Abstract
Plant trait measurements are needed for evaluating ecological responses to environmental conditions and for ecosystem process model development, parameterization, and testing. We present a standardized dataset integrating measurements from projects conducted by the Terrestrial Ecosystem Research and Regional Analysis- Pacific Northwest (TERRA-PNW) research group between 1999 and 2014 across Oregon and Northern California, where measurements were collected for scaling and modeling regional terrestrial carbon processes with models such as Biome-BGC and the Community Land Model. The dataset contains measurements of specific leaf area, leaf longevity, leaf carbon and nitrogen for 35 tree and shrub species derived from more than 1,200 branch samples collected from over 200 forest plots, including several AmeriFlux sites. The dataset also contains plot-level measurements of forest composition, structure (e.g., tree biomass), and productivity, as well as measurements of soil structure (e.g., bulk density) and chemistry (e.g., carbon). Publically-archiving regional datasets of standardized, co-located, and geo-referenced plant trait measurements will advance the ability of earth system models to capture species-level climate sensitivity at regional to global scales.
Subject terms: Ecological modelling, Environmental chemistry, Forestry, Plant physiology, Biogeochemistry
Background & Summary
Earth system models (ESMs) play an important role in climate change mitigation and adaptation efforts, enabling evaluation of potential future climate impacts and management decisions on ecological systems; however, these models have typically been parameterized with generalized Plant Functional Types (PFTs), which makes it difficult to predict species impacts and shifts within regions. Parameterizing ESMs with species or genus-level traits is more desirable, yet model development, testing and applications have been limited by the availability of sufficient field measurements for regional to global modeling1. Thus, there is a pressing need for standardized, spatially-extensive measurement of select plant traits and associated plot-level characteristics.
We have developed a dataset that includes ESM-relevant leaf trait measurements (specific leaf area, nitrogen, carbon, lifespan) for 35 tree and shrub species from 239 sites in Oregon and northern California (Fig. 1a,b). The dataset also includes plot-level characteristics (e.g., biomass, productivity, soil depth) for the sites. The dataset incorporates measurements from projects in the Pacific Northwest from 1999–2014 that focused on evaluation of ecosystem processes and model development and testing (Table 1). Protocols for field sampling, laboratory analysis, computations, and data submission were developed and implemented in 1999-2000 (ref. 2). Below we provide a brief description of each research project.
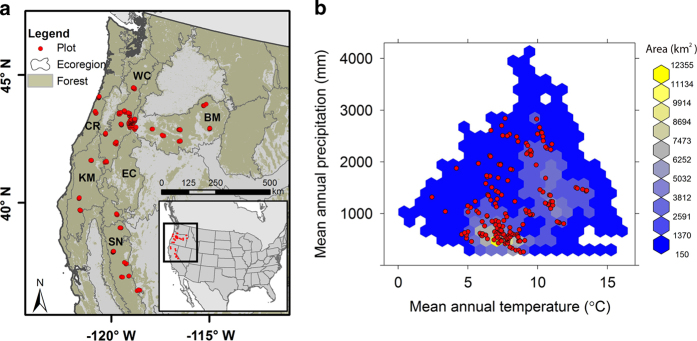
Figure 1. Location of sampling sites in (a) geographic space and (b) climate space.
Sampling sites (n=239) were spread among six forested ecoregions located in Oregon and northern California. The sampling sites covered much of the climate space encounter across the forested parts of the ecoregions. Only climate spaces occupying at least 150 km2 are shown in the figure. Climate data were from PRISM and were averaged from 1984 to 2013.
Table 1. Summary of TERRA-PNW projects that have been combined into the dataset.
