Abstract
Background:
To develop a vaccine-based immunotherapy for sarcoma, we evaluated a mixture of heat shock proteins (mHSPs) as a vaccine for sarcoma treatment in a mouse model. Heat shock protein/peptides (HSP/Ps) are autoimmune factors that can induce both adaptive and innate immune responses; HSP/Ps isolated from tumors can induce antitumor immune activity when used as vaccines.
Methods:
In this study, we evaluated the effects of mHSP/Ps on prophylactic antitumor immunity. We extracted mHSP/Ps, including HSP60, HSP70, GP96, and HSP110, from the mouse sarcoma cell lines S180 and MCA207 using chromatography. The immunity induced by mHSP/Ps was assessed using flow cytometry, ELISPOT, lactate dehydrogenase release, and enzyme-linked immunosorbent assay.
Results:
Of S180 sarcoma-bearing mice immunized with mHSP/Ps isolated from S180 cells, 41.2% showed tumor regression and long-term survival, with a tumor growth inhibition rate of 82.3% at 30 days. Of MCA207 sarcoma-bearing mice immunized with mHSP/Ps isolated from MCA207 cells, 50% showed tumor regression and long-term survival with a tumor growth inhibition rate of 79.3%. All control mice died within 40 days. The proportions of natural killer cells, CD8+, and interferon-γ-secreting cells and tumor-specific cytotoxic T-lymphocyte activity were increased in the immunized group.
Conclusions:
Vaccination with a polyvalent mHSP/P cancer vaccine can induce an immunological response and a marked antitumor response to autologous tumors. This mHSP/P vaccine exerted greater antitumor effects than did HSP70, HSP60, or tumor lysates alone.
Keywords: Heat Shock Protein/Peptides, Immunotherapy, Prophylactic, Sarcoma, Tumor Vaccine
INTRODUCTION
Molecular chaperones, including heat shock proteins (HSPs) and glucose-regulated proteins (GRPs), are some of the most abundant cellular proteins. These proteins can be classified into three major groups by mass (i.e., the 90-, 70-, and 50-κDa to 60-κDa proteins), and can be further subdivided based on localization within the cell.[1] During increased protein production (e.g., tumor formation) and stress, these chaperones bind to exposed hydrophobic sites within nascent polypeptides. The tumor peptide antigens can bind to HSPs to form HSP/peptides (HSP/Ps), which induce immune responses.
The use of HSPs as tumor vaccines was first reported by Srivastava et al., who showed that HSPs, in particular GRP94, bind peptides that are immunogenic in vitro and in vivo. These HSP/Ps induce both specific and innate immune responses, as demonstrated by numerous studies. HSP/Ps can present antigenic fingerprints/peptides to major histocompatibility complex antigens and activate dendritic cells, the most efficient antigen-presenting cells, which when used as a vaccine can cause tumor regression.[2,3] More recently, HSP70, HSP110, and GRP170 purified from tumors were shown to function as tumor vaccines and cause tumor regression in animal models,[4,5,6] which resulted in CD8+ T-cell-dependent tumor clearance.[7,8] HSPs can also elicit innate immune responses.[9,10] Immune recognition does not originate from HSP itself, but from the binding peptides.[6] These encouraging results in animals have led to cancer vaccine trials of metastatic melanoma,[11,12] renal cell carcinoma,[13,14] colorectal cancer,[15] and chronic myelogenous leukemia.[16] The preliminary results from phase II and III clinical trials showed that a GRP96 (oncophage) vaccine has low to no toxicity, although the antitumor effect was not ideal. The challenge is to produce more effective tumor vaccines.
HSP/Ps have been shown to deliver tumor antigens as a fingerprint genome.[9] The various HSP subtypes can deliver different tumor antigens. For example, HSP110 delivers different antigens than does HSP70.[4] To date, all animal studies and clinical trials have used a single HSP subtype, such as Gp96, HSP70, or HSP110. Thus, we hypothesized that mixed HSP/Ps may deliver more specific tumor antigens and so exhibit greater efficacy. In one animal tumor model, the antitumor activity of the combination of Gp96 and HSP70 was superior to that of each protein alone.[17] Here, we developed a vaccine comprising a mixture of HSP/Ps (mHSP/Ps), which included HSP60 and HSP110 in addition to HSP70 and Gp96, and evaluated its antitumor effects in two mouse sarcoma models.
METHODS
Animals
Female 6–8-week-old BALB/C mice and C57 mice were purchased from the Military Medical Academy (China) and bred in the Chinese PLA General Hospital. The Institutional Animal Care and Use Committee approved the protocol.
Cell lines
The ascetic mouse S180 sarcoma cell line (ATCC TIB-66) was obtained from the Military Medical Academy and the mouse MCA207 sarcoma cell line was obtained from Dr. ZL Zong at Stanford Medical Center and reproduced in our laboratory. The S180 line was maintained by several passages through the BALB/C mouse peritoneal cavity. The MCA207 line was expanded subcutaneously in C57 mice.
Reagents
Anti-HSP60, -HSP70, -HSP110, and -GRP96/94 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Sephacryl S-200HR, Concanavalin A (ConA), and adenosine 5'-diphosphate affinity columns were purchased from Pharmacia (Dorval, Quebec, Canada).
