Abstract
Background:
Vascular endothelial growth factor-targeted agents are standard treatments in advanced clear-cell renal cell carcinoma (ccRCC), but biomarkers of activity are lacking. The aim of this study was to investigate the association of Von Hippel-Lindau (VHL) gene status, vascular endothelial growth factor receptor (VEGFR) or stem cell factor receptor (KIT) expression, and their relationships with characteristics and clinical outcome of advanced ccRCC.
Methods:
A total of 59 patients who received targeted treatment with sunitinib or pazopanib were evaluated for determination at Cancer Hospital and Institute, Chinese Academy of Medical Sciences between January 2010 and November 2012. Paraffin-embedded tumor samples were collected and status of the VHL gene and expression of VEGFR and KIT were determined by VHL sequence analysis and immunohistochemistry. Clinical-pathological features were collected and efficacy such as response rate and Median progression-free survival (PFS) and overall survival (OS) were calculated and then compared based on expression status. The Chi-square test, the Kaplan–Meier method, and the Lon-rank test were used for statistical analyses.
Results:
Of 59 patients, objective responses were observed in 28 patients (47.5%). The median PFS was 13.8 months and median OS was 39.9 months. There was an improved PFS in patients with the following clinical features: Male gender, number of metastatic sites 2 or less, VEGFR-2 positive or KIT positive. Eleven patients (18.6%) had evidence of VHL mutation, with an objective response rate of 45.5%, which showed no difference with patients with no VHL mutation (47.9%). VHL mutation status did not correlate with either overall response rate (P = 0.938) or PFS (P = 0.277). The PFS was 17.6 months and 22.2 months in VEGFR-2 positive patients and KIT positive patients, respectively, which was significantly longer than that of VEGFR-2 or KIT negative patients (P = 0.026 and P = 0.043).
Conclusion:
VHL mutation status could not predict the efficacy of sunitinib or pazopanib. Further investigation of VHL/VEGFR pathway components is needed.
Keywords: Clear-cell Renal Cell Carcinoma, Kidney Cancer, Prognosis, Vascular Endothelial Growth Factors, Von Hippel–Lindau
INTRODUCTION
Clear-cell renal cell carcinoma (ccRCC) is the most common subtype of kidney cancer and accounts for approximately 80–90% of cancers that arise in the renal parenchyma. For many patients, the disease is localized when it is found. However, about 30–40% of the patients who either present with metastatic disease or develop distant relapse after surgery.[1] ccRCC is obviously resistant to traditional cytotoxic chemotherapy. Multiple therapeutic approaches for vascular endothelial growth factor (VEGF) blockade have recently been investigated in metastatic ccRCC and have yielded successful results.
Von Hippel-Lindau (VHL) gene played a significantly role in occurrence and progression of ccRCC. VHL mutations in patients with sporadic ccRCC varies from 18% to 82%. Hypermethylation of the VHL promoter resulting in gene silencing has been detected in 5–20% of ccRCC. VHL's predominant function is to regulate the cell's response to oxygen availability in the local microenvironment.[2] VHL gene encoded pVHL19 and pVHL30, which participated in the substrate recognition component of an E3 ubiquitin ligase complex that also contains elongin B, elongin C, cullin 2, and rbx1. Then, E3 ubiquitin ligase complex combine with hypoxia-inducible factor alpha (HIF-α). In the presence of normal local oxygen microenvironment, the binding of HIF-α to VHL and to the E3 ubiquitin ligase complex causes HIF-α to be degraded.[3] Therefore, in the normal microenvironment with normal local oxygen availability, HIF-α levels are kept low in the cell. Under hypoxic physiological conditions, the HIF-α is transferred to the nucleus, and combine with HIF-β, which induce some gene expression contains hypoxia-responsive element (HRE), VEGF, glucose transporter-1 (GLUT-1), and transforming growth factor-α (TGF-α) exist similar HIF binding sites as HRE. VHL gene mutation or promoter methylation result in gene inactivation and pVHL express properly, therefore, cannot form E3 ubiquitin ligase complex. The levels of HIF increased that lead to VEGF, GLUT-1, and TGF-α accreted. Resulting in the occurrence and development of the tumor.[4] However, it is unclear whether this genomic profile correlates with the response to targeted therapy.
Pazopanib and sunitinib are potent multi-target receptor tyrosine kinase inhibitor (TKI) of vascular endothelial growth factor receptors (VEGFR)-1, -2, and -3, platelet-derived growth factor receptors (PDGFR)-α/β, and stem cell factor receptor (KIT). They have been approved by both the Food and Drug Administration and the European Commission for the indication of advanced/metastatic ccRCC in the first line setting. However, currently prognostic and predictive biomarkers for response to TKI treatment are still lacking. It was reported that adverse effects like hypertension and the hand-foot skin reaction appear to be associated with a better response to sunitinib and longer overall survival (OS).[5,6] However, whether the VHL mutation and expression of VEGFR and KIT are associated with higher response rates to anti-VEGF agents in ccRCC is still largely unknown.