| Project | Year |
Number of… |
Key references | |||
|---|---|---|---|---|---|---|
| Sites | Genera | Species | Branches | |||
| The primary sampling year and key references are provided for each project, as are number of sites, genera, species, and branch samples. | ||||||
| RADAR | 1999 | 20 | — | — | — | Law et al.6 |
| EPA | 2001 | 90 | 19 | 24 | 562 | Law et al.7 |
| COHO | 2002 | 3 | 5 | 5 | 86 | Schwarz et al.8 |
| ORCA | 2004 | 73 | 13 | 18 | 397 | Law et at.9 |
| METOFIRE | 2007 | 64 | 2 | 2 | 88 | Meigs et al.11 |
| CADIS | 2014 | 15 | 4 | 6 | 163 | Berner & Law12 |
The RADAR project (1999) focused on the East Cascades and sought to characterize forest canopy structure and biomass, as well as develop and test methods for estimating biomass from fusion of radar and AVIRIS hyperspectral imagery3,4. Measurements were made on 20 plots, largely dominated by ponderosa pine (Pinus ponderosa), but several or grand fir (Abies grandis). The leaf area index (LAI) sampling was more intensive than the standard protocol2 given the focus on 3-dimensional canopy modeling5,6.
The EPA project (2001) was a regional study over Oregon and northern California that aimed to quantify current biomass and net ecosystem production (NEP) by integrating remote sensing, intensive plots, extensive plots, inventories and modeling using a spatially nested hierarchical design2,7. Measurements were made on 96 plots, with 36 plots spread among three intensive clusters (Coast Range, West Cascades, East Cascades), each of which included 4 age classes x 3 replications. The remaining 60 plots were distributed regionally to capture Landsat spectral variability.
The COHO project (2002) focused on measuring and modeling carbon stocks and fluxes at five sites it the East Cascades using biometric and eddy covariance measurements in conjunction with the Soil-Plant-Atmosphere (SPA) model8. Measurements were made at young, mature and old ponderosa pine sites, as well as at mature grand fir and western juniper (Juniperus occidentalis) sites.
The Oregon and California (ORCA) project (2004–2005) sought to broaden the regional sampling network and tree species representation though extensive sampling in the East Cascades, Blue Mountains, Klamath Mountains, northern Great Basin, and Sierra Nevada, with measurements made on 80 plots9,10. In 2005, measurements were made on 14 of these plots in the Sierra Nevada as part of the Forest Hill thinning study, where the impacts of thinning on productivity and carbon allocation by trees and shrubs were assessed both 3- and 16-years after thinning in relation to unthinned plots.
The Metolius Fire (METOFIRE) project (2007–2008) focused on quantifying pre- and post-fire carbon pools and productivity on four mixed-severity wildfires (2002–2003) in mixed fir and ponderosa pine forests in East Cascades11. Measurements were made on 64 plots, with burned and unburned stands measured in 2007 and 2008, respectively.
The Cascade Drought Impact Study (CADIS; 2014) evaluated the role of water availability in shaping tree morphological traits and forest carbon cycling along a steep climatic gradient in the East Cascades12. Measurements were made on 15 sites spread evenly among western juniper, ponderosa pine, and grand fir.
Portions of this dataset have furthermore been used to (1) parameterize and test ecological models (e.g., Biome-BGC13, CLM14); (2) evaluate satellite algorithms15 and eddy covariance measurements16; (3) assess regional carbon budgets10 and consequences of forest management (e.g., harvest regimes13,17, bioenergy production18,19); and (4) explore relationships between soil carbon and detritus20. We are confident that the research community will find additional uses for this dataset. This article introduces the dataset and associated methods, describes each variable, and provides statistical summaries of the leaf traits by species, and summaries of the remaining variables (e.g., biomass, productivity, soil characteristics) at the plot level.
Methods
This section provides brief descriptions of the field and laboratory measurement protocols, as well as of the leaf, stand, and soil variables included in the dataset. The field, lab and computational methods are described in detail in an FAO protocol document that was subsequently developed for the AmeriFlux network and FLUXNET2.
Field sampling
The plot design consisted of a 1 ha plot containing four subplots (center, north, southwest, southeast) that were spaced at 35 m between subplot centers, with subplot diameter ranging from 10 to 17 m, depending on tree density. Tree height and diameter at breast height (DBH) were measured on each subplot for all stems that were 10–80 cm DBH. All large trees >80 cm DBH were measured on the entire 1 ha plot, while sapling surveys (stems 1–10 cm DBH) were conducted inside the subplots with radii of 2–5 m depending on tree density. Around twenty tree cores were collected per plot, with half used to estimate annual growth and age, and the remaining used to determine wood density. Furthermore, overstory leaf area index (LAI, one-sided) was measured at 35–45 locations on each subplot using an LAI-2000 or LAI-2200 (LiCor, Lincoln, NE).
