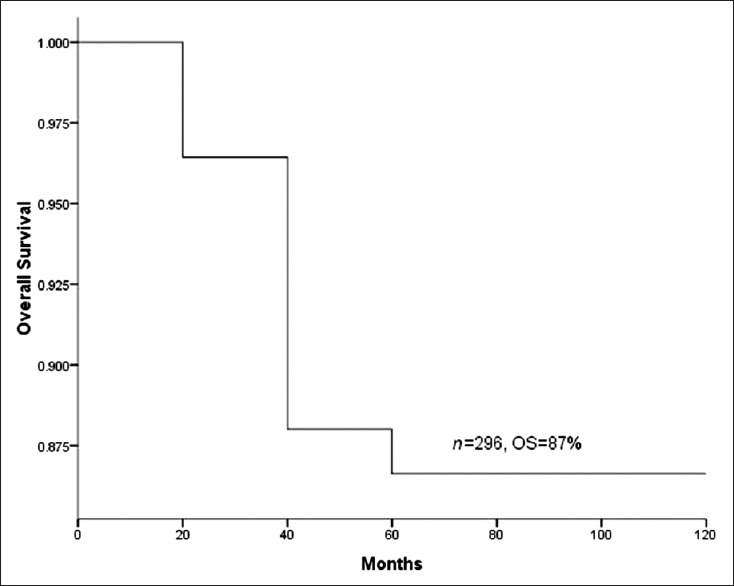
Survival curve of total 296 patients.
Figure 2.
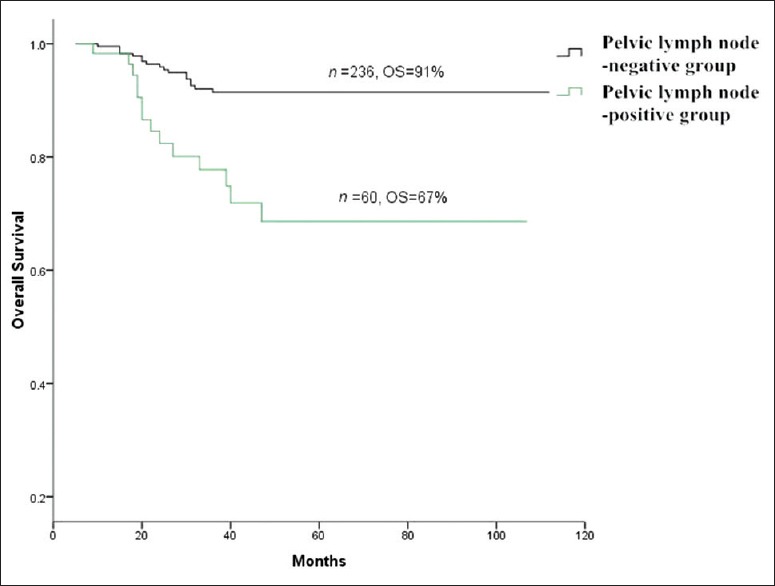
Survival curves of lymph node-negative positive group versus pelvic lymph node-positive group.
Figure 3.
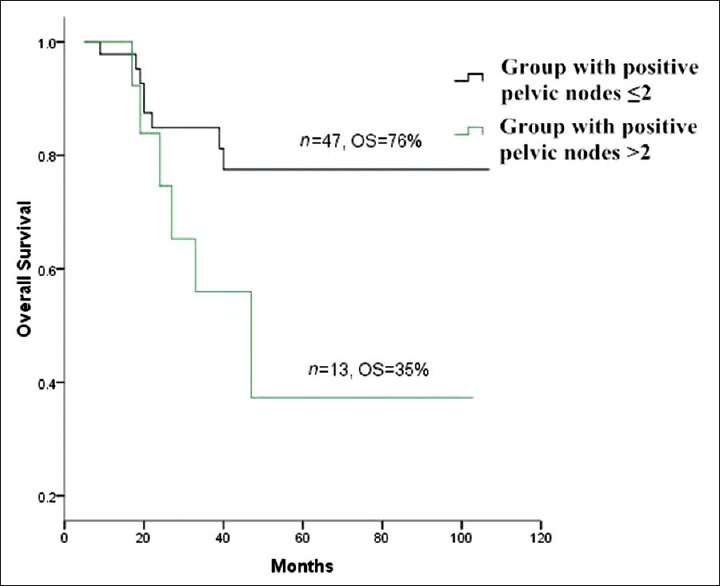
Survival curves of group with positive pelvic lymph nodes (LNs) ≤2 versus group with positive pelvic LNs >2.
