Abstract
Background:
Systemic chemotherapy (SC) is the recommended treatment for gastric cancer with liver metastasis. However, the improvement in survival has been disappointing. The aim of this study was to compare the therapeutic efficacy of gastrectomy with transarterial chemoembolization plus SC (GTC) and SC alone for gastric cancer with synchronous liver metastasis.
Methods:
From January 2008 to December 2013, 107 gastric cancer patients with synchronous liver metastasis attending the four participating centers were enrolled in this multicenter, ambispective, controlled cohort study. Patients who underwent GTC (n = 32) were compared with controls who were received SC alone (n = 75). The primary endpoints of the study were overall survival (OS) and progression-free survival (PFS). The secondary endpoints were response rate to treatment and treatment-related adverse effects.
Results:
The median OS was 14.0 months (95% confidence interval [CI]: 13.1–14.9 months) in the GTC treatment group and 8.0 months (95% CI: 6.6–9.4 months) in SC group, this difference being statistically significant (P < 0.001). The median PFS was significantly longer in the GTC than in the SC group (5 months, 95% CI: 2.2–7.8 months vs. 3 months, 95% CI: 2.3–3.4 months, respectively) (P < 0.001). The rate of response to treatment was significantly better in the GTC than the SC group (59.4% vs. 37.4%, respectively) (P = 0.035). According to multivariate analysis, OS in patients receiving combination treatment was significantly correlated with the size (P = 0.037) and extent of liver metastases (P < 0.001). PFS was also correlated with the extent of liver metastases (P = 0.003).
Conclusions:
GTC is more effective than SC alone in patients with gastric cancer with synchronous liver metastasis. GTC therapy prolongs the survival of selected gastric cancer patients with synchronous liver metastasis.
Keywords: Chemotherapy, Gastrectomy, Gastric Cancer, Liver Metastases, Transcatheter Arterial Chemoembolization
INTRODUCTION
The presence of liver metastasis is one of the most important prognostic factors in patients with gastric cancer.[1,2,3,4] Because this has usually been considered as systemic disease;[5] these patients have been treated palliatively with therapy including chemotherapy, best supportive care, or admission to a clinical trial.[6] However, their survival is far from satisfactory.
In recent decades, multimodality approaches using various combinations of chemotherapy, radiotherapy, and surgery have been evaluated in patients with gastric cancer with liver metastases.[6,7,8,9,10,11,12] Many studies have shown that curative surgical resection for liver metastasis can result in long-term survival in some highly selected patients with a solitary hepatic metastasis from gastric cancer.[6,8,11,12,13] Unfortunately, most gastric cancer patients with liver metastasis are not candidates for liver surgery because they have multiple, scattered, and bilobar hepatic lesions or also have other distant metastases or extensive lymph node metastases.[1,14] Patients with gastric cancer and isolated liver metastasis are rare, comprising only 0.5% of patients in a series reported by Linhares et al.[15] Transarterial chemoembolization (TACE) is an alternative therapy for patients with gastric cancer and liver metastasis in whom hepatic resection cannot be performed. Hirasawa et al.[9] reported the effects of TACE using degradable starch microspheres after prior systemic chemotherapy (SC) in gastric cancer patients with liver metastasis. TACE has frequently been used to treat unresectable liver metastases from gastric cancer, and the outcomes have been promising.
Whether combination TACE therapy confers survival benefit in patients with gastric cancer and synchronous liver metastasis (GCSLM) is still an unanswered question. To answer it, we compared the therapeutic efficacy of gastrectomy with TACE plus SC (GTC) and SC alone (SC) for GCSLM. We also identified the predictors of outcome after GTC treatment.
METHODS
Eligibility and enrollment
This was a multicenter, ambispective, controlled cohort study. From January 2008 to December 2013, 107 patients with GCSLM attending the Chinese People's Liberation Army General Hospital, Beijing Cancer Hospital, Peking University People's Hospital, and Beijing Friendship Hospital undergoing GTC treatment or SC were enrolled in this study. The data were collected retrospectively from January 2008 to May 2012 and have been collected prospectively since June 2012. Thirty-two patients received GTC therapy, whereas 75 received SC therapy. We defined synchronous metastases as metastases detected before or during surgery, or those which occur within 6 months after gastrectomy. The inclusion criteria were as follows: (1) Age between 18 and 75 years; (2) clinical performance status according to Eastern Cooperative Oncology Group (ECOG) criteria ≤2; (3) longevity >3 months; (4) histologically or cytologically proven primary gastric cancer; (5) liver metastasis was examined by computed tomography (CT) and/or magnetic resonance imaging (MRI); (6) no extrahepatic metastasis; (7) laboratory findings within acceptable limits for undergoing gastrectomy, TACE and/or SC; (8) achievement of a microscopically margin-negative (R0) gastrectomy; (9) no other malignancies; (10) no serious associated medical diseases. The exclusion criteria included: (1) Longevity <3 months, (2) portal vein occlusion; (3) passive ascites; (4) Child–Pugh grade C liver function; and (5) weight <40 kg. The study was approved by the Chinese People's Liberation Army General Hospital Ethics Committee. Informed consent was obtained from all patients in the prospective cohort prior to entering the study and from all patients in the retrospective cohort at follow-up visits.