Mid-canopy, south-facing branches with high, but vertically-variable light exposure were harvested for each species present on a plot, with sample size per species (generally 5 to 10) determined by the species prevalence within a plot. Leaf samples from this canopy position tend to be a reasonable approximation for canopy-average trait characteristics21, which are necessary in most ecosystem models. Additionally, soil cores were collected from multiple layers (e.g., 0–20, 20–50, 50–100 cm).
Foliage analysis
Leaf carbon and nitrogen content
Leaf carbon or nitrogen content per unit of dry mass, expressed as a percentage of leaf dry mass. Leaf carbon and nitrogen content were measured on one-year old foliage, except in the case of deciduous species, where measurements were based on current-year, fully-expanded and hardened foliage. Leaf samples were oven-dried at 70 °C for at least 48 h and then finely ground using a coffee-grinder and/or mortar and pestle. Carbon and nitrogen concentrations were then measured with a LECO CNS2000 analyzer by Central Analytical Laboratory at Oregon State University. Genus-level graphical summaries of leaf nitrogen are presented in Fig. 2, while species-level and genus-level statistical summaries of both leaf carbon and nitrogen are given in Tables 2 and 3.
Figure 2. Box and whisker plots summarizing leaf nitrogen measurements for each plant genera represented in the dataset.
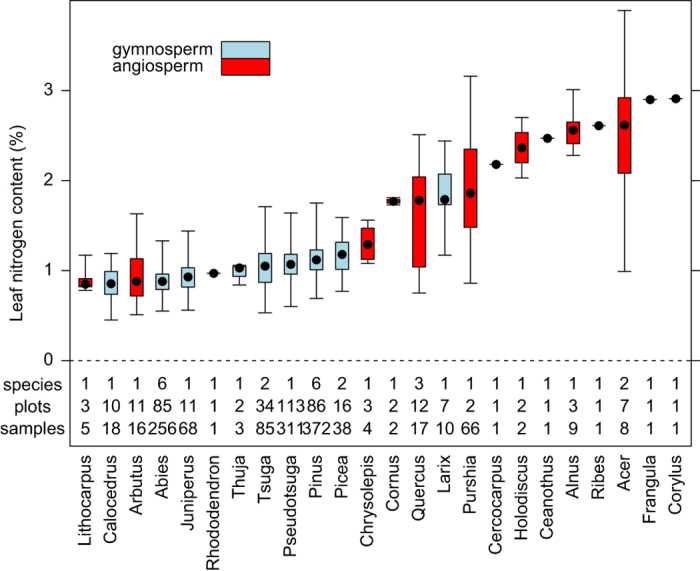
Intrabox dots denote medians, box edges denote 25th and 75th percentiles, and whiskers denote minimum and maximum values. The number of species, sites, and samples representing each genera is also provided. Species-level and genus-level statistical summaries are given in Tables 2 and 3.
Table 2. Species-level statistical summaries (average, s.d., sample size) of specific leaf area (SLA; cm2 HSA g−1 C), leaf carbon (%), leaf nitrogen (%), and leaf lifespan (years) for tree and shrub species sampled in Oregon and Northern California.