More investigation of tumor characteristics that may select the patient population that can benefit the most from VEGF-targeted therapy is urgently needed. In view of the roles of VHL in ccRCC, VHL mutation could render a tumor more VEGF-dependent, and more susceptible to VEGF-targeted therapy. The overwhelming majority of present basic data of VHL status, expression of VEGFR and KIT, and respond to targeted therapy is from Europe or the United States. The situation in the Asian population, especially Chinese, is still uncertain. Thus, we examined the clinical characteristics, VHL status, expression of VEGFR and KIT, and clinical outcomes in Chinese patients with advanced ccRCC receiving VEGF-targeted therapy.
METHODS
Patients
Totally, 59 patients with advanced/metastatic ccRCC who received therapy with sunitinib or pazopanib at Cancer Hospital and Institute, Chinese Academy of Medical Sciences from January 2010 to November 2012 were evaluated. The patients eligible for enrolment must meet all criteria: Age ≥18 years old; ECOG 0 or 1; absolute neutrophil count ≥1.5 × 109/L; hemoglobin ≥100 g/L; platelets ≥100 × 109/L; tobal bilirubin ≤1.5 × upper limit of normal (ULN); alanine aminotransferase and aspartate transaminase ≤2.5 × ULN; calculated creatinine clearance ≥30 ml/min. Data regarding clinical characteristics such as age, gender, tumor histology, number of metastases, stage, and drug-related toxicities were collected. All patients were pathologically diagnosed with ccRCC from the nephrectomy specimen. Forty-eight patients of all were treated with sunitinib orally in 6 weeks cycles: 50 mg orally daily for 4 weeks followed by 2 weeks off treatment. Dose may be reduced for toxicity to 37.5 mg and 25 mg. The rest 11 patients received 800 mg of pazopanib once daily and reduced to 600 mg and 400 mg in case of toxicity. Subjects receiving sunitinib or pazopanib may dose interruptions for up to 2 weeks to recover from treatment emergent toxicity. Tumor assessments were performed every 6 weeks with RECIST 1.0 criteria until disease progression, death or unacceptable toxicity.
DNA extraction and Von Hippel-Lindau sequence analysis
For DNA extraction from paraffin-embedded tissue, 5 μm thick sections were manually microdissected as previously described. DNA was extracted using the QIAamp DNA Mini kit (Qiagen, Hilgen, Germany) according to manufacturer's instructions. Polymerase chain reaction (PCR) based amplification of each of the exons was performed using 6 primer sets overlapping fragments of the coding region of VHL. The amplification of exon 1, 2, and 3 were completed by 3, 2, and 1 pairs of primer, respectively [Table 1]. Reaction mixtures were incubated at 95°C for 10 min before 38 cycles of two-step PCR (30 s at 95°C, 30 s at 56°C, 45 s at 72°C), followed by 10 min at 72°C. Amplification products were treated with Exonuclease I/Antarctic Phosphatase (New England Biolabs, MA, USA) at 37°C for 30 min and 80°C for 15 min. The sequencing primers were the same with the PCR primers. Purified sequencing PCR products were sequenced on an ABI 3500XL analyzer (Applied Biosystems, Carlsbad, CA, USA) using POP7 polymer.
Table 1.
The sequencing primers
| Exons | Name of primer | Primer sequence |
|---|---|---|
| Exon 1A | ||
| Forward | VHL 1AF | TGGTCTGGATCGCGGAGGGAAT |
| Reverse | VHL 1AR | GACTGCGATTGCAGAAGATGACCTGGG |
| Exon 1B | ||
| Forward | VHL 1BF | GGCCCGTGCTGCGCTCGGTGAACT |
| Reverse | VHL 1BR | CCCTGCTGGGTCGGGCCTAAGCGC CGGGCCCGT |
| Reverse | VHL 1BR2 | CCCGTCTGCAAAATGGAC |
| Exon 2 | ||
| Forward | VHL 2AF | GTGGCTCTTTAACAACCTTTGC |
| Forward | VHL 2AF2 | TCCCAAAGTGCTGGGATTAC |
| Reverse | VHL 2AR | CCTGTACTTACCACAACAACCTTATC |
| Exon 3 | ||
| Forward | VHL 3AF | TTCCTTGTACTGAGACCCTAGT |
| Reverse | VHL 3AR | TACCATCAAAAGCTGAGATGAAACA GTGTAAGT |
VHL: Von Hippel-Lindau.