Treatments
Gastric tube cancer group
A total, distal, or proximal gastrectomy with D2 lymphadenectomy was performed, depending on the tumor location, and the metastatic lesions left untouched. Negative margins were ensured to achieve a microscopically margin-negative (R0) gastrectomy. The D category was evaluated in accordance with the 7th edition of the Japanese Classification of Gastric Carcinoma.
TACE, using the Seldinger technique, was performed before or after gastrectomy. Depending on the size and location of the tumor and its arterial supply, a catheter was selectively inserted into the appropriate segmental feeding arteries to perform embolization with an emulsion containing pirarubicin (30 mg/m2), oxaliplatin (130 mg/m2), 5-fluoro-uracil (500 mg/m2), and lipiodol 10–30 ml (at 1–2 ml/cm of the tumor diameter). Depending on the status of the blood supply, additional embolization using 1- to 2-mm-diameter gelatin sponge particles was performed. TACE was repeated at 8- to 12-week intervals for as long as the patient was able to tolerate this procedure. A follow-up abdominal CT was performed 2 months after undergoing TACE to assess the treatment response. In three patients, radiofrequency ablation was performed after TACE.
All patients began systemic XELOX chemotherapy within 8 weeks of undergoing surgery. This regimen comprised intravenous oxaliplatin (130 mg/m2) on day 1, and oral capecitabine (1000 mg∙m−2∙d−1) for 2 consecutive weeks followed by a 1-week rest. This regimen was repeated every 3 weeks for no fewer than six cycles. Second-line chemotherapy based on irinotecan or paclitaxel was recommended in cases of disease progression or intolerable toxicity.
Systemic chemotherapy group
These patients received the same chemotherapy as described above but did not undergo surgery or TACE.
Follow-up
Follow-up was performed every 6 weeks during chemotherapy, every 3 months for the first 2 years, every 6 months for the subsequent 3 years and yearly thereafter. Follow-up evaluation included clinical examination, standard blood chemistry, serum carcinoembryonic antigen (CEA) concentration, serum sialyl Lewisa antigen (CA19-9) concentration, abdominal ultrasound, and abdomino-pelvic CT or MRI. The patient follow-up lasted until death or the cut-off date of December 31, 2014. Four patients (3.8%) were lost to follow-up. Two (1.9%) patients were still alive and were censored at the cut-off date.
Evaluation criteria
Tumor response to treatment was assessed according to the modified response evaluation criteria in solid tumors[16] by a radiologist in each hospital. Complete response (CR) was defined as disappearance of all target lesions; partial response (PR) as not less than 30% decrease in the sum of diameters of viable lesions; progressive disease (PD) as an increase of 20% in the sum of diameters of viable lesions or any new tumor lesion; and stable disease (SD) as all cases that did not qualify as CR, PR, or PD. Response rate (RR) was defined as complete plus PR.
Extent of liver metastases was classified by the Japanese Research Society for Gastric Cancer, which are as follows: H1, liver metastases limited to one lobe of the liver; H2, isolated metastases in both lobes of the liver; and H3, multiple spread of metastases in both lobes of the liver.
Adverse effects were recorded during treatment and for 28 days after the last dose of study medication and graded according to the National Cancer Institute - Common Toxicity Criteria scale version 3.0.
Statistical analysis
All statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Clinicopathologic variables were compared between the two groups using the Student's t-test and the Chi-square test. Survival curves were constructed according to the Kaplan–Meier method and compared with the log-rank test. Rates of treatment response and treatment-related adverse effects were compared by the Chi-square or Fisher's exact test. Prognostic variables with a significance level of P < 0.1 according to univariate analysis were entered into the model. Independent prognostic factors were verified using the Cox proportional hazards model with stepwise models. P < 0.05 was considered as statistically significant.
RESULTS
Patients’ clinical characteristics
This study included 100 men and 7 women. The mean age was 59.0 ± 1.7 years (range, 33–75 years). Relevant clinicopathological data of the patients according to the two patient groups are summarized in Table 1 (GTC therapy, 32 patients; SC therapy, 75 patients). There were no significant differences between the two groups in age, sex, ECOG performance status, primary gastric cancer-related factors, liver metastases-related factors, or Child–Pugh grade.
Table 1.