Figure 4.
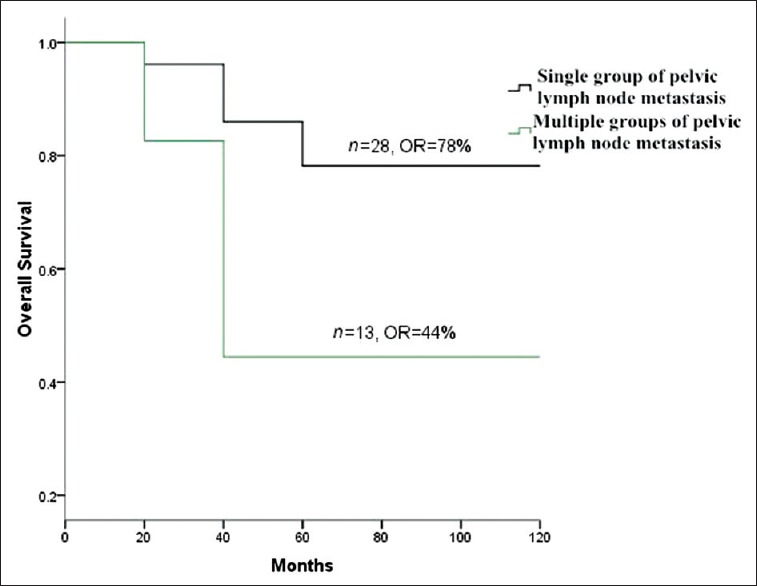
Survival curves of single group of pelvic lymph node metastasis (LNM) versus multiple groups of pelvic LNM.
High-risk factors for pelvic lymph node metastasis
A univariate analysis revealed that clinical stage, histological grade, tumor diameter, LVSI, depth of cervical stromal invasion, uterine invasion, parametrial invasion, and NACT were correlated with pelvic LNM (P < 0.05) [Table 3]. In a multivariate analysis, LVSI (P = 0.00), the depth of cervical stromal invasion (P = 0.00) and parametrial invasion (P = 0.03) were independently associated with pelvic LNM in patients with Stage IA–IIB cervical squamous cell carcinoma [Table 4].
Table 3.
Clinicopathological characteristics of patients with cervical squamous cell carcinoma in relation to pelvic LNM (n = 296)
| Characteristics | Pelvic LNM negative, n (%) | Pelvic LNM positive, n (%) | P |
|---|---|---|---|
| Age | 0.28 | ||
| ≤35 years | 25 (89.3) | 3 (10.7) | |
| >35 years | 211 (78.7) | 57 (21.3) | |
| Gravidity | 0.79 | ||
| ≤2 | 95 (80.51) | 23 (19.49) | |
| >2 | 141 (79.21) | 37 (20.79) | |
| Clinical stage | 0.00 | ||
| IA | 33 (100) | 0 (0.00) | |
| IB | 118 (82.52) | 25 (17.48) | |
| IIA | 46 (75.41) | 15 (24.59) | |
| IIB | 39 (66.10) | 20 (33.90) | |
| Histological grade | 0.00 | ||
| G1 | 52 (96.30) | 2 (3.70) | |
| G2–G3 | 184 (76.03) | 58 (23.97) | |
| Tumor diameter (cm) | 0.01 | ||
| ≤4 | 187 (83.11) | 38 (16.89) | |
| >4 | 49 (69.01) | 22 (30.99) | |
| LVSI | 0.00 | ||
| Negative | 169 (90.86) | 17 (9.14) | |
| Positive | 67 (60.91) | 43 (39.09) | |
| The depth of cervical stromal invasion | 0.00 | ||
| <1/2 | 145 (92.95) | 11 (7.05) | |
| ≥1/2 | 91 (65.00) | 49 (35.00) | |
| Uterine invasion | 0.00 | ||
| Negative | 223 (83.21) | 45 (16.79) | |
| Positive | 13 (46.43) | 15 (53.57) | |
| Parametrial invasion | 0.00 | ||
| Negative | 234 (81.53) | 53 (18.47) | |
| Positive | 2 (22.22) | 7 (77.78) | |
| NACT | 0.02 | ||
| Yes | 79 (72.48) | 30 (27.52) | |
| No | 157 (83.96) | 30 (16.04) |
LVSI: Lymph-vascular space involvement; NACT: Neoadjuvant chemotherapy; LNM: Lymph node metastasis.