Von Hippel–Lindau methylation
Methylation status of the VHL promoter was assessed by methylation-specific PCR following DNA bisulfite treatment using the EZ DNA Methylation Kit (Zymo Research). The modified templates were amplified using methylated and unmethylated-specific primers [Table 2] and results were visualized on a 2% agarose gel.
Table 2.
The methylation primers
| Items | Primer sequence |
|---|---|
| VHL MF | TGGAGGATTTTTTTGCGTACGC |
| VHL MR | GAACCGAACGCCGCGAA |
| VHL UF | GTTGGAGGATTTTTTTGTGTATGT |
| VHL UR | CCCAAACCAAACACCACAAA |
VHL: Von Hippel-Lindau.
Immunohistochemistry
The specimens were fixed in 10% neutral buffered formalin and subsequently embedded in paraffin. The paraffin-embedded tissues were cut at 3 μm. Sections were deparaffinized and rehydrated for further hematoxylin and eosin staining or immunohistochemistry staining. Following a brief proteolytic digestion and a peroxidase blocking of tissue slides, the slides were incubated with the following primary antibodies: Antihuman VEGFR-1.2.3 rabbit monoclonal antibody (Santa Cruz Biotechnology, dilution rate: 2/100), antihuman KIT rabbit polyclonal antibody (Dako, Diagnostics, dilution of 1:100) against respective target proteins overnight at 4°C. After washing, peroxidase-labeled polymer and substrate-chromogen were employed in order to visualize the staining of the interested proteins.
Immunohistochemical staining was interpreted by two independent pathologists, who were blinded to the pathological and clinical data of the patients. Cells with yellow to brown granules in the cell membrane or plasma were calculated as positive staining. The highest intensity of immunohistochemical staining for each protein was scored on several fields of each section with semiquantitative fashion: Negative, weak, moderate, and strong. According to the previous study,[7,8] the staining result of VEGFR-1.2.3 and KIT were scored positive with moderate or strong staining intensity in >10% of tumor cells.
Statistical analysis
The software SPSS 20.0 for Windows (SPSS Inc., IL, USA) was used for statistical analysis. Correlations between categorical values were performed using the Chi-square and Fisher's exact test. Survival analyses were estimated by the Kaplan-Meier method and the Lon-rank test was used to compare different survival curves. The prognostic significance of certain factors was assessed by the COX proportional hazards regression model. Differences were considered statistically significant when P < 0.05.
RESULTS
Clinical characteristics
The median follow-up time was 15.8 months (range 4.7–61.8 months), all 59 patients had clear-cell histology from the nephrectomy specimen. The patient cohort of the present study included 46 males and 13 females, with a median age of 56.2 years (range 20–75 years at initiation of therapy). Of all patients, 48 patients received therapy with sunitinib and 11 patients with pazopanib. Median progression-free survival (PFS) and OS were estimated to be 13.8 and 39.9 months. Twenty-eight patients achieved partial response leading to an overall response rate (ORR) of 47.5% and 30 patients achieved stable disease. Univariate analysis of clinical characteristics showed the PFS of male gender, number of metastatic sites 2 or less, VEGFR-2 positive or KIT positive were longer than control groups [Table 3].
Table 3.
Univariate analysis of clinical parameters and potential markers with regard to PFS and OS
| Variables | Number | Median PFS (months) | P | Median OS (months) | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 46 | 16.2 | 0.015 | 39.2 | 0.033 |
| Female | 13 | 12.0 | 26.2 | ||
| Age (years) | |||||
| ≤60 | 39 | 13.8 | 0.961 | 37.6 | 0.261 |
| >60 | 20 | 13.7 | 46.8 | ||
| Pathological grade | |||||
| G1 | 12 | 21.3 | 0.135 | 42.0 | 0.636 |
| G2 | 24 | 13.8 | 38.6 | ||
| G3 | 23 | 12.6 | 37.6 | ||
| Number of disease sites | |||||
| 2 or less | 48 | 16.6 | 0.010 | 39.9 | 0.008 |
| >2 | 11 | 9.7 | 21.7 | ||
| Hypertension | |||||
| No | 19 | 9.5 | 0.095 | 38.4 | 0.055 |
| Yes | 40 | 16.2 | 39.9 | ||
| Hand-foot skin reaction | |||||
| No | 19 | 13.7 | 0.861 | 39.2 | 0.812 |
| Yes | 40 | 14.0 | 38.6 | ||
| Hypothyroidism | |||||
| No | 32 | 13.8 | 0.702 | 39.9 | 0.229 |
| Yes | 27 | 14.0 | 34.0 | ||
| Hyperlipidemia | |||||
| No | 18 | 13.1 | 0.650 | 38.6 | 0.456 |
| Yes | 41 | 16.2 | 37.6 | ||
| Fatigue | |||||
| No | 26 | 13.7 | 0.580 | 38.6 | 0.793 |
| Yes | 33 | 13.8 | 37.6 | ||
| VHL gene | |||||
| Wild-type | 48 | 13.8 | 0.257 | 39.2 | 0.873 |
| Mutation | 11 | 21.3 | 34.6 | ||
| VEGFR-1 | |||||
| Negative | 19 | 12.1 | 0.056 | 38.4 | 0.052 |
| Positive | 40 | 16.2 | 39.2 | ||
| VEGFR-2 | |||||
| Negative | 17 | 12.9 | 0.026 | 46.8 | 0.626 |
| Positive | 42 | 17.6 | 38.6 | ||
| VEGFR-3 | |||||
| Negative | 38 | 13.8 | 0.586 | 38.6 | 0.259 |
| Positive | 21 | 17.6 | 37.6 | ||
| KIT | |||||
| Negative | 38 | 12.6 | 0.043 | 38.6 | 0.382 |
| Positive | 21 | 22.2 | 38.4 | ||
| Therapy | |||||
| Sunitinib | 48 | 13.8 | 0.912 | 38.4 | 0.534 |
| Pazopanib | 11 | 16.6 | 45.8 |
VEGFR: Vascular endothelial growth factor receptors; VHL: Von Hippel-Lindau; PFS: Progression-free survival; OS: Overall survival.