Baseline characteristics of patients with gastric cancer and synchronous liver metastasis
| Characteristics | GTC (n = 32) | SC (n = 75) | P |
|---|---|---|---|
| Patient-related factors | |||
| Age (years) | 60.5 ± 18.0 | 58.4 ± 17.8 | 0.277 |
| Sex, n (%) | 0.612 | ||
| Male | 31 (96.9) | 69 (92.0) | |
| Female | 1 (3.1) | 6 (8.0) | |
| ECOG performance status, n (%) | 0.270 | ||
| 0–1 | 27 (84.4) | 56 (74.7) | |
| 2 | 5 (15.6) | 19 (25.3) | |
| Primary gastric cancerrelated factors | |||
| Size (cm), n (%) | 0.534 | ||
| <6 | 18 (56.2) | 47 (62.7) | |
| ≥6 | 14 (43.8) | 28 (37.3) | |
| Tumor location, n (%) | 0.269 | ||
| Lower | 16 (50.0) | 30 (40.0) | |
| Middle | 4 (12.5) | 23 (30.7) | |
| Upper | 11 (34.4) | 20 (26.7) | |
| Whole | 1 (3.1) | 2 (2.6) | |
| Histologic classification, n (%) | 0.814 | ||
| Well differentiated | 1 (3.1) | 3 (4.0) | |
| Moderately differentiated | 6 (18.8) | 19 (25.3) | |
| Poorly differentiated | 24 (75.0) | 52 (69.3) | |
| Undifferentiated | 1 (3.1) | 1 (1.3) | |
| Liver metastases-related factors | |||
| Tumor size (cm), n (%) | 0.816 | ||
| <3 | 15 (46.9) | 38 (50.7) | |
| ≥3 | 17 (53.1) | 37 (49.3) | |
| Extent of liver metastases, n (%) | 0.108 | ||
| H1 | 7 (21.9) | 11 (14.7) | |
| H2 | 12 (37.5) | 17 (22.7) | |
| H3 | 13 (40.6) | 47 (62.7) | |
| Child–Pugh grade, n (%) | 0.409 | ||
| A | 27 (84.4) | 58 (77.3) | |
| B | 5 (15.6) | 17 (22.7) |
GTC: Gastrectomy, TACE, Chemotherapy; SC: Systemic chemotherapy; ECOG: Eastern cooperative oncology group.
Survival analysis
Figure 1 shows overall survival (OS) curves for the two therapy groups. The 1-, 2-, and 3-year OS rates in the GTC group were 62.5%, 9.9%, and 3.3%, respectively, whereas they were 21.2%, 1.5%, and 0%, respectively, in the SC group. The median OS was 14 months (95% confidence interval [CI]: 13.1–14.9 months) in the GTC group and 8 months (95% CI: 6.6–9.4 months) in the SC group; this difference is statistically significant (P < 0.001).
Figure 1.
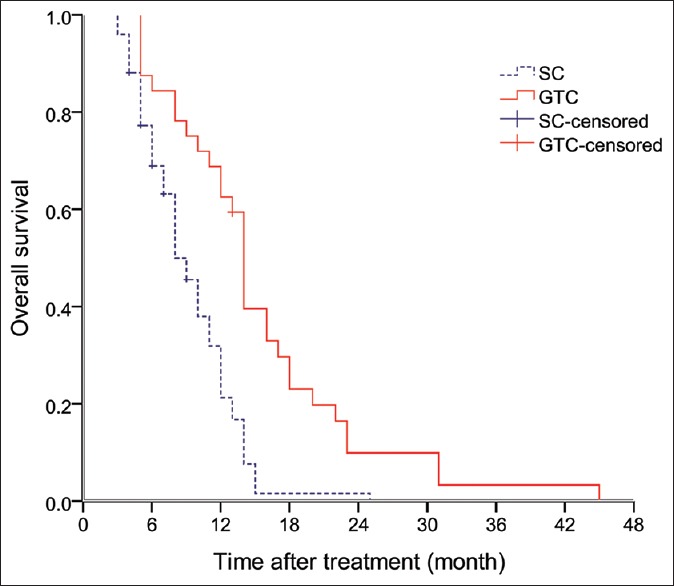
Overall survival by treatment group.
Figure 2 shows progression-free survival (PFS) curve for the two therapy groups. The 6- and 12-month PFS were 46.4% and 9.4%, respectively, for the patients who underwent GTC therapy, with a median PFS of 5 months (95% CI: 2.2–7.8 months). The 6- and 12-month PFS were 8.0% and 1.3%, respectively, for the patients who underwent SC therapy, with a median PFS of 3 months (95% CI: 2.3–3.4 months). The difference between the two therapy groups in PFS was statistically significant (P < 0.001).
Figure 2.