| Div. | Genus | Species |
SLA |
Leaf C |
Leaf N |
Leaf lifespan |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avg. | s.d. | N | Avg. | s.d. | N | Avg. | s.d. | N | Avg. | s.d. | N | |||
| Divisions (Div.) include angiosperms and gymnosperms. | ||||||||||||||
| angio. | Acer | circinatum | 689 | 263 | 4 | 49.58 | 1.77 | 4 | 2.23 | 0.94 | 4 | 1 | 0 | 4 |
| angio. | Acer | macrophyllum | 406 | 121 | 4 | 47.45 | 2.30 | 4 | 2.81 | 0.78 | 4 | 1 | 0 | 4 |
| angio. | Alnus | rubra | 326 | 136 | 9 | 51.03 | 1.07 | 9 | 2.56 | 0.22 | 9 | 1 | 0 | 9 |
| angio. | Arbutus | menziesii | 168 | 35 | 16 | 47.57 | 2.10 | 16 | 0.93 | 0.31 | 16 | 1 | 0 | 0 |
| angio. | Ceanothus | velutinus | 121 | NA | 1 | 51.20 | NA | 1 | 2.47 | NA | 1 | 1 | NA | 1 |
| angio. | Cercocarpus | unknown | 86 | NA | 1 | 46.56 | NA | 1 | 2.18 | NA | 1 | NA | NA | 0 |
| angio. | Chrysolepis | chrysophylla | 137 | 28 | 4 | 49.77 | 0.34 | 4 | 1.30 | 0.23 | 4 | 1 | 0 | 4 |
| angio. | Cornus | unknown | 928 | 107 | 2 | 39.98 | 0.13 | 2 | 1.77 | 0.06 | 2 | NA | NA | 2 |
| angio. | Corylus | cornuta | 1,146 | NA | 1 | 48.50 | NA | 1 | 2.91 | NA | 1 | 1 | NA | 1 |
| angio. | Frangula | purshiana | 488 | NA | 1 | 47.70 | NA | 1 | 2.90 | NA | 1 | 1 | NA | 1 |
| angio. | Holodiscus | discolor | 484 | 355 | 2 | 49.00 | 0.57 | 2 | 2.37 | 0.47 | 2 | 1 | 0 | 2 |
| angio. | Lithocarpus | densiflorus | 162 | 36 | 5 | 43.14 | 4.29 | 5 | 0.91 | 0.16 | 5 | 3.4 | 2.1 | 5 |
| angio. | Purshia | tridentate | 202 | 86 | 66 | 50.09 | 1.20 | 66 | 1.93 | 0.52 | 66 | NA | NA | 66 |
| angio. | Quercus | chrysolepis | 130 | 30 | 7 | 43.25 | 2.82 | 7 | 0.96 | 0.15 | 7 | 3.6 | 1.4 | 7 |
| angio. | Quercus | garryana | 586 | 205 | 3 | 46.51 | 1.59 | 3 | 2.10 | 0.62 | 3 | 1 | NA | 3 |
| angio. | Quercus | kelloggii | 260 | 59 | 7 | 45.54 | 0.78 | 7 | 1.95 | 0.14 | 7 | 1 | NA | 7 |
| angio. | Rhododendron | macrophyllum | 116 | NA | 1 | 52.30 | NA | 1 | 0.97 | NA | 1 | 1 | NA | 1 |
| angio. | Ribes | divaricatum | 232 | NA | 1 | 48.40 | NA | 1 | 2.61 | NA | 1 | 1 | NA | 1 |
| gymno. | Abies | amabilis | 87 | 18 | 25 | 52.13 | 0.65 | 25 | 0.89 | 0.12 | 25 | 5.3 | 1.6 | 25 |
| gymno. | Abies | concolor | 102 | 18 | 89 | 48.08 | 1.97 | 89 | 0.88 | 0.13 | 89 | 8.7 | 2.6 | 89 |
| gymno. | Abies | grandis | 91 | 19 | 102 | 48.87 | 1.71 | 71 | 0.88 | 0.16 | 71 | 8.2 | 2.5 | 97 |
| gymno. | Abies | lasiocarpa | 98 | 13 | 7 | 50.59 | 2.45 | 7 | 0.92 | 0.08 | 7 | 10.1 | 5.0 | 7 |
| gymno. | Abies | magnifica | 73 | 11 | 11 | 48.89 | 2.10 | 11 | 0.90 | 0.18 | 11 | 19.6 | 4.7 | 11 |
| gymno. | Abies | procera | 72 | 20 | 22 | 51.72 | 1.66 | 22 | 0.85 | 0.15 | 22 | 3.9 | 2.1 | 22 |
| gymno. | Calocedrus | decurrens | 82 | 24 | 18 | 48.04 | 1.62 | 18 | 0.84 | 0.21 | 18 | 2.5 | 2.1 | 1 |