The COX proportional hazards regression model displayed number of disease sites (P = 0.009, odds ratio [OR]: 3.807, 95% confidence interval [CI]: 1.390–10.429) and VEGFR-2 (P = 0.000, OR: 0.164, 95% CI: 0.059–0.451) were independent predictive factor of PFS. On the other hand, number of metastatic sites was associated with prolonged OS (P = 0.000, OR: 9.915, 95% CI: 2.749–35.757).
Von Hippel-Lindau gene status
Overall, 11 of 59 patients (18.6%) had at least one VHL mutation and no one in all patients showed promoter hypermethylation. Mutations occurred across all exons, most commonly in exon 3 (6/11), 4 in exon 2, 1 in exon 1. VHL mutation types included frame shift (2, 18.2%) and missense (9, 81.8%) [Table 4].
Table 4.
Characteristics of VHL mutations
| Exon 1 | Exon 2 | Exon 3 | Type of mutation |
|---|---|---|---|
| c. 349T>A(TGG>AGG) | Missense | ||
| c. 412C>G(CCA>GCA) | Missense | ||
| c. 485G>A(TGC>TAC) | Missense | ||
| c. 515C>T(CCT>CTT) | Missense | ||
| c. 548C>A(TCG>TAG) | Missense | ||
| c. 607C>A(CAG>AAG) | Missense | ||
| Del415416>TC | Frameshift | ||
| Del358361>AGAG | Frameshift | ||
| c. 484T>C(TGC>CGC) | Missense | ||
| c. 101G>A(GGC>GAC) | Missense | ||
| c. 545G>A(AGG>AAG) | Missense |
VHL: Von Hippel-Lindau.
There was no difference in the frequency of VHL mutation with gender, age, pathological grade, number of metastatic sites, adverse events of targeted therapy (hypertension, hand-foot skin reaction, hypothyroidism, hyperlipidemia or fatigue), or status of VEGFR or KIT. However, the incidence rate of VHL mutation in high pathological grade (G3) patients was 2 times of that in low pathological grade (G1 or G2) patients (P = 0.245). Interestedly, the incidence of VHL mutation of patients with hand-foot skin reaction was 6 times in control groups ([10/30 vs. 1/18] [10/31 vs. 1/17] P = 0.090). Similarly, the incidence of VHL mutation of patients with hyperlipidemia was 5.5 times in control groups (10/31 vs. 1/17) [Table 5].
Table 5.
The VHL mutation status analysis
| Variables | VHL gene | P | |
|---|---|---|---|
| Mutation | Wild-type | ||
| Gender | |||
| Male | 8 | 38 | 0.645 |
| Female | 3 | 10 | |
| Age (years) | |||
| ≤60 | 8 | 31 | 0.610 |
| >60 | 3 | 17 | |
| Pathological grade | |||
| G1 or G2 | 5 | 31 | 0.245 |
| G3 | 6 | 17 | |
| Number disease sites | |||
| 2 or less | 9 | 39 | 0.965 |
| >2 | 2 | 9 | |
| Hypertension | |||
| No | 3 | 16 | 0.700 |
| Yes | 8 | 32 | |
| Handfoot skin reaction | |||
| No | 1 | 18 | 0.071 |
| Yes | 10 | 30 | |
| Hypothyroidism | |||
| No | 4 | 28 | 0.191 |
| Yes | 7 | 20 | |
| Hyperlipidemia | |||
| No | 1 | 17 | 0.090 |
| Yes | 10 | 31 | |
| Fatigue | |||
| No | 4 | 22 | 0.572 |
| Yes | 7 | 26 | |
| VEGFR-1 | |||
| Negative | 2 | 17 | 0.274 |
| Positive | 9 | 31 | |
| VEGFR-2 | |||
| Negative | 3 | 14 | 0.901 |
| Positive | 8 | 34 | |
| VEGFR-3 | |||
| Negative | 5 | 33 | 0.149 |
| Positive | 6 | 15 | |
| KIT | |||
| Negative | 7 | 31 | 0.953 |
| Positive | 4 | 17 | |
VHL: Von Hippel-Lindau; VEGFR: Vascular endothelial growth factor receptors.