Table 4.
Multivariate analysis of the clinicopathological factors associated with pelvic LNM
| Items | P | OR | 95% CI for OR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Clinical stage | 0.61 | 1.14 | 0.70 | 1.85 | |
| Histological grade | 0.07 | 4.42 | 0.87 | 22.42 | |
| Tumor diameter | 0.13 | 1.76 | 0.85 | 3.64 | |
| LVSI | 0.00 | 4.86 | 2.35 | 10.03 | |
| The depth of cervical stroma invasion | 0.00 | 3.35 | 1.51 | 7.44 | |
| Uterine invasion | 0.22 | 1.80 | 0.71 | 4.56 | |
| Parametrial invasion | 0.03 | 8.56 | 1.24 | 58.98 | |
| NACT | 0.07 | 2.22 | 0.94 | 5.27 | |
| Constant | 0.00 | 0.01 | – | – | |
LNM: Lymph node metastasis; LVSI: Lymph-vascular space involvement; NACT: Neoadjuvant chemotherapy; OR: Odds ratio; CI: Confidence interval.
DISCUSSION
Once LNM occurs, cervical cancer patients have a poor prognosis.[8,9] Thus, it is necessary to investigate the clinicopathological characteristics of LNM in cervical squamous cell carcinoma. It is difficult to detect or predict LNM before surgery, and postoperative pathological examination of sectioned LNs commonly shows LNM. Benedetti-Panici et al.[10] reported that the incidences of LNM in pelvic LNs and para-aortic LNs were 33% and 5%, respectively, in patients with locally advanced cervical cancer treated with NACT. Similarly, Marana et al.[11] reported that 38.9% of patients with locally advanced cervical cancer had pelvic LNM, and only 8.3% of patients had pelvic and para-aortic LNM. In our study, a small fraction of patients underwent para-aortic lymphadenectomy; therefore, only pelvic LNM was studied.
Sakuragi et al.[4] reported that the incidences of pelvic LNM in Stage IB, Stage IIA, and Stage IIB cervical carcinoma were 11.5%, 26.7%, and 39.2%, respectively. Patients with advanced cervical cancer are at high risk for pelvic LNM. In the present study, the incidences of pelvic LNM in Stage IA, Stage IB, Stage IIA, and Stage IIB cervical squamous cell carcinoma were 0%, 17.5%, 24.6%, and 33.9%, respectively. Our study demonstrated that pelvic LNM was a critical factor for both recurrence and survival of cervical squamous cell carcinoma. This finding was similar to many other reports on cervical cancer.[12,13] Our results suggested that cervical squamous cell carcinoma patients with pelvic LNM had a poor prognosis.
The number of positive pelvic LNs was significantly associated with survival of cervical squamous cell carcinoma. Takeda et al.[14] found that the survival of patients with ≥3 positive pelvic LNMs was quite poor, and the estimated 5-year survival rate was 20.2% in Stage IB–IIB cervical carcinomas. In our study, the OS of the ≤2 positive pelvic LNs group was 35%, half of the OS of the >2 positive pelvic LNs group. These results suggested that cervical squamous cell carcinoma patients with positive pelvic LNs >2 had poor survival.