| gymno. | Juniperus | occidentalis | 64 | 9 | 68 | 48.86 | 1.71 | 68 | 0.95 | 0.20 | 68 | NA | NA | 0 |
| gymno. | Larix | occidentalis | 309 | 33 | 10 | 46.13 | 2.31 | 10 | 1.87 | 0.37 | 10 | 1 | 0 | 10 |
| gymno. | Picea | engelmannii | 94 | 30 | 11 | 50.01 | 2.23 | 11 | 0.89 | 0.12 | 11 | 7.2 | 2.5 | 11 |
| gymno. | Picea | sitchensis | 138 | 47 | 27 | 53.02 | 0.76 | 27 | 1.27 | 0.16 | 27 | 4.4 | 0.8 | 27 |
| gymno. | Pinus | contorta | 83 | 10 | 40 | 50.06 | 1.67 | 40 | 1.06 | 0.21 | 40 | 8 | 4.1 | 40 |
| gymno. | Pinus | flexilis | 107 | 5 | 3 | 52.90 | 0.20 | 3 | 1.29 | 0.12 | 3 | NA | NA | 0 |
| gymno. | Pinus | jeffreyi | 90 | 27 | 45 | 49.56 | 0.94 | 45 | 1.00 | 0.16 | 45 | 6.6 | 2.5 | 45 |
| gymno. | Pinus | lambertiana | 103 | 32 | 6 | 47.63 | 0.93 | 6 | 1.05 | 0.08 | 6 | 7 | 1.7 | 6 |
| gymno. | Pinus | monticola | 103 | 15 | 11 | 50.71 | 1.03 | 11 | 1.40 | 0.23 | 11 | 6.5 | 1.2 | 11 |
| gymno. | Pinus | ponderosa | 81 | 11 | 267 | 50.77 | 2.17 | 267 | 1.15 | 0.17 | 267 | 4.5 | 1.2 | 185 |
| gymno. | Pseudotsuga | menziesii | 119 | 24 | 310 | 51.40 | 1.64 | 253 | 1.08 | 0.19 | 253 | 5.5 | 2.0 | 306 |
| gymno. | Thuja | plicata | 95 | 18 | 3 | 51.03 | 1.99 | 3 | 0.98 | 0.12 | 3 | 5 | NA | 1 |
| gymno. | Tsuga | heterophylla | 175 | 46 | 82 | 52.74 | 0.98 | 82 | 1.05 | 0.23 | 82 | 5.2 | 1.3 | 82 |
| gymno. | Tsuga | mertensiana | 193 | 61 | 3 | 50.87 | 0.81 | 3 | 0.89 | 0.21 | 3 | 5.3 | 1.5 | 3 |
Table 3. Genus-level statistical summaries (average, s.d., sample size) of specific leaf area (SLA; cm2 HSA g−1 C), leaf carbon (%), leaf nitrogen (%), and leaf lifespan (years) for tree and shrub species sampled in Oregon and Northern California Divisions.
| Div. | Genus |
SLA |
Leaf C |
Leaf N |
Leaf lifespan |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avg. | s.d. | N | Avg. | s.d. | N | Avg. | s.d. | N | Avg. | s.d. | N | ||
| Divisions (Div.) include angiosperms and gymnosperms. | |||||||||||||
| angio. | Acer | 548 | 243 | 8 | 48.51 | 2.22 | 8 | 2.52 | 0.86 | 8 | 1 | 0 | 8 |
| angio. | Alnus | 326 | 136 | 9 | 51.03 | 1.07 | 9 | 2.56 | 0.22 | 9 | 1 | 0 | 9 |
| angio. | Arbutus | 168 | 35 | 16 | 47.57 | 2.10 | 16 | 0.93 | 0.31 | 16 | NA | NA | 0 |
| angio. | Ceanothus | 121 | NA | 1 | 51.20 | NA | 1 | 2.47 | NA | 1 | 1 | NA | 1 |
| angio. | Cercocarpus | 86 | NA | 1 | 46.56 | NA | 1 | 2.18 | NA | 1 | NA | NA | 0 |
| angio. | Chrysolepis | 137 | 28 | 4 | 49.77 | 0.34 | 4 | 1.30 | 0.23 | 4 | 1 | 0 | 4 |
| angio. | Cornus | 928 | 107 | 2 | 39.98 | 0.13 | 2 | 1.77 | 0.06 | 2 | NA | NA | 2 |
| angio. | Corylus | 1,146 | NA | 1 | 48.50 | NA | 1 | 2.91 | NA | 1 | 1 | NA | 1 |
| angio. | Frangula | 488 | NA | 1 | 47.70 | NA | 1 | 2.90 | NA | 1 | 1 | NA | 1 |
| angio. | Holodiscus | 484 | 355 | 2 | 49.00 | 0.57 | 2 | 2.37 | 0.47 | 2 | 1 | 0 | 2 |