In terms of relationship with clinical outcomes, VHL mutation status did not correlate with either ORR (ORR was 45.5% [4/11] in patients with VHL gene mutations vs. 47.9% [23/48] in patients without VHL gene mutation, P = 0.938) or PFS (median PFS was 21.3 months vs. 13.8 months in patients with or without VHL mutation, P = 0.257), respectively [Table 3 and Figure 1].
Figure 1.
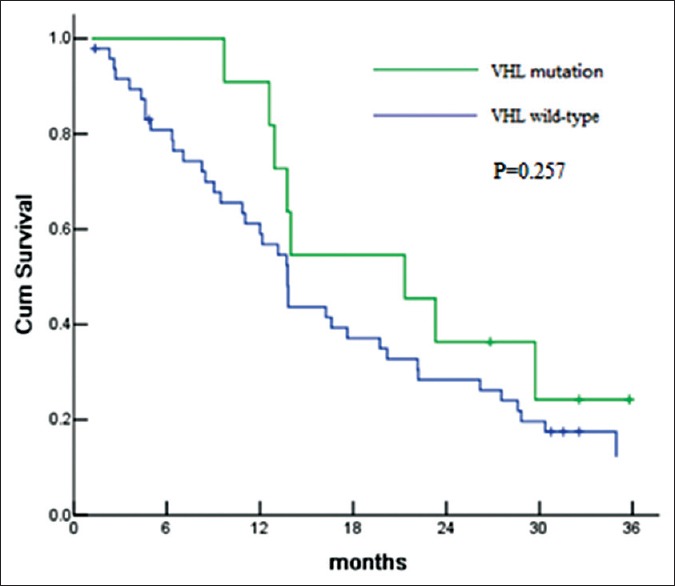
Progression-free survival according to Von Hippel-Lindau (VHL) gene status for comparison of VHL mutation versus VHL wild-type.
Immunohistochemistry examinations
Tumor cells with brown granules in membrane or cytoplasm were calculated as VEGFR positive staining. The expression rates of VEGFR-1 and VEGFR-2 were similar, 40/59 (67.8%) and 42/59 (71.2%), respectively. VEGFR-3 expression rate was relatively lower, about 21/58 (35.6%). According to the previous study,[9] KIT positive was defined as nucleus staining in this study. Thus, KIT expression was positive in 35.6% (21/59) patients. No staining was found in membrane or cytoplasm in all KIT slides.
The patients with VHL mutation were more likely to have higher expression of VEGFR, especially, VEGFR-1 (81.8% [9/11] vs. 64.6% [31/48]) and VEGFR-3 (54.5% [6/11] vs. 31.2% [15/48]). VEGFR-2 and KIT expression appeared to be associated with PFS. The PFS of VEGFR-2 positive patients and KIT positive patients was 17.6 months and 22.2 months, which was significantly longer than that of VEGFR-2 or KIT negative (P = 0.026 and P = 0.043) [Figures 2 and 3]. No staining was found in membrane or cytoplasm in all KIT slides [Figure 4].
Figure 2.
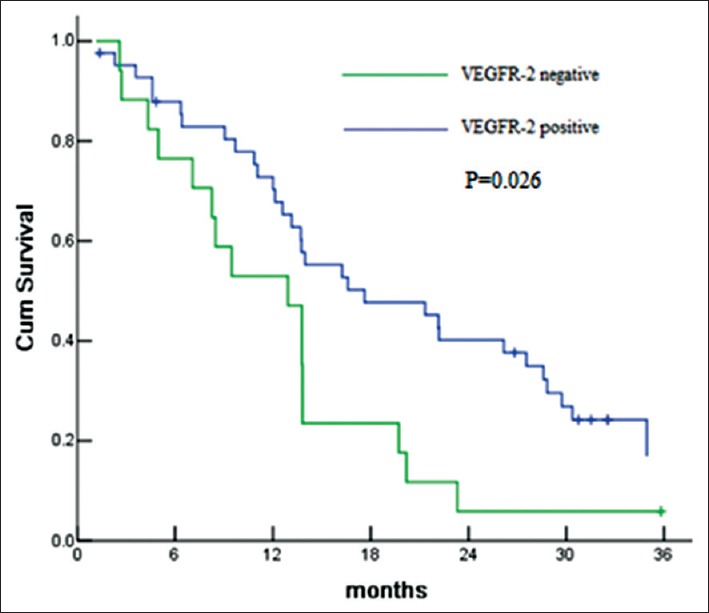
Progression-free survival according to vascular endothelial growth factor receptor-2 (VEGFR) expression for comparison of VEGFR-2 positive versus VEGFR-2 negative.