In addition, multiple groups of pelvic LNM had a significant influence on survival. The OS for patients with single group pelvic LNM was 78%, whereas that of patients with multiple groups pelvic LNM was down to 44%. This suggested that cervical squamous cell carcinoma patients with multiple groups of pelvic LNM had low survival.
Multivariate analysis demonstrated that only LVSI, the depth of cervical stromal invasion and parametrial invasion were independent risk factors for pelvic LNM in patients with Stage IA–IIB cervical squamous cell carcinoma. According to the risk assessments, patients with LVSI had a risk of pelvic LNM five times that of patients without LVSI; patients with a depth of cervical stromal invasion >1/2 had a risk of pelvic LNM three times that of patients with a depth of cervical stromal invasion ≤1/2, and patients with parametrial invasion had a risk of pelvic LNM nine times that of patients without parametrial invasion. Delgado et al.[15] published a study of 645 patients with Stage I cervix squamous carcinoma that underwent radical hysterectomy and pelvic and para-aortic lymphadenectomy. The authors found that the depth of invasion, parametrial involvement, LVSI, tumor grade, and gross versus occult primary tumor were significantly associated with pelvic LNM. Further multivariate analysis found that LVSI, the depth of invasion, parametrial involvement, and age remained independent risk factors for pelvic LNM. This result was similar to the reports of Milam et al. and Sakuragi et al. that LVSI and the depth of cervical stromal invasion are independent risk factors for pelvic LNM.[4,16] Togami et al.[17] noted that parametrial invasion and tumor diameter >2 cm were independently associated with nodal metastasis in Stage IA2–IIB cervical cancer. However, in Narayan et al.'s study, only uterine invasion appeared to be associated with an increased risk of nodal metastasis in cervical cancer.[18]
The limitations of our study were its retrospective nature and inherent bias. However, two gynecologic pathologists helped to limit this bias by ensuring consistency in diagnosis. Our study was also limited by the NACT scheme. Data were collected for the study from 2004 to 2013. The NACT courses ranged from one to three. Overall, it is unclear whether these differences in NACT played a significant role in the results.
In conclusion, pelvic LNM is an important prognostic factor for disease recurrence and survival. More than two positive pelvic LNs and multiple groups of pelvic LNM appeared to identify patients with worse survival outcomes in node-positive IA–IIB cervical squamous cell carcinoma. LVSI, parametrial invasion, and the depth of cervical stromal invasion are independent clinicopathological characteristics of pelvic LNM in Stage IA–IIB cervical squamous cell carcinoma. Our study results suggested that pelvic LNM should be added in stage, and pelvic lymphadenectomy should be recommended for patients with high-risk factors of pelvic LNM. In the future, we will use the significant clinicopathological risk factors found in our study for trials to establish a prognosis scoring system to subclassify patients and change therapy accordingly.
Footnotes
Edited by: Xin Chen
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Trattner M, Graf AH, Lax S, Forstner R, Dandachi N, Haas J, et al. Prognostic factors in surgically treated stage ib-iib cervical carcinomas with special emphasis on the importance of tumor volume. Gynecol Oncol. 2001;82:11–6. doi: 10.1006/gyno.2001.6252. [DOI] [PubMed] [Google Scholar]
- 2.Suprasert P, Srisomboon J, Kasamatsu T. Radical hysterectomy for stage IIB cervical cancer: A review. Int J Gynecol Cancer. 2005;15:995–1001. doi: 10.1111/j.1525-1438.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Cai J, Kuang Y, Cao J, Wang Z. Surgical-pathologic risk factors of pelvic lymph node metastasis in stage Ib1-IIb cervical cancer. Acta Obstet Gynecol Scand. 2012;91:802–9. doi: 10.1111/j.1600-0412.2012.01415.x. [DOI] [PubMed] [Google Scholar]