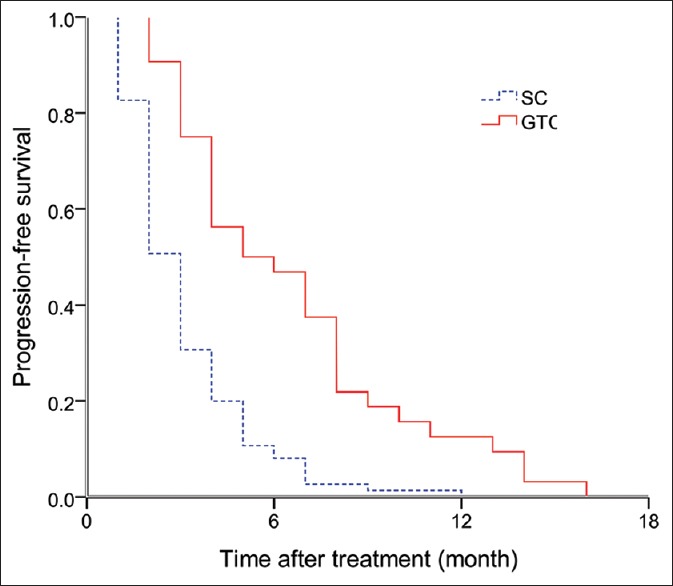
Progression-free survival by treatment group.
Rate of response to treatment
In the GTC group, 32 patients received 87 cycles of TACE (median of three cycles). Three (9.4%) and 16 patients (50.0%) achieved CR and PR, respectively. Eight patients (25.0%) achieved SD and 5 (15.6%) developed PD. The overall RR in the GTC group was 59.4%. In the SC group, 2 (2.7%) and 26 patients (34.7%) achieved CR and PR, respectively. Nineteen patients (25.3%) achieved SD and 28 (37.3%) developed PD. The overall RR in the SC group was 37.4%. The rate of response to treatment was significantly higher in the GTC than in the SC group (P = 0.035).
Adverse events
Table 2 lists treatment-related adverse effects according to treatment group and Common Toxicity Criteria scale version 3.0. Most patients in the GTC group developed postembolization complications, including abnormal liver function, abdominal pain, fever, and nausea, within 1-month. These patients received supportive treatment with antiemetics, analgesics, and antipyretics, corticosteroids being reserved for the more severe cases. Three patients in the GTC group underwent only one cycle of TACE because of abnormal serum bilirubin or alanine transaminase levels. No patients in the SC group stopped treatment because of treatment-related adverse events.
Table 2.
Treatment-related adverse effects according to treatment group and Common Toxicity Criteria scale version 3.0
| Adverse events | Grades 1–2, n (%) | Grades 3–4, n (%) | ||||
|---|---|---|---|---|---|---|
| GTC (n = 32) | SC (n = 75) | P | GTC (n = 32) | SC (n = 75) | P | |
| Fever | 23 (71.9) | 19 (25.3) | 0.001† | 2 (6.3) | 3 (4.0) | 0.634 |
| Fatigue | 18 (56.2) | 39 (52.0) | 0.687 | 3 (9.4) | 3 (4.0) | 0.517 |
| Hand-foot skin reaction | 11 (34.4) | 27 (36.0) | 0.872 | 0 | 0 | – |
| Nausea | 21 (65.6) | 43 (57.3) | 0.423 | 3 (9.4) | 5 (6.7) | 0.694 |
| Vomiting | 18 (56.3) | 37 (49.3) | 0.512 | 1 (3.1) | 3 (4.0) | 0.735 |
| Diarrhea | 13 (40.6) | 21 (28.0) | 0.199 | 0 | 0 | – |
| Abdominal pain | 23 (71.9) | 9 (12.0) | 0.001 | 7 (21.9) | 1 (1.3) | 0.001† |
| Anemia | 14 (43.8) | 24 (32.0) | 0.245 | 3 (9.4) | 5 (6.7) | 0.694 |
| Neutropenia | 11 (34.4) | 25 (33.3) | 0.916 | 2 (6.3) | 6 (8.0) | 0.931 |
| Bilirubin abnormality | 19 (59.2) | 9 (12.0) | 0.001† | 3 (9.4) | 0 | 0.025* |
| Alanine transaminase abnormality | 20 (62.5) | 11 (14.7) | 0.001† | 5 (15.6) | 2 (2.7) | 0.040* |
*P<0.05; †P<0.001. –: Not applicable; GTC: Gastrectomy, TACE, Chemotherapy; SC: Systemic chemotherapy.
Prognostic factors in patients undergoing gastric tube cancer treatment
Univariate analysis showed the following three factors to be associated with OS: Size of liver metastases (P = 0.025), extent of liver metastases (P = 0.002), and use of radiofrequency ablation (P = 0.013) [Table 3]. PFS was correlated with serum CEA concentrations (P = 0.093), lymph node metastases (P = 0.045), extent of liver metastases (P = 0.001), and use of radiofrequency ablation (P = 0.009) [Table 4]. The significant variables listed above were entered into a stepwise Cox regression analysis. Multivariate analysis revealed that size (P = 0.037) and extent of liver metastases (P < 0.001) were independent predictors of OS. Extent of liver metastases (P = 0.003) was also an independent predictor of PFS [Table 5].
Table 3.