Figure 3.
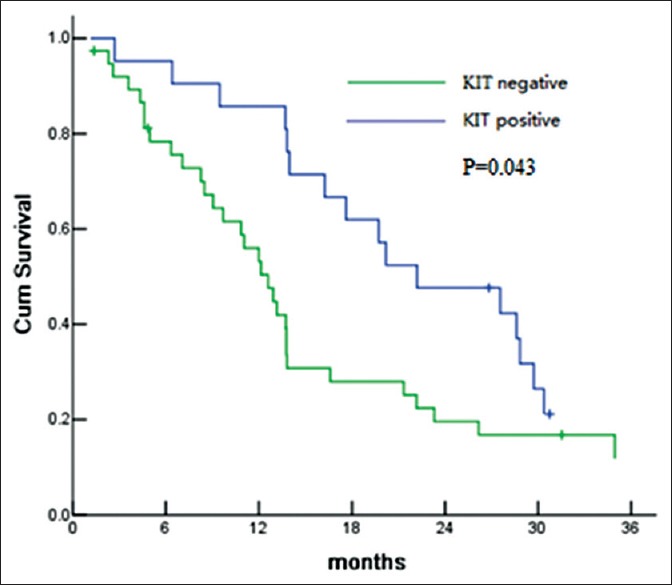
Progression-free survival according to KIT expression for comparison of KIT positive versus KIT negative.
Figure 4.
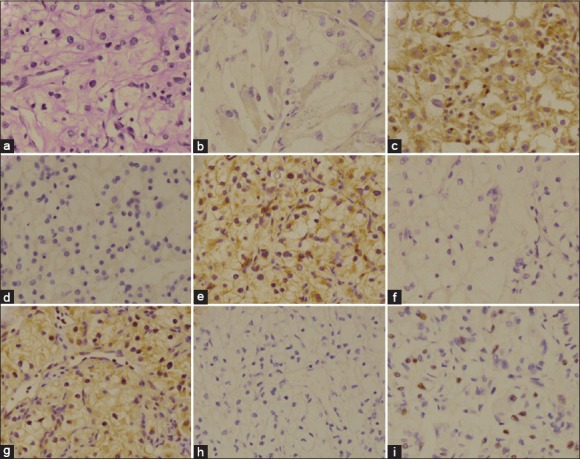
H and E staining (Original magnification ×200) of radical nephrectomy specimens (a); Immunohistochemical staining of radical nephrectomy specimens with a vascular endothelial growth factor receptor (VEGFR) antibody: VEGFR-1 negative (b); VEGFR-1 positive (c); VEGFR-2 negative (d); VEGFR-2 positive (e); VEGFR-3 negative (f); VEGFR-3 positive (g); Immunohistochemical staining of radical nephrectomy specimens with a KIT antibody: KIT negative (h); KIT positive (i).
DISCUSSION
Clear-cell renal cell carcinoma histology comprises more than 90% of patients with advanced renal cell carcinoma (RCC) and is characterized by VHL inactivation through VHL mutation or promoter methylation in most patients. VHL inactivation leads to the production of VEGFR and PDGFR by the tumor cell, and acceleration of angiogenesis, tumor growth, and metastases. Recently, therapies against VEGF protein or the VEGFR and PDGFR showed substantial clinical activity in RCC. Identifying clinical and/or tissue-based predictors of response might help to treat more patients and further the understanding of the biology underlying RCC and response to VEGF-targeted agents. Metastatic ccRCC patients urgently need molecular markers for the prediction of response to TKI treatment. Therefore, we systematically evaluated predictive factors involved in angiogenic pathways in tumor tissues from Chinese patients with metastatic ccRCC who were treated with sunitinib or pazopanib.