Isolation of heat shock protein/peptides and vaccine
Isolation of mixture of heat shock protein/peptide using molecular chromatography
Tumor tissue (S180 or MCA207) was homogenized at 4°C in buffer (30 mmol/L NaHCO3, pH 7.1), with freshly added protease inhibitor, phenylmethylsulfonyl fluoride (PMSF, 0.5 mmol/L). The homogenate was centrifuged at 10,000 × g for 30 min at 4°C, after which the supernatant was centrifuged at 100,000 × g at 4°C for 2 h. The supernatant (tumor lysate) was dialyzed against 20 mmol/L Tris-HCl and 150 mmol/L NaCl, pH 7.2; the tumor supernatant was then applied to Sephacryl S-200 HR. After equilibration, the protein was eluted with the same buffer and monitored using an ultraviolet monitor. The collected eluted protein fractions were subjected to resolution by SDS-PAGE; fractions 3–6 contained 40- to 200-kDa proteins; thus, we combined these four fractions as the mHSP/Ps. mHSP/Ps were isolated from skeletal muscles and liver of wild-type mice using the same methods. Purification of GRP96, HSP70, and HSP60 was performed as described previously.[17,18]
Purification of GRP96
mHSP/Ps were applied to a ConA-Sepharose 4B column (Pharmacia Biotech, USA) equilibrated with binding buffer (20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 10 mmol/L MgCl2, 10 mmol/L CaCl2, 15 mmol/L 2-ME); the binding proteins were eluted with binding buffer containing 15% α-D-methylmannoside (Sigma).
Purification of heat shock protein 70 and heat shock protein 60
The nonbinding fraction of ConA was used to purify HSP70 and HSP60, applied to an ADT-Sepharose column, and equilibrated with binding buffer (20 mmol/L Tris-HCl, pH 7.5, 20 mmol/L NaCl, 3 mmol/L MgCl2, 15 mmol/L 2-ME, 0.5 mmol/L PMSF). After 30 min, the solution was eluted with 500 mmol/L NaCl. The bound and eluted components were collected separately.
Peptides were identified by SDS-PAGE and Western blot analysis. Each protein sample was subjected to 10% SDS-PAGE and transferred to membranes (Millipore, USA). Membranes were blocked with 5% nonfat milk in TBST for 1 h at room temperature and then incubated with primary antibodies and horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG. Immunoreactivity was detected using the enhanced chemiluminescence detection system (SyZhi.com, Beijing).
Immunization and tumor challenge
The antitumor effects were evaluated in prophylactic models. S180 or MCA207 mHSP/Ps or NS (2, 5, 10, 20, or 30 µg) was injected subcutaneously in all mice 3 times at weekly intervals. Seven days after the second injection, BALB/C mice were injected subcutaneously with S180 tumor cells (4 × 104) in the back hind quarters s.c., and C57 mice were injected with MCA207 tumor cells (5 × 106).
The antitumor potency was evaluated based on tumor volume, tumor growth inhibition rates, and survival time. The shortest diameter (A) and longest diameter (B) were measured using a caliper every 3 days to monitor tumor growth. The tumor volume (V) was calculated using the formula V = (A2B/2). Tumor growth inhibition rates were measured 30 days after tumor transplantation. The rate was calculated using the formula R = tumor volume of immunized mice/tumor volume of phosphate-buffered saline-treated mice. Survival rate or complete regression was considered when the tumor did not appear after more than 3 months.
Immune response
Treatment of mice for the analysis of immune responses was the same as for the prophylactic protocol. Three days after the third immunization with S180 mHSP/Ps, all mice were euthanized and blood and spleen samples collected. Control group mice were euthanized at the same time; each group comprised three mice.
Assay for T-cell subgroups
Subgroups of T-lymphocytes in sera from each group of S180-immunized mice were analyzed using a FACScan instrument (Becton Dickinson, USA). Cell staining involved the use of a fluorescein isothiocyanate- or phycoerythrin-conjugated goat antibody against mouse CD3+, CD4+, CD8+, or natural killer (NK) cells. The antibodies were purchased from Serotect (UK).
Cytotoxicity assay (cytotoxic T-lymphocytes)
The in vitro tumor-specific cytotoxic T-lymphocyte (CTL) response elicited by immunization with mHSP/P was analyzed by means of lactate dehydrogenase release from damaged cells. Splenocytes of mHSP/P-immunized mice were isolated and purified using Ficoll-Paque density centrifugation and used as effector cells. After re-stimulating with ConA and mHSP/P in vitro for 4 days, S180 cells as target cells were first seeded on 96-well plates, after which expanded lymphocytes were serially diluted in 96-well plates in triplicate with effector: Target(E: T) ratios of 40:1, 20:1, and 5:1. Culture medium was added to a final volume of 200 µl/well. Wells containing only target cells or lymphocytes with culture medium were spontaneous-release wells; target cells treated with 0.5% Triton X-100 served as maximal release controls. After 24-h incubation at 37°C and 5% CO2, 150 µl of the supernatant from each well were transferred to a new 96-well plate, scanned using a 96-well plate reader (Bio-Rad), and optical density read at a wavelength of 490 nm. The percent specific lysis was calculated using the following formula: % specific lysis = 100 × (experimental release − spontaneous release/maximum release − spontaneous release).