Clinicopathological and treatment-related factors associated with OS after GTC treatment
| Characteristics | Number of patients | Median OS (months) | Survival rate (%) | P | ||
|---|---|---|---|---|---|---|
| 1-year | 2 years | 3 years | ||||
| Age (years) | 0.624 | |||||
| ≤60 | 15 | 14.0 | 60.0 | 6.7 | 0.0 | |
| >60 | 17 | 14.0 | 64.7 | 12.9 | 6.5 | |
| CEA concentration (ng/ml) | 0.275 | |||||
| ≤5 | 20 | 13.0 | 55.0 | 11.1 | 5.6 | |
| >5 | 12 | 17.0 | 75.0 | 8.3 | 0.0 | |
| CA19-9 concentration (ng/ml) | 0.835 | |||||
| ≤15 | 21 | 14.0 | 76.2 | 10.3 | 5.2 | |
| >15 | 11 | 14.0 | 54.5 | 9.1 | 0.0 | |
| Child–Pugh grade | 0.381 | |||||
| A | 27 | 14.0 | 55.6 | 8.0 | 4.0 | |
| B | 5 | 16.0 | 100.0 | 20.0 | 0.0 | |
| Gastric cancer | ||||||
| Tumor size (cm) | 0.358 | |||||
| <6 | 18 | 14.0 | 72.2 | 18.1 | 6.0 | |
| ≥6 | 14 | 12.0 | 50.0 | 14.3 | 0.0 | |
| Tumor location | 0.294 | |||||
| Upper | 11 | 6.0 | 56.3 | 7.1 | 7.1 | |
| Not upper | 21 | 12.0 | 68.8 | 25.0 | 0.0 | |
| Depth of invasion | 0.744 | |||||
| Non-T4b | 20 | 14.0 | 65.0 | 5.0 | 5.0 | |
| T4b | 12 | 12.0 | 50.0 | 20.0 | 0.0 | |
| Lymph node metastasis | 0.211 | |||||
| Absent | 5 | 17.0 | 80.0 | 20.0 | 20.0 | |
| Present | 27 | 14.0 | 55.6 | 7.9 | 0.0 | |
| Histological differentiation | 0.338 | |||||
| Well, moderately | 7 | 12.0 | 42.9 | 14.3 | 0.0 | |
| Poorly, undifferentiated | 25 | 14.0 | 68.0 | 12.8 | 4.3 | |
| Liver metastases | ||||||
| Size of liver metastases (cm) | 0.025* | |||||
| <3 | 17 | 12.0 | 70.6 | 9.3 | 6.4 | |
| ≥3 | 15 | 6.0 | 46.7 | 6.7 | 0.0 | |
| Extent of liver metastases | 0.002* | |||||
| H1 | 7 | 23.0 | 71.4 | 42.9 | 4.3 | |
| H2 | 12 | 13.0 | 58.3 | 8.3 | 0.0 | |
| H3 | 13 | 10.0 | 46.0 | 0.0 | 0.0 | |
| Radiofrequency ablation | 0.013* | |||||
| Not performed | 29 | 14.0 | 58.6 | 14.7 | 0.0 | |
| Performed | 3 | 31.0 | 100.0 | 33.3 | 33.3 | |
OS: Overall survival; GTC: Gastrectomy, TACE, Chemotherapy; CEA: Carcinoembryonic antigen. *P<0.05.
Table 4.
Clinicopathological and treatment-related factors associated with PFS after GTC treatment
| Characteristics | Number of patients | Median PFS (months) | Survival rate (%) | P | ||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| Ages (years) | 0.508 | |||||
| ≤60 | 15 | 7.0 | 53.0 | 6.7 | ||
| >60 | 17 | 5.0 | 19.0 | 6.3 | ||
| CEA concentration (ng/ml) | 0.093 | |||||
| ≤5 | 20 | 4.0 | 37.5 | 5.4 | ||
| >5 | 12 | 8.0 | 66.7 | 16.7 | ||
| CA19-9 concentration (ng/ml) | 0.896 | |||||
| ≤15 | 21 | 7.0 | 50.6 | 5.1 | ||
| >15 | 11 | 5.0 | 36.4 | 9.1 | ||
| Child–Pugh grade | 0.345 | |||||
| A | 27 | 5.0 | 40.7 | 7.4 | ||
| B | 5 | 9.0 | 60.0 | 20.0 | ||
| Gastric cancer | ||||||
| Tumor size (cm) | 0.209 | |||||
| <6 | 18 | 7.0 | 44.4 | 5.6 | ||
| ≥6 | 14 | 4.0 | 35.7 | 7.1 | ||
| Tumor location | 0.419 | |||||
| Upper | 11 | 4.0 | 31.3 | 6.3 | ||
| Not upper | 21 | 7.0 | 56.3 | 12.5 | ||
| Depth of invasion | 0.373 | |||||
| Non-T4b | 20 | 7.0 | 40.0 | 5.0 | ||
| T4b | 12 | 5.0 | 33.3 | 8.3 | ||
| Lymph node metastasis | 0.045* | |||||
| Absent | 5 | 8.0 | 60.0 | 20.0 | ||
| Present | 27 | 8.0 | 40.7 | 3.7 | ||
| Histological differentiation | 0.879 | |||||
| Well, moderately | 7 | 3.0 | 28.6 | 14.3 | ||
| Poorly, undifferentiated | 25 | 7.0 | 40.0 | 4.0 | ||
| Liver metastases | ||||||
| Size of liver metastases (cm) | 0.198 | |||||
| <3 | 17 | 8.0 | 52.9 | 11.8 | ||
| ≥3 | 15 | 5.0 | 20.0 | 6.7 | ||
| Extent of liver metastases | 0.001† | |||||
| H1 | 7 | 11.0 | 100.0 | 28.6 | ||
| H2 | 12 | 4.0 | 25.0 | 8.3 | ||
| H3 | 13 | 4.0 | 23.1 | 0.0 | ||
| Radiofrequency ablation | 0.009* | |||||
| Not performed | 29 | 5.0 | 41.4 | 3.4 | ||
| Performed | 3 | 14.0 | 100.0 | 33.3 | ||
PFS: Progression-free survival; GTC: Gastrectomy, TACE, Chemotherapy; CEA: Carcinoembryonic antigen. *P<0.05, †P<0.01.