In this study, we explored VHL mutation, VEGFR or KIT expression that could possibly be associated with sunitinib or pazopanib activity. It is noted that a low incidence of VHL mutations in the 59 patients tested (18.6%). Guo et al. suggested a prevalence of VHL mutations varying from 27% to 55%.[10] Compared with most of the reports, VHL mutation rate is low in this study. This result may be related to the limited number of cases in this study, however, it does not rule out that there might be racial differences between east and west, the possibility of VHL mutation in east is low. No difference was found between the frequency of VHL mutation with not only clinical characteristic (gender, age, pathological grade, number of metastatic sites), but adverse event of targeted therapy (hypertension, hand-foot skin reaction, hypothyroidism, hyperlipidemia or fatigue). Similarly, the expression of VEGFR or KIT was not different whatever VHL mutation status was. Although not statistically significant, but the incidence rate of VHL mutation in high pathological grade (G3) was 2 times that in low pathological grade (G1 or G2). Ma et al. reported the VHL mutation rate was 33% in G3, significantly higher than that in G1 (22%) or G2 (9%).[11] Caused by VHL mutations, the tumor cell should be more aggressive and worse prognosis, so VHL mutation rate may be higher in poorly differentiated carcinoma. Similarly, the patients with hand-foot skin reaction or hyperlipidemia had a high incidence of VHL mutation than control groups. Perhaps, the patients with VHL mutation are more likely to appear hand-foot skin reaction or hyperlipidemia in targeted therapy of sunitinib or pazopanib. The exact mechanism of relationship of VHL mutation and the adverse event remains unclear, may be the factor caused VHL mutation in tumor tissues can also lead to VHL mutation in other organizations. Through the detection of VHL, adverse event of targeted therapy can be determined ahead of time, reduce the risk of treatment. However, the VEGFR-1, VEGFR-2, and VEGFR-3 expression were not significantly correlated with VHL mutation. Theoretically, VHL mutation leads to VEGFR expression increased, but that is not the case. Of course, the VHL pathway is complex. Perhaps unknown factors in VHL pathway make VEGFR expression and VHL mutation not consistent.
Currently, there is no consensus in the relationship between VHL inactivation and ccRCC targeted therapy. Kondo et al. showed that the presence of VHL mutation was associated with better cancer-specific survival in a multivariate Cox proportional hazard analysis (P = 0.023).[12] Yao et al. study failed to associate VHL mutations with a better cancer-specific survival in a subset of patients with RCC and metastatic disease.[13] In our study, the VHL mutation did not affect objective response to VEGF-targeted therapy. The patients with VHL mutation had a 45.5% response rate to VEGF-targeted agents. Similarly, the response rate of patients without VHL mutation is 47.9% (P = 0.938).
The presence of VERFR or KIT was not significantly associated with VHL mutation, but the patients with VHL mutation had a higher incidence of VEGFR-1 (81.8% vs. 64.6%) and VEGFR-3 (54.5% vs. 31.2%). VHL mutation may result in changes of HIF/VEGF pathway, leading to the downstream VEGFR expression increase. Na et al. considered that VHL gene inactivation leads to HIF up-regulation and increased VEGFR expression.[14] Of course, the VHL pathway is complex. The impact of a given VHL mutation or methylation on subsequent VHL protein structure and function and thus, on VEGFR expression, may not be straightforward.
In our study, the median PFS and OS were 13.8 and 39.9 months, respectively. Motzer et al. reported the median PFS of 11 months and median OS of 26.4 months in the subset of 375 sunitinib-treated patients.[15] In accordance with our results, the study by Choueiri et al. also demonstrated a median PFS and OS of 10.8 and 29.8 months though their patients received different VEGF-targeted therapies (63% sunitinib, 28% sorafenib, 14% axitinib, and 17% bevacizumab).[16] Although VHL gene status was not obviously correlated with PFS and OS, but median PFS in patients with VHL mutation extend for 7.5 months than that without VHL mutation. Perhaps with the increase of sample size, we may get a positive result. Rini et al. reported that the median TTP in patients with VHL mutation or methylation was 10.8 months versus 5.5 with no VHL mutation or methylation.[17] The PFS of VEGFR positive or KIT positive was significantly extended. However, only VEGFR-2 was an independent predictive factor of PFS. Despite inhibitory effects on multiple tyrosine kinases, sunitinib was demonstrated to most efficaciously inactivate the kinase activate of VEGFR-2.[18] Therefore, this finding would support the present outcome that patients with positive expression of VEGFR-2 in the primary specimen might be likely to derive greater clinical benefits from treatment with sunitinib or pazopanib than those with negative VEGFR-2 expression.