| angio. | Lithocarpus | 162 | 36 | 5 | 43.14 | 4.29 | 5 | 0.91 | 0.16 | 5 | 3.40 | 2.07 | 5 |
| angio. | Purshia | 194 | 73 | 66 | 50.10 | 1.20 | 66 | 1.93 | 0.52 | 66 | 1 | 0 | 66 |
| angio. | Quercus | 264 | 185 | 17 | 44.77 | 2.31 | 17 | 1.57 | 0.58 | 17 | 2.06 | 1.56 | 17 |
| angio. | Rhododendron | 116 | NA | 1 | 52.30 | NA | 1 | 0.97 | NA | 1 | 1 | NA | 1 |
| angio. | Ribes | 232 | NA | 1 | 48.40 | NA | 1 | 2.61 | NA | 1 | 1 | NA | 1 |
| gymno. | Abies | 92 | 20 | 256 | 49.26 | 2.30 | 225 | 0.88 | 0.14 | 225 | 8.27 | 3.92 | 251 |
| gymno. | Calocedrus | 82 | 24 | 18 | 48.04 | 1.62 | 18 | 0.84 | 0.21 | 18 | 4 | NA | 1 |
| gymno. | Juniperus | 64 | 9 | 68 | 48.88 | 1.71 | 68 | 0.94 | 0.18 | 68 | NA | NA | 0 |
| gymno. | Larix | 309 | 33 | 10 | 46.13 | 2.31 | 10 | 1.87 | 0.37 | 10 | 1 | 0 | 10 |
| gymno. | Picea | 125 | 47 | 38 | 52.15 | 1.92 | 38 | 1.16 | 0.23 | 38 | 5.21 | 1.95 | 38 |
| gymno. | Pinus | 84 | 16 | 372 | 50.51 | 2.04 | 372 | 1.13 | 0.19 | 372 | 5.41 | 2.47 | 287 |
| gymno. | Pseudotsuga | 120 | 24 | 310 | 51.40 | 1.64 | 253 | 1.08 | 0.19 | 253 | 5.42 | 1.96 | 306 |
| gymno. | Thuja | 95 | 18 | 3 | 51.03 | 1.99 | 3 | 0.98 | 0.12 | 3 | 5.00 | NA | 1 |
| gymno. | Tsuga | 175 | 46 | 85 | 52.68 | 1.04 | 85 | 1.04 | 0.23 | 85 | 5.20 | 1.27 | 85 |
Specific leaf area
Specific leaf area is the ratio of leaf surface area to carbon mass. We provide SLA estimates calculated using both leaf projected surface area (PSA; cm2 PSA g−1 C) and leaf hemi-surface area (i.e., one-half total leaf area; HSA; cm2 HSA g−1 C). As with leaf chemistry, specific leaf area was measured on one-year old foliage, except in the case of deciduous species, where measurements were based on current-year, fully-expanded and hardened foliage. Fresh leaf PSA was measured using a LI-3100C Area Meter (LiCor, Lincoln, NE). For broad-leaf angiosperms, leaf PSA was assumed to be equivalent to HSA; while for conifers, leaf PSA was converted to HSA using published conversion coefficients22–26 (Table 4). Species with unknown conversion coefficients were assigned values from similar species. For Pinus species, leaf HSA was estimated from measurements of needle length and maximum fascicle diameter, except for samples collected as part of the CADIS project, which were scanned using the LI-3100C. After measuring leaf surface area, the samples were oven-dried at 70 °C for at least 48 h and then weighed. Leaf dry mass was converted to carbon based on elemental analysis of sample carbon content (described above). The SLA of each sample was then calculated by dividing leaf PSA and/or HSA by carbon mass. Genus-level graphical summaries of SLA are presented in Fig. 3, while species-level and genus-level statistical summaries are given in Tables 2 and 3.