Table 5.
Cox's multivariate analysis of factors contributing to survival of the 32 patients who underwent GTC treatment
| Characteristics | OS | PFS | ||
|---|---|---|---|---|
| P | HR (95.0% CI) | P | HR (95.0% CI) | |
| CEA concentration | – | – | 0.092 | 1.000 (0.996–1.000) |
| Lymph node metastasis | – | – | 0.775 | 1.224 (0.308–4.850) |
| Size of liver metastasis | 0.037* | 1.524 (1.108–2.707) | – | – |
| Extent of liver metastases | 0.001† | 3.182 (1.645–6.127) | 0.003* | 2.331 (1.324–4.092) |
| Radiofrequency ablation | 0.086 | 0.148 (0.018–1.304) | 0.146 | 0.169 (0.016–1.852) |
–: Not applicable; CEA: Carcinoembryonic antigen; CI: Confidence interval; HR: Hazard ratio; GTC: Gastrectomy, TACE, Chemotherapy; PFS: Progression-free survival; OS: Overall survival. *P<0.05, †P<0.01.
DISCUSSION
Liver metastases are lethal in patients with gastric cancer. Approximately, 4.0–9.9% of gastric cancer patients have synchronous liver metastases;[14] the prognosis is very poor. In recent decades, surgical, interventional, and ablation treatment techniques have improved remarkably, and their use in patients with liver metastases from gastric cancer may improve prognosis. This study has shown that it is feasible to administer GTC treatment to patients with GCSLM and that GTC treatment achieves better outcomes than SC treatment in such patients.
Several studies have shown that gastrectomy for gastric cancer with concomitant liver metastases confers survival benefits.[12,17,18] Gastrectomy achieves a median survival time of 8.0–16.3 months compared with 2.4–6.8 months without this treatment.[18,19] The rationale for offering gastrectomy to patients with GCSLM is that it reduces the tumor load, thus rendering the residual tumor more responsive to adjuvant treatment, decreases tumor metabolic demands, reduces the risk of potential life-threatening complications and postpones their appearance, and reduces tumor secretion of immunosuppressive cytokines.[19,20,21]
There are two primary mechanisms for TACE's beneficial effects.[22] First, arterial embolization preferentially interrupts the tumor blood supply and stalls growth. Second, TACE maximizes the concentration of chemotherapeutic drugs within the hepatic lesions while simultaneously minimizing systemic exposure, thus achieving greater therapeutic efficacy with less toxicity than conventionally administered chemotherapy. This effect is potentiated by arterial embolization. Thus, TACE can decrease the size and number of hepatic lesions. In our study, the treatment RR was 59.4% in the GTC group, which was significantly better than in the SC group (37.4%). In three patients with critical and inoperable hepatic metastases, the tumor size and number were reduced by repeated sessions of TACE, after which the tumors were ablated by radiofrequency ablation. All three of these patients achieved satisfactory outcomes [Table 6]. In addition, repeated TACE may treat undetected micrometastases that would otherwise cause intrahepatic recurrence;[7] complete eradication of metastases may improve the prognosis in patients with GCSLM. In our experience, TACE is a minimally invasive treatment and repeated TACE achieves a better prognosis. In the late stage of gastric cancer, liver metastases may reflect generalized disease. In addition to surgical resection and local control, SC is a prime option for prolonging survival. However, the best chemotherapy regimen for gastric cancer with liver metastases is uncertain. A multicenter phase III study (CLASSIC trial) found that capecitabine plus oxaliplatin (XELOX) is promising enough to become the standard first-line treatment for patients with gastric cancer who have undergone curative D2 gastrectomy.[23] Park et al.[24] noted that XELOX is effective and well-tolerated as a first-line therapy for advanced gastric cancer. Capecitabine, with its mild side effects and ease of administration, has been the standard choice for adjuvant chemotherapy for advanced gastric cancer.[25,26] In light of the above studies, we surmised that the XELOX regimen might improve the outcomes of patients with GCSLM and, therefore, chose it as the SC after gastrectomy. In our study, SC alone achieved about 37.4% RR in patients with GCSLM.