In its most generic sense, KIT positive is defined as cells with yellow to brown granules in the cell membrane or plasma in not only ccRCC but gastrointestinal stromal tumors. KIT expression in sarcomatoid RCC is common, but is rare in pure ccRCC, reported to be <5%. Sengupta et al. reported that KIT expression was observed in four patients of 175 ccRCC patients (2.3%).[19] Krüger et al. found none of 20 ccRCC patients was KIT positive.[8] Zhang et al. demonstrated a high KIT positive rate in 10.9% (13/119) ccRCC.[20] This study showed no expression in 59 patients. On the other hand, we observed 21 of 59 sections had brown granules in the nucleus. Although the real meaning of nucleus staining is not clear at present, we find out the median PFS of 22.2 months of KIT positive (nucleus staining) was significantly extended than that of 12.6 months of KIT negative. In Zhang's study, cell with yellow to brown granules in the membrane, plasma, or nuclear were calculated as KIT positive staining. Therefore KIT positive rate increased significantly (12/17), median PFS was 46 weeks in KIT positive patients and only 6 weeks in KIT negative ones.[9]
In conclusion, targeted therapies are the standard of treatments for patients with advanced ccRCC. Analysis of clinical and molecular characteristic of targeted therapeutics may provide an understanding of the mechanism of response and resistance, increase the benefit of current agents, and identify additional therapeutic targets. The VHL/VEGFR pathway may play an important role in the treatment of advanced ccRCC. Thus, efforts to identify predictive biomarkers and develop new drugs are critical for long-term survival of patients with ccRCC.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Choueiri TK, Fay AP, Gagnon R, Lin Y, Bahamon B, Brown V, et al. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res. 2013;19:5218–26. doi: 10.1158/1078-0432.CCR-13-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–81. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–5. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark PE. The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int. 2009;76:939–45. doi: 10.1038/ki.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XS, Wu X, Zhao PJ, Huang LH, Song Y, Gong K, et al. Efficacy and safety of sunitinib in the treatment of metastatic renal cell carcinoma. Chin Med J. 2011;124:2920–4. [PubMed] [Google Scholar]
- 7.Terakawa T, Miyake H, Kusuda Y, Fujisawa M. Expression level of vascular endothelial growth factor receptor-2 in radical nephrectomy specimens as a prognostic predictor in patients with metastatic renal cell carcinoma treated with sunitinib. Urol Oncol. 2013;31:493–8. doi: 10.1016/j.urolonc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Krüger S, Sotlar K, Kausch I, Horny HP. Expression of KIT (CD117) in renal cell carcinoma and renal oncocytoma. Oncology. 2005;68:269–75. doi: 10.1159/000086783. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HL, Zhu Y, Qin XJ, Wang CF, Yao XD, Zhang SL, et al. c-KIT: Potential predictive factor for the efficacy of sorafenib in metastatic renal cell carcinoma with sarcomatoid feature. Clin Genitourin Cancer. 2013;11:134–40. doi: 10.1016/j.clgc.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Guo G, Gui Y, Gao S, Tang A, Hu X, Huang Y, et al. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet. 2011;44:17–9. doi: 10.1038/ng.1014. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Yang K, Lindblad P, Egevad L, Hemminki K. VHL gene alterations in renal cell carcinoma patients: Novel hotspot or founder mutations and linkage disequilibrium. Oncogene. 2001;20:5393–400. doi: 10.1038/sj.onc.1204692. [DOI] [PubMed] [Google Scholar]
- 12.Kondo K, Yao M, Yoshida M, Kishida T, Shuin T, Miura T, et al. Comprehensive mutational analysis of the VHL gene in sporadic renal cell carcinoma: Relationship to clinicopathological parameters. Genes Chromosomes Cancer. 2002;34:58–68. doi: 10.1002/gcc.10054. [DOI] [PubMed] [Google Scholar]
- 13.Yao M, Yoshida M, Kishida T, Nakaigawa N, Baba M, Kobayashi K, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94:1569–75. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 14.Na X, Wu G, Ryan CK, Schoen SR, di’Santagnese PA, Messing EM. Overproduction of vascular endothelial growth factor related to von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol. 2003;170:588–92. doi: 10.1097/01.ju.0000074870.54671.98. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Vaziri SA, Jaeger E, Elson P, Wood L, Bhalla IP, et al. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180:860–5. doi: 10.1016/j.juro.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Jaeger E, Weinberg V, Sein N, Chew K, Fong K, et al. Clinical response to therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: Impact of patient characteristics and Von Hippel-Lindau gene status. BJU Int. 2006;98:756–62. doi: 10.1111/j.1464-410X.2006.06376.x. [DOI] [PubMed] [Google Scholar]
- 18.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 19.Sengupta S, Cheville JC, Corless CL, Lohse CM, Heinrich MC, Kwon ED, et al. Rare expression of KIT and absence of KIT mutations in high grade renal cell carcinoma. J Urol. 2006;175:53–6. doi: 10.1016/S0022-5347(05)00059-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Ye D, Yao X, Dai B, Zhang S, Shen Y, et al. Role of KIT expression in the prognosis of clear cell renal cell carcinomas in Chinese patients. J Cancer Res Clin Oncol. 2009;135:249–53. doi: 10.1007/s00432-008-0447-6. [DOI] [PubMed] [Google Scholar]