EISPOT assays for interferon-γ
Splenocytes of immunized mice were obtained and expanded as described for the CTL assay. Half of the cells were incubated with ConA and half were additionally re-stimulated with mHSP/P for 5 days in 96-well ELISPOT plates. The assays were performed according to the manufacturer's instructions (Utrecht CJ, U-CyTech B.V., The Netherlands). All experiments were performed in triplicate.
Antibody assay
The serum was separated from the blood of immunized mice in each group, diluted, incubated with antibodies against HSP60 and HSP70, and assayed using ELISA.
Statistical analysis
Statistical analysis used SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Data are shown as means ± standard deviation (SD). Log-rank testing was used to evaluate survival. A two-tailed paired t-test with Welch correction was used for comparison of interferon-γ (IFN-γ) levels. P < 0.05 was considered to indicate statistical significance.
RESULTS
Isolation of heat shock protein/peptides
mHSP/Ps were obtained from S180 and MCA207 tumors and liver or muscles of wild-type mice. HSP60, HSP70, Gp96, and HSP110 isolated from S180 tumors were subjected to SDS-PAGE [Figure 1a] and identification by Western blotting analysis [Figure 2]. In total, 2–2.5-mg mHSP/P was obtained from each gram (wet weight) of the tumor. This yield was higher than that of pure HSP70 (100–150 µg) and pure HSP60 (400–500 µg). mHSP/P obtained from wild-type mice liver is shown in Figure 1b.
Figure 1.
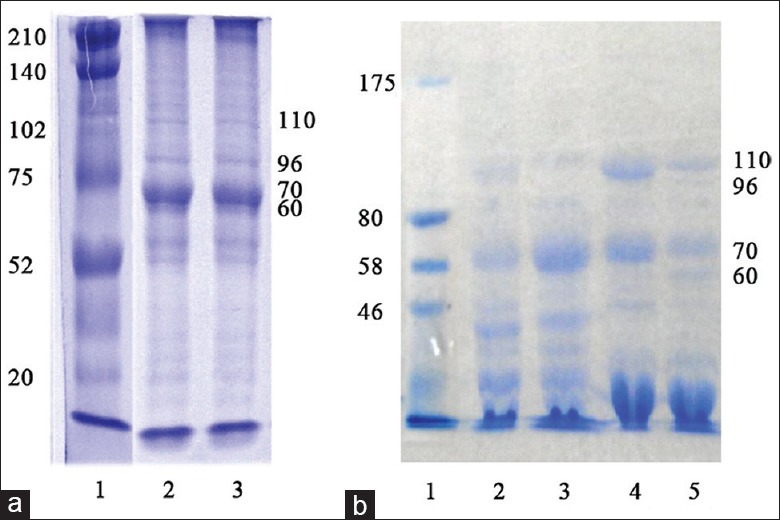
(a) SDS-PAGE of mixed heat shock proteins/peptides from (a) S180 sarcoma cells and (b) tissue of wild-type mice. (a) S180 cells: Lane 1, standard; lanes 2 and 3, bands for HSP110, GRP96, HSP70, and HSP60. (b) Wild-type mice: Lane 1, standard; lanes 2 and 3, bands for HSP110, GRP96, HSP70, and HSP60 in muscle. Lanes 4 and 5, bands for HSP110, GRP96, HSP70, and HSP60 in liver (HSP: Heat shock protein).
Figure 2.
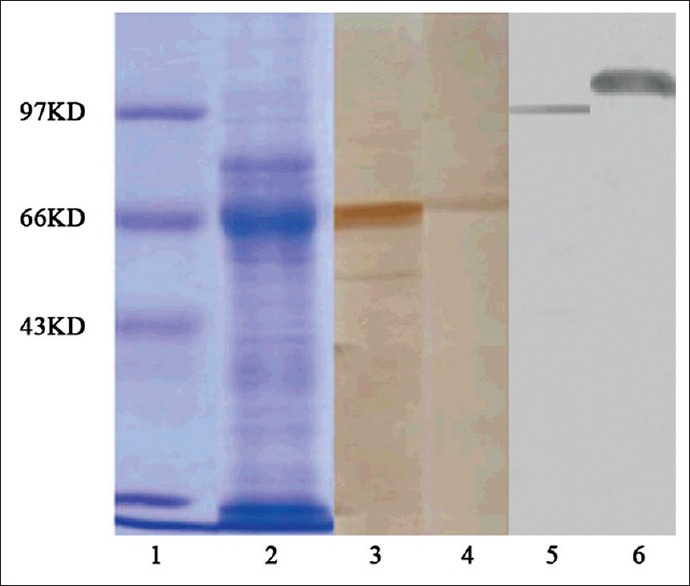
SDS-PAGE and Western blotting analysis of heat shock protein/peptides of S180 cells after Concanavalin A affinity chromatography. Coomassie light-blue staining of a 10% SDS-PAGE gel. Lane 1, standard; lane 2, fractions 3-6. Western blot analysis: Lane 3, proteins that did not bind the ATP column; lane 4, protein that bound to ATP; lane 5, anti-GRP96 antibody staining; lane 6, anti-heat shock protein 110 antibody staining.
Antitumor effects of mixture of heat shock protein/peptides
S180 model
BALB/C mice were administered S180 mHSP/Ps at 2, 5, 10, 20, and 30 µg. After vaccination, tumors transiently developed in some vaccinated mice, while no measurable tumor mass developed in others. All doses produced antitumor effects. Tumor growth volume was significantly smaller in vaccinated than unvaccinated mice at 30 days (P < 0.01). The most effective dose was 20 µg: The growth inhibition rate was 82.3% and complete regression occurred in 7 of 17 mice (41.2%) [Table 1].