Table 6.
Details of three patients who achieved long survival after TACE plus radiofrequency ablation
| Case | Age/sex | Primary tumor | Liver metastasis | TACE | Radiofrequency ablation | ||||
|---|---|---|---|---|---|---|---|---|---|
| Histological differentiation | TNM*, classification | Main tumor size (cm) | Tumor number | Number of TACEs | Therapeutic response | Tumor enhancement | Survival (months) | ||
| 1 | 62/male | Moderately | T4bN1M1 | 2.6 | 7 | 5 | PR | None | 45 |
| 2 | 66/male | Moderately | T4bN2M1 | 0.8 | 1 | 3 | PR | None | 31 |
| 3 | 70/male | Moderately | T4bN1M1 | 3.4 | 5 | 1 | SD | None | 20 |
*The TNM classification according to the 7th edition of the AJCC. TACE: Transarterial chemoembolization; AJCC: American joint committee on cancer; TNM: Tumor, node, metastasis; PR: Partial response; SD: Stable disease.
Several studies have reported that various pathological characteristics of primary gastric cancer, such as depth of tumor invasion, lymph node metastasis, and histological differentiation, are independent predictors of survival of patients with gastric cancer and liver metastases.[27,28] However, these factors had no significant impact on survival in our study. The probable explanation of this apparent discrepancy is that our study focused only on gastric cancer patients with synchronous liver metastases, whereas most previous studies have included patients with both synchronous and metachronous metastases. Several reports[2,8,28] have confirmed that the number of liver metastases is a major prognostic factor. Our study supported these findings: We found that the extent of liver metastases (H1, H2, and H3) was a predictor of both OS and PFS after GTC treatment. Taking the present findings and those of previous reports together, the size and extent of liver metastases in patients with GCSLM might be significant predictors of survival after GTC treatment. In our study, according to univariate analysis, radiofrequency ablation after TACE was associated with significantly improved OS and PFS. However, multivariate analysis did not identify radiofrequency ablation as a significant predictor of survival, possibly because too few patients underwent radiofrequency ablation. Nevertheless, we recommend radiofrequency ablation after TACE when possible.
The present study has several limitations. First, there were too few patients in the GTC group, preventing a powerful interpretation of our findings. Second, this was an ambispective cohort study, with all the disadvantages associated with retrospective studies. Third, depending on the treatment RR, the postoperative chemotherapy regimens were varied in some patients. Fourth, the efficacy of TACE with radiofrequency ablation postoperation was supposed to be compared in future study.
In conclusion, the present study has shown that gastrectomy with TACE plus SC is more effective in patients with GSCLM than SC alone. GTC therapy results in a better treatment RR than SC. Small and few liver metastases have favorable outcomes following GTC treatment. Therefore, we believe that GTC therapy prolongs survival in selected gastric cancer patients with synchronous liver metastases.
Footnotes
Edited by: Yi Cui
Source of Support: The trial was supported by grants from the Capital Health Research and Development of Special (No. 2011-5001-01), PLA Medical and Health Research Fund Project (No. 11BJZ17), and PLA Medical Technology Key Project of Scientific Research in the 12th Research Projects in 12th five-year-Plan (No. BWS12J049).
Conflict of Interest: None declared.