- 4.Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85:1547–54. doi: 10.1002/(sici)1097-0142(19990401)85:7<1547::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Aoki Y, Sasaki M, Watanabe M, Sato T, Tsuneki I, Aida H, et al. High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol. 2000;77:305–9. doi: 10.1006/gyno.2000.5788. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Sun FQ, Wang ZH. Radical trachelectomy versus radical hysterectomy for the treatment of early cervical cancer: A systematic review. Acta Obstet Gynecol Scand. 2011;90:1200–9. doi: 10.1111/j.1600-0412.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- 7.Yeh SA, Wan Leung S, Wang CJ, Chen HC. Postoperative radiotherapy in early stage carcinoma of the uterine cervix: Treatment results and prognostic factors. Gynecol Oncol. 1999;72:10–5. doi: 10.1006/gyno.1998.5217. [DOI] [PubMed] [Google Scholar]
- 8.Hosaka M, Watari H, Mitamura T, Konno Y, Odagiri T, Kato T, et al. Survival and prognosticators of node-positive cervical cancer patients treated with radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol. 2011;16:33–8. doi: 10.1007/s10147-010-0123-0. [DOI] [PubMed] [Google Scholar]
- 9.Kasamatsu T, Onda T, Sawada M, Kato T, Ikeda S, Sasajima Y, et al. Radical hysterectomy for FIGO stage I-IIB adenocarcinoma of the uterine cervix. Br J Cancer. 2009;100:1400–5. doi: 10.1038/sj.bjc.6605048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetti-Panici P, Maneschi F, Scambia G, Greggi S, Cutillo G, D’Andrea G, et al. Lymphatic spread of cervical cancer: An anatomical and pathological study based on 225 radical hysterectomies with systematic pelvic and aortic lymphadenectomy. Gynecol Oncol. 1996;62:19–24. doi: 10.1006/gyno.1996.0184. [DOI] [PubMed] [Google Scholar]
- 11.Marana HR, de Andrade JM, Dos Reis FJ, Tiezzi DG, Zola FE, Clagnan WS, et al. Impact of surgical staging in locally advanced cervical cancer and subsequent chemotherapy. J Surg Oncol. 2009;100:505–10. doi: 10.1002/jso.21360. [DOI] [PubMed] [Google Scholar]
- 12.Kamura T, Tsukamoto N, Tsuruchi N, Saito T, Matsuyama T, Akazawa K, et al. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer. 1992;69:181–6. doi: 10.1002/1097-0142(19920101)69:1<181::aid-cncr2820690130>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Yuan C, Wang P, Lai C, Tsu E, Yen M, Ng H. Recurrence and survival analyses of 1,115 cervical cancer patients treated with radical hysterectomy. Gynecol Obstet Invest. 1999;47:127–32. doi: 10.1159/000010076. [DOI] [PubMed] [Google Scholar]
- 14.Takeda N, Sakuragi N, Takeda M, Okamoto K, Kuwabara M, Negishi H, et al. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand. 2002;81:1144–51. doi: 10.1034/j.1600-0412.2002.811208.x. [DOI] [PubMed] [Google Scholar]
- 15.Delgado G, Bundy BN, Fowler WC, Jr, Stehman FB, Sevin B, Creasman WT, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: A Gynecologic Oncology Group Study. Gynecol Oncol. 1989;35:314–20. doi: 10.1016/0090-8258(89)90070-x. [DOI] [PubMed] [Google Scholar]
- 16.Milam MR, Frumovitz M, dos Reis R, Broaddus RR, Bassett RL, Jr, Ramirez PT. Preoperative lymph-vascular space invasion is associated with nodal metastases in women with early-stage cervical cancer. Gynecol Oncol. 2007;106:12–5. doi: 10.1016/j.ygyno.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Togami S, Kamio M, Yanazume S, Yoshinaga M, Douchi T. Can pelvic lymphadenectomy be omitted in stage IA2 to IIB uterine cervical cancer? Int J Gynecol Cancer. 2014;24:1072–6. doi: 10.1097/IGC.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 18.Narayan K, McKenzie AF, Hicks RJ, Fisher R, Bernshaw D, Bau S. Relation between FIGO stage, primary tumor volume, and presence of lymph node metastases in cervical cancer patients referred for radiotherapy. Int J Gynecol Cancer. 2003;13:657–63. doi: 10.1046/j.1525-1438.2003.13026.x. [DOI] [PubMed] [Google Scholar]