Table 1.
Prophylactic antitumor effects of varying doses of mHSP/Ps in S180 model
| Indices | Injection dose (μg) | |||||
|---|---|---|---|---|---|---|
| Saline control | 2 | 5 | 10 | 20 | 30 | |
| Number of animals tested | 10 | 10 | 7 | 7 | 17 | 10 |
| Number of complete regression | 0 | 4 | 2 | 1 | 7 | 2 |
| Growth inhibition (%) | – | 66.5 | 75.2 | 54.8 | 82.3 | 42.3 |
mHSP/Ps: Mixed heat shock protein/peptides.
MCA207 model
C57 mice were administered MCA207 mHSP/Ps at 5, 10, 20, and 30 µg. All doses produced antitumor effects; the mean tumor regression was 50%, and the most effective dose was 30 µg (70% tumor regression). Tumor growth volume at 30 days was significantly smaller than that in the controls (P < 0.01) [Table 2 and Figure 3]. In this model, no mouse showed metastasis.
Table 2.
Prophylactic antitumor effects of varying doses of mHSP/Ps in MCA207 model
| Indices | Injection dose (μg) | ||||
|---|---|---|---|---|---|
| Saline control | 5 | 10 | 20 | 30 | |
| Number of animals tested | 10 | 10 | 10 | 10 | 10 |
| Number of complete regression | 0 | 3 | 6 | 4 | 7 |
| Growth inhibition (%) | – | 68.2 | 76.8 | 58.3 | 79.3 |
mHSP/Ps: Mixed heat shock protein/peptides.
Figure 3.
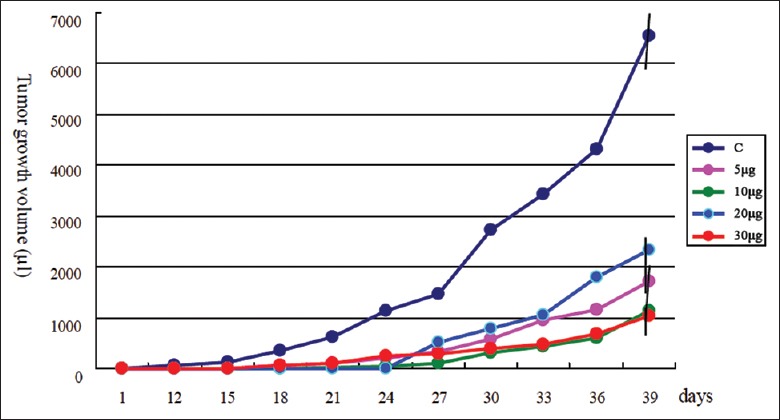
Effect of immunization with MCA207 tumor-cell derived mixture of heat shock protein/peptides on tumor growth. Three mixture of heat shock protein/peptide treatments in MCA207 tumor-bearing mice compared with the saline-treated group (C); the tumor growth volume in the mixture of heat shock protein/peptides group was significantly lower than that in the control group (P < 0.01).
In these two mouse models, the antitumor effects were not in parallel with the vaccinated doses, which showed that a large dose range of antigen could stimulate an immune reaction.
To determine whether this antitumor activity induces long-term immunity against tumors, we challenged mice that survived with 104 S180 cells 12 months after the first challenge, but no mouse developed tumors.
To explore the specificity of mHSP/P vaccine, three mice were vaccinated with the MCA207 mHSP/P, and the MCA207 tumor was rejected. Mice were then re-challenged with MCA207 and S180. The MCA207 tumors did not regrow, but all S180 tumors regrew. Thus, the mHSP/P-induced immune reaction was tumor specific.
We next compared the antitumor effects of mHSP/Ps and single HSP/P (HSP60 or HSP70) vaccines in the S180 model. All mice were subcutaneously vaccinated with 20-µg HSP/Ps (as this amount exerted the greater antitumor effects) 3 times at weekly intervals. The antitumor effects of mHSP/Ps were superior to those of HSP60, HSP70, and tumor lysates [Table 3 and Figure 4], with no significant differences between mHSP/Ps and single HSPs or tumor lysates (P > 0.05). Thus, mHSP/P may enhance the antitumor effect.
Table 3.
Comparison of antitumor effects of various HSPs of S180
| Indices | Untreated | mHSP/P | HSP70 | HSP60 | Tumor lysate |
|---|---|---|---|---|---|
| Number of animals | 10 | 10 | 10 | 5 | 10 |
| tested | |||||
| Number of complete | 0 | 4 (40) | 3 (33.3) | 1 (20) | 2 (20) |
| regression (%) | |||||
| Growth inhibition (%) | – | 82.3 | 62.3 | 72.6 | 66.2 |
HSPs: Heat shock proteins; mHSP/P: Mixed heat shock protein/peptide.
Figure 4.