REFERENCES
- 1.Fujisaki S, Tomita R, Nezu T, Kimizuka K, Park E, Fukuzawa M. Prognostic studies on gastric cancer with concomitant liver metastases. Hepatogastroenterology. 2001;48:892–4. [PubMed] [Google Scholar]
- 2.Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86–91. doi: 10.1097/00000658-200201000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, et al. Surgical resection of liver metastases of gastric cancer: An analysis of a 17-year experience with 22 patients. Surgery. 2003;133:507–11. doi: 10.1067/msy.2003.147. [DOI] [PubMed] [Google Scholar]
- 4.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: Priorities for prevention. Carcinogenesis. 2010;31:100–10. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochi M, Fujii M, Kanamori N, Kaiga T, Okubo R, Hagiwara K, et al. Irinotecan plus S-1 for liver metastases of gastric cancer. Hepatogastroenterology. 2009;56:1755–9. [PubMed] [Google Scholar]
- 6.Qiu JL, Deng MG, Li W, Zou RH, Li BK, Zheng Y, et al. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol. 2013;39:694–700. doi: 10.1016/j.ejso.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Yamakado K, Nakatsuka A, Takaki H, Mori Y, Tonouchi H, Kusunoki M, et al. Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastasis of gastric cancer. J Vasc Interv Radiol. 2005;16:1747–51. doi: 10.1097/01.RVI.0000188738.84911.3B. [DOI] [PubMed] [Google Scholar]
- 8.Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146–53. doi: 10.1093/annonc/mdn026. [DOI] [PubMed] [Google Scholar]
- 9.Hirasawa T, Asahara S, Fujisaki S, Kuraoka K, Takano K, Kamei A, et al. Transcatheter arterial chemoembolization (TACE) using degradable starch microspheres (DSM) for metastatic liver tumors in patients with gastric cancer. Nihon Shokakibyo Gakkai Zasshi. 2008;105:367–72. [PubMed] [Google Scholar]
- 10.Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia. 2010;26:305–15. doi: 10.3109/02656730903555696. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, et al. Outcomes for patients following hepatic resection of metastatic tumors from gastric cancer. Hepatol Int. 2010;4:406–13. doi: 10.1007/s12072-009-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Li JH, Zhai RJ, Wei B, Shao MZ, Chen L. Predictive factors improving survival after gastric and hepatic surgical treatment in gastric cancer patients with synchronous liver metastases. Chin Med J. 2012;125:165–71. [PubMed] [Google Scholar]
- 13.Wang YN, Shen KT, Ling JQ, Gao XD, Hou YY, Wang XF, et al. Prognostic analysis of combined curative resection of the stomach and liver lesions in 30 gastric cancer patients with synchronous liver metastases. BMC Surg. 2012;12:20. doi: 10.1186/1471-2482-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda K, Iwahashi M, Nakamori M, Nakamura M, Naka T, Ishida K, et al. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394:647–53. doi: 10.1007/s00423-008-0311-9. [DOI] [PubMed] [Google Scholar]
- 15.Linhares E, Monteiro M, Kesley R, Santos CE, Filho OS, Simões JH. Major hepatectomy for isolated metastases from gastric adenocarcinoma. HPB (Oxford) 2003;5:235–7. doi: 10.1080/13651820310015815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Kwok CM, Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY. Survival of gastric cancer with concomitant liver metastases. Hepatogastroenterology. 2004;51:1527–30. [PubMed] [Google Scholar]
- 18.Li C, Yan M, Chen J, Xiang M, Zhu ZG, Yin HR, et al. Survival benefit of non-curative gastrectomy for gastric cancer patients with synchronous distant metastasis. J Gastrointest Surg. 2010;14:282–8. doi: 10.1007/s11605-009-1095-0. [DOI] [PubMed] [Google Scholar]
- 19.Saidi RF, ReMine SG, Dudrick PS, Hanna NN. Is there a role for palliative gastrectomy in patients with stage IV gastric cancer? World J Surg. 2006;30:21–7. doi: 10.1007/s00268-005-0129-3. [DOI] [PubMed] [Google Scholar]
- 20.Pollock RE, Roth JA. Cancer-induced immunosuppression: Implications for therapy? Semin Surg Oncol. 1989;5:414–9. doi: 10.1002/ssu.2980050607. [DOI] [PubMed] [Google Scholar]
- 21.McCarter MD, Fong Y. Role for surgical cytoreduction in multimodality treatments for cancer. Ann Surg Oncol. 2001;8:38–43. doi: 10.1007/s10434-001-0038-0. [DOI] [PubMed] [Google Scholar]
- 22.Miraglia R, Pietrosi G, Maruzzelli L, Petridis I, Caruso S, Marrone G, et al. Efficacy of transcatheter embolization/chemoembolization (TAE/TACE) for the treatment of single hepatocellular carcinoma. World J Gastroenterol. 2007;13:2952–5. doi: 10.3748/wjg.v13.i21.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 24.Park YH, Lee JL, Ryoo BY, Ryu MH, Yang SH, Kim BS, et al. Capecitabine in combination with Oxaliplatin (XELOX) as a first-line therapy for advanced gastric cancer. Cancer Chemother Pharmacol. 2008;61:623–9. doi: 10.1007/s00280-007-0515-7. [DOI] [PubMed] [Google Scholar]
- 25.Kang Y, Lee J, Min Y, Lee K, Zang D, Ryoo B, et al. A randomized multi-center phase II trial of capecitabine (X) versus S-1 (S) as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. J Clin Oncol. 2007;25:4546. doi: 10.1038/sj.bjc.6604536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamoto J, Chin K, Kondo K, Kojima H, Terashima M, Yamamura Y, et al. Phase II study of a 4-week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs. 2006;17:231–6. doi: 10.1097/00001813-200602000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181:279–83. doi: 10.1016/s0002-9610(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 28.Shirabe K, Shimada M, Matsumata T, Higashi H, Yakeishi Y, Wakiyama S, et al. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: A multi-institutional study of the indications for resection. Hepatogastroenterology. 2003;50:1560–3. [PubMed] [Google Scholar]