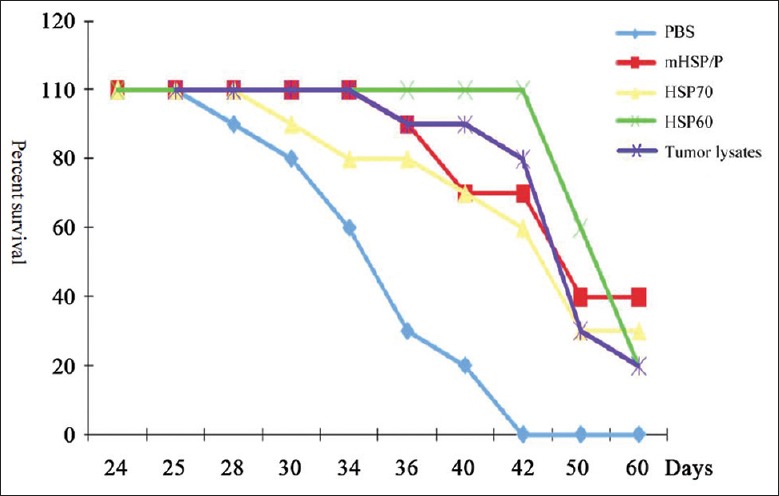
Effect of immunization with various tumor-derived mixtures of heat shock protein/peptides, HSP70, and HSP60 on the survival of S180 tumor-bearing mice. Comparison of immunization with mixture of heat shock protein/peptide, HSP70, HSP60, and tumor lysates in S180 tumor-bearing mice; survival following vaccination with mixture of heat shock protein/peptides (40%) was higher than that with HSP70 (33.3%), HSP60 (20%), and tumor lysates (20%) when compared to mixture of heat shock protein/peptide and phosphate-buffered saline control treatment, P < 0.01. No significant difference between mixture of heat shock protein/peptide and HSP70, HSP60, and tumor lysates (P > 0.01), (HSP: Heat shock protein).
Immune reaction induced by mixture of heat shock protein/peptides
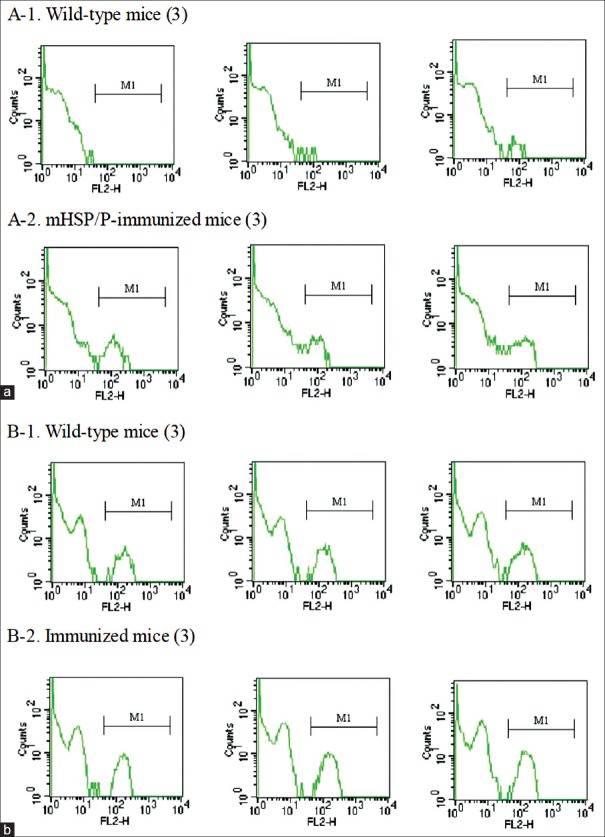
Numbers of CD8+ cells and NK cells were elevated following S180 mHSP/P treatment in BALB/C mice, as determined using flow cytometry analysis. The mean proportion of CD8+ cells among total mononuclear cells was 5.89% ± 0.36% in wild-type mice [Figure 5]. After mHSP/P immunization, the mean proportion of CD8+ cells among total mononuclear cells increased to 9.21% ± 1.45%. The mean proportion of NK cells among total mononuclear cells in wild-type mice was 1.69% ± 0.32%. After mHSP/P immunization, the mean proportion of NK cells among total mononuclear cells increased to 5.29% ± 0.25%.
Figure 5.
(a and b) Analysis of natural killer cells (a) and CD8+ cells (b) in S180 mixture of heat shock protein/peptide-immunized mice using flow cytometry.
INF-γ level increased in S180 mixture of heat shock protein/peptide-treated mice
The level of IFN-γ from splenocytes expanded with ConA and stimulated with mHSP/P in vitro was higher than that of cells expanded with ConA alone in vitro. Upon stimulation with ConA and mHSP/Ps, the mean IFN-γ level in mHSP/P-immunized mice was 246.6 ± 7.45/106 cells, higher than that of wild-type (180 ± 3.082/106 cells, P < 0.05) and tumor-bearing (121.3 ± 1.00/106, P < 0.01) mice [Figure 6]. The level of IFN-γ from splenocytes stimulated with ConA alone was significantly higher in mHSP/P-immunized than in untreated tumor-bearing mice and wild-type mice.
Figure 6.
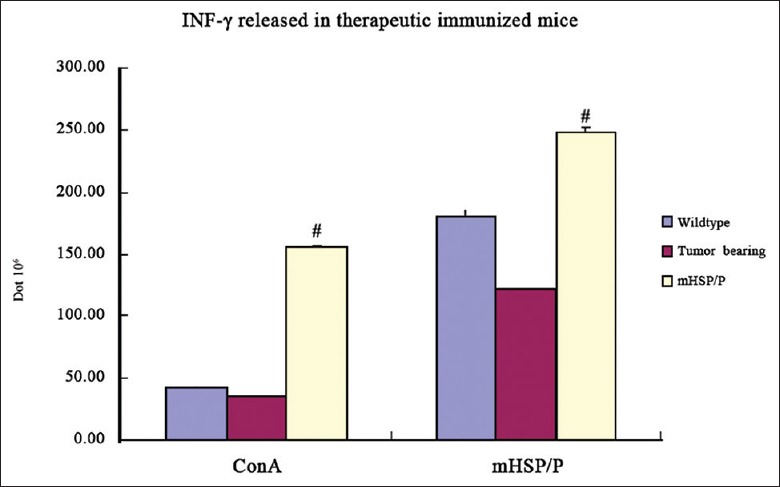
Interferon-γ release in S180 mixture of heat shock protein/peptide-immunized mice evaluated by ELISPOT. Interferon-γ released from splenocytes stimulated with both Concanavalin A and mixture of heat shock protein/peptide in vitro in mixture of heat shock protein/peptide immunized S180-bearing mice (246.6 ± 7.45/106 cells) was higher than that in untreated tumor-bearing (121.3 ± 1.00/106, #P < 0.01) or wild-type (180 ± 3.082/106 cells, #P < 0.05) mice.
Cytotoxic T-lymphocyte generated by mixture of heat shock protein/peptide were capable of killing target cells
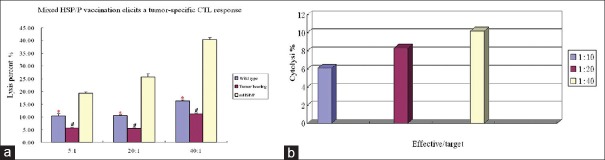
To assess the functional effector properties of CTLs generated by mHSP/P, we performed in vitro cytotoxicity assays of T-lymphocytes isolated from mice vaccinated with S180 mHSP/Ps. Target cells (S180) pulsed with effector T-cells were killed in an E/T ratio-dependent manner: 1:5 = 21%, 1:20 = 27%, 1:40 = 44%, which is significantly higher than that of tumor-bearing mice that did not receive mHSP/Ps (P < 0.01) and wild-type mice (P < 0.05) [Figure 7a]. T-lymphocytes isolated from mice vaccinated with S180 mHSP/Ps did not kill MCA207 cells at E/T ratios of 5:1, 20:1, and 40:1. The cytolytic ratios were 6.1%, 8.3%, and 10.0% [Figure 7b]. In addition, T-lymphocytes isolated from liver or muscle of wild-type mice vaccinated with mHSP/Ps did not kill the S180 sarcoma cells; all cytolytic ratios were lower than 1%.
Figure 7.
Cytotoxicity of T-lymphocytes from S180 mixture of heat shock protein/peptide-immunized mice based on lactate dehydrogenase release. (a) Comparison of mixture of heat shock protein/peptide-immunized mice with tumor-bearing mice and wild-type mice. The cytotoxicity of mixed lymphocytes against S180 tumor cells in mice immunized with mixture of heat shock protein/peptide was higher than that in untreated tumor-bearing (E/T = 5:1, 20:1 and 40:1, *P < 0.01) and wild-type (E/T = 5:1, 20:1 and 40:1, #P < 0.05) mice. (b) Cytotoxicity of lymphocytes from mice vaccinated with S180 mixture of heat shock protein/peptides against MCA207.
Antibody detected in immunized mice
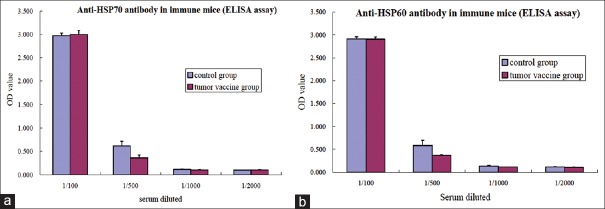
All mice in the control and mHSP/P groups were positive for anti-HSP70 and -HSP60 antibodies using serum dilutions of 1:500–1:2000. Moreover, there were no differences in levels of antibodies against HSP60 and HSP70 [Figure 8].
Figure 8.
(a and b) Cytotoxicity of lymphocytes from mice vaccinated with S180 mixture of heat shock protein/peptides.
DISCUSSION
The efficacy of current standard therapies for the treatment of sarcoma remains limited. To facilitate the development of vaccine-based immunotherapy for sarcoma, we evaluated mHSP/Ps as a vaccine treatment for sarcoma in mice. HSP/Ps can induce protection against auto-tumors and generate specific CTLs. HSP/P vaccines may be used to treat a wide range of cancers. Vaccination with Gp96 (oncophage) and HSP70 as immunotherapy has been tested in clinical trials. However, use of a mixture of HSP60, HSP70, HSP110, and Gp96 (mHSP/P) as a vaccine for immunotherapy of sarcoma has not been reported. Here, we examined the capacity of mHSP/P, a mixture of HSP60, HSP70, HSP110, and Gp96, as a vaccine for immunotherapy of sarcoma in mouse models. mHSP/P vaccination induced an antitumor immune response and significantly inhibited S180 and MCA207 tumor growth in mice, and significantly prolonged their survival. The antitumor effects of mHSP/Ps may improve the antitumor effects of vaccination with HSP70, HSP60, or tumor lysate.
The HSP/P profile expressed in sarcoma has rarely been reported. We examined 110 cases of human orthopedic tumors, including osteosarcoma, chondrosarcoma, fibrosarcoma, liposarcoma, giant cell sarcoma, and synovial sarcoma. HSP60 was 100% highly expressed in those tumors based on immunohistochemistry,[19] and was abundant in S180 and MCA207 cell lines, as shown by SDS-PAGE and Western blot analysis. HSP110 was 90% expressed, besides HSP70, and Gp96, which are highly expressed in cancer cells. Thus, we isolated all subtypes of HSP/Ps from mouse sarcoma.
In our experiments, the antitumor effect of mHSP/Ps was greater than that of any single HSP; HSP60 is a potent adjuvant that induces innate immune responses and has been used in vaccines as an effective adjuvant.[20,21] Another major component, HSP110, was more effective than HSP70 in binding the peptide chain and can bind various peptides originating from HSP70 or Gp96.[4] Our mixture of HSP/Ps included HSP60 and HSP110, in addition to HSP70 and Gp96, and could bind more antigen peptides and potentially induce multifarious immune responses. This may explain why use of multiple mHSP/Ps results in greater antitumor effects compared to use of single HSP70, HSP60 alone, although the differences between mHSP/P and HSP70 or HSP60 were not significant. In addition, mHSP/Ps isolated from MCA207 cells were more effective than those isolated from S180 cells, possibly because S180 is more aggressive than MCA207. In the S180 model, metastasis of the tumor to lung occurred in 90% of the mice, compared to 0% in the MCA207 model. The mean survival of S180 tumor-bearing mice was 35 days, and that of MCA207 tumor-bearing mice was 54 days. Thus, tumor malignancy may be associated with immune antitumor effects.
We investigated the specificity of the mHSP/P vaccine in our experiments because the mHSP/P of the S180 model did not induce cytolysis of MCA207 (<10% cytolysis), and mice vaccinated with the MCA207 mHSP/Ps showed protection against challenge only with MCA207, not S180, in vivo. In addition, mHSP/Ps isolated from liver and muscle of wild-type mice had no cytolytic effect against S180 sarcoma in vitro. Thus, the mHSP/P induced an immune reaction was against only an autologous tumor-specific antigen, as with individual vaccines.
In addition to tumor antigenic peptides, a vaccine may contain an array of self-peptide complexes, thus raising the possibility of eliciting auto-immunity; in particular, HSP60 may be associated with the development of arthritis, systemic lupus erythematosus, atherosclerosis, autoimmune thyroid disease, and diabetes.[22,23,24,25] However, in such cases the autoantibody level was considerably higher than that in normal individuals. In our experiment, the levels of antibody against HSP60 and HSP70 in immunized mice were similar to those in wild-type mice. The levels of antibodies against HSP60 and HSP70 were elevated in wild-type mice, perhaps because of natural bacterial infection, which resulted in the production of anti-HSP antibodies against xenogeneic organisms. Antibody against HSP60 can be detected in normal humans;[26] however, our results indicated that immunization with mHSP/P did not increase the level of antibodies against HSP60 or HSP70, which suggested that mHSP/Ps may not induce autoimmune diseases. However, this hypothesis requires further investigation.
NK cells can directly induce tumor cell lysis and enhance CTL activity. We found that the number of NK cells and the INF-γ level were elevated in mHSP/P-treated mice, which increased the frequency of IFN-γ-producing cells and IFN-γ-secreting NK cells. Thus, mHSP-mediated NK cell activation could play a role in eliciting antitumor responses. This finding is consistent with the use of HSP70 as a vaccine in a clinical trial.[27]
Vaccination with whole tumor cells or tumor cell lysates have been investigated in phase III clinical trials of colon cancer and melanoma.[28,29,30,31,32] In our mouse model, vaccination with mHSP/Ps showed better results than tumor lysates. The mHSP/Ps contained mainly high-molecular-weight proteins (about 40–200 kDa), as determined by chromatography. Small molecules – such as transforming growth factor β, interleukin 10, prostaglandin, or immunosuppressive cytokines – were excluded.
In summary, this preliminary study of a mHSP/P tumor vaccine showed induction of effective immune responses in immunized mice. The use of mHSP/Ps as a vaccine can provide a broad antigen repertoire of tumor cell peptides, and sufficient quantities of mHSP/P can be prepared from a small amount of tumor tissue. Overall, mHSP/P functions as a potent antitumor vaccine and will facilitate future clinical studies of autologous, patient-specific HSP-based vaccines for immunotherapy of human sarcoma.
Footnotes
Edited by: Xiu-Yuan Hao
Source of Support: This work was supported by the National High technique Research and Development Program of China (863 No. 2007AA021806).
Conflict of Interest: None declared.
REFERENCES
- 1.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–8. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 2.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–8. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986;83:3407–11. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166:490–7. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 5.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–20. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 6.Hoos A, Levey DL. Vaccination with heat shock protein-peptide complexes: From basic science to clinical applications. Expert Rev Vaccines. 2003;2:369–79. doi: 10.1586/14760584.2.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Ciupitu AM, Petersson M, Kono K, Charo J, Kiessling R. Immunization with heat shock protein 70 from methylcholanthrene-induced sarcomas induces tumor protection correlating with in vitro T cell responses. Cancer Immunol Immunother. 2002;51:163–70. doi: 10.1007/s00262-002-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janetzki S, Blachere NE, Srivastava PK. Generation of tumor-specific cytotoxic T lymphocytes and memory T cells by immunization with tumor-derived heat shock protein gp96. J Immunother. 1998;21:269–76. doi: 10.1097/00002371-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia. 2002;18:576–85. doi: 10.1080/0265673021000017109. [DOI] [PubMed] [Google Scholar]
- 10.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: Clinical and immunologic findings. J Clin Oncol. 2002;20:4169–80. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 12.Eton O, Ross MI, East MJ, Mansfield PF, Papadopoulos N, Ellerhorst JA, et al. Autologous tumor-derived heat-shock protein peptide complex-96 (HSPPC-96) in patients with metastatic melanoma. J Transl Med. 2010;8:9. doi: 10.1186/1479-5876-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aalamian M, Fuchs E, Gupta R, Levey DL. Autologous renal cell cancer vaccines using heat shock protein-peptide complexes. Urol Oncol. 2006;24:425–33. doi: 10.1016/j.urolonc.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: A multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–54. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 15.Pilla L, Squarcina P, Coppa J, Mazzaferro V, Huber V, Pende D, et al. Natural killer and NK-Like T-cell activation in colorectal carcinoma patients treated with autologous tumor-derived heat shock protein 96. Cancer Res. 2005;65:3942–9. doi: 10.1158/0008-5472.CAN-04-3493. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Qiao Y, Liu B, Laska EJ, Chakravarthi P, Kulko JM, et al. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin Cancer Res. 2005;11:4460–8. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 17.Graner M, Raymond A, Akporiaye E, Katsanis E. Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother. 2000;49:476–84. doi: 10.1007/s002620000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ménoret A. Purification of recombinant and endogenous HSP70s. Methods. 2004;32:7–12. doi: 10.1016/s1046-2023(03)00180-4. [DOI] [PubMed] [Google Scholar]
- 19.Tang D, Khaleque MA, Jones EL, Theriault JR, Li C, Wong WH, et al. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo . Cell Stress Chaperones. 2005;10:46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu NR, Wu HB, Wu TC, Boux LJ, Mizzen LA, Siegel MI. Immunotherapy of a human papillomavirus type 16 E7-expressing tumor by administration of fusion protein comprised of Mycobacterium bovis BCG Hsp65 and HPV16 E7. Cell Stress Chaperones. 2000;5:401–5. doi: 10.1379/1466-1268(2000)005<0401:ioahpt>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterloh A, Meier-Stiegen F, Veit A, Fleischer B, von Bonin A, Breloer M. Lipopolysaccharide-free heat shock protein 60 activates T cells. J Biol Chem. 2004;279:47906–11. doi: 10.1074/jbc.M408440200. [DOI] [PubMed] [Google Scholar]
- 22.van Eden W. Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol Rev. 1991;121:5–28. doi: 10.1111/j.1600-065x.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 23.Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, et al. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: Immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–6. doi: 10.1161/01.cir.99.12.1560. [DOI] [PubMed] [Google Scholar]
- 24.McGregor AM. Heat shock proteins and autoimmune thyroid disease; too hot to handle? J Clin Endocrinol Metab. 1992;74:720–3. doi: 10.1210/jcem.74.4.1548333. [DOI] [PubMed] [Google Scholar]
- 25.Jones DB, Hunter NR, Duff GW. Heat-shock protein 65 as a beta cell antigen of insulin-dependent diabetes. Lancet. 1990;336:583–5. doi: 10.1016/0140-6736(90)93390-b. [DOI] [PubMed] [Google Scholar]
- 26.Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Y, Chen X, Larmonier N, Larmonier C, Li G, Sepassi M, et al. Natural killer cells play a key role in the antitumor immunity generated by chaperone-rich cell lysate vaccination. Int J Cancer. 2006;119:2624–31. doi: 10.1002/ijc.22150. [DOI] [PubMed] [Google Scholar]
- 28.Bohle W, Schlag P, Liebrich W, Hohenberger P, Manasterski M, Möller P, et al. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor-cell vaccine. First clinical results with tumor-cell vaccines modified with live but avirulent Newcastle disease virus. Cancer. 1990;66:1517–23. doi: 10.1002/1097-0142(19901001)66:7<1517::aid-cncr2820660714>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Uyl-de Groot CA, Vermorken JB, Hanna MG Jr, Verboom P, Groot MT, Bonsel GJ, et al. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: A prospective study of medical and economic benefits. Vaccine. 2005;23:2379–87. doi: 10.1016/j.vaccine.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Hanna MG Jr, Hoover HC Jr, Vermorken JB, Harris JE, Pinedo HM. Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: First randomized phase III trials show promise. Vaccine. 2001;19:2576–82. doi: 10.1016/s0264-410x(00)00485-0. [DOI] [PubMed] [Google Scholar]
- 31.Berd D, Sato T, Maguire HC Jr, Kairys J, Mastrangelo MJ. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol. 2004;22:403–15. doi: 10.1200/JCO.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 32.Sondak VK, Sosman JA. Results of clinical trials with an allogenic melanoma tumor cell lysate vaccine: Melacine. Semin Cancer Biol. 2003;13:409–15. doi: 10.1016/j.semcancer.2003.09.004. [DOI] [PubMed] [Google Scholar]