Table 4. Summary of literature-derived coefficients used to convert projected surface area (PSA) to hemisurface area (HSA) when determining specific leaf area.
| Species | Coef | Source | Note |
|---|---|---|---|
| Abies amabilis | 1.09 | Barclay and Goodman22 | Coef for Abies grandis |
| Abies concolor | 1.09 | Barclay and Goodman22 | Coef for Abies grandis |
| Abies grandis | 1.09 | Barclay and Goodman22 | |
| Abies lasiocarpa | 1.09 | Smith et al.23 | Interpreted from Fig. 1 and bias-corrected by 0.16 based on systematic offset in comparison of PINPON, PINCON, and PSEMEN from refs 22,26 |
| Abies magnifica | 1.09 | Barclay and Goodman22 | Coef for Abies grandis |
| Abies procera | 1.09 | Barclay and Goodman22 | Coef for Abies grandis |
| Calocedrus decurrens | 1.15 | Barclay and Goodman22 | Coef for Tsuga heterophylla |
| Juniperus occidentalis | 1.57 | Hicks and Dugas24 | |
| Larix occidentalis | 1.29 | Gower and Norman25 | Coef for Lairx decidua |
| Picea engelmannii | 1.19 | Smith et al.23 | See note for Abies lasiocarpa |
| Picea sitchensis | 1.16 | Barclay and Goodman22 | |
| Pinus contorta | 1.29 | Barclay and Goodman22 | |
| Pinus flexilis | 1.19 | Smith et al.23 | See note for Abies lasiocarpa |
| Pinus jeffreyi | 1.18 | Law et al.26 | Coef for Pinus ponderosa |
| Pinus lambertiana | 1.18 | Law et al.26 | Coef for Pinus ponderosa |
| Pinus monticola | 1.18 | Law et al.26 | Coef for Pinus ponderosa |
| Pinus ponderosa | 1.18 | Law et al.26 | Coef for Pinus ponderosa |
| Pseudotsuga menziesii | 1.19 | Barclay and Goodman22 | |
| Thuja plicata | 1.14 | Barclay and Goodman22 | |
| Tsuga heterophylla | 1.15 | Barclay and Goodman22 | |
| Tsuga mertensiana | 1.15 | Barclay and Goodman22 | Coef for Tsuga heterophylla |
Figure 3. Box and whisker plots summarizing specific leaf area measurements for each plant genera represented in the dataset.
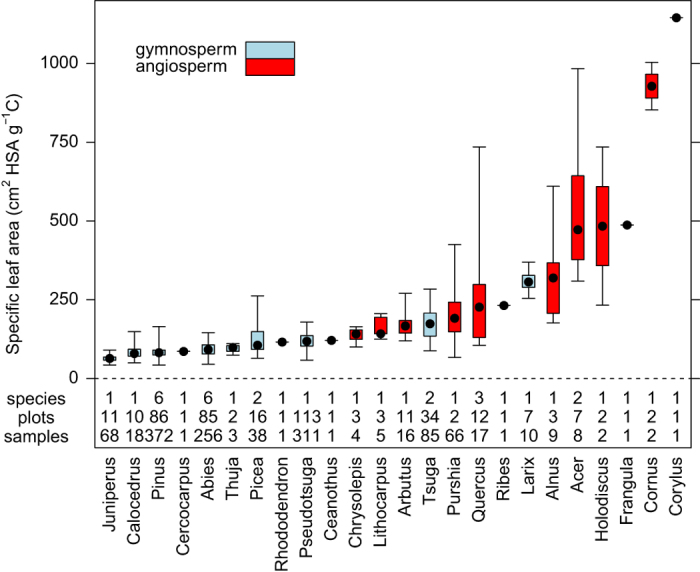
Intrabox dots denote medians, box edges denote 25th and 75th percentiles, and whiskers denote minimum and maximum values. The number of species, sites, and samples representing each genera is also provided. Species-level and genus-level statistical summaries are given in Tables 2 and 3.
Leaf lifespan
Leaf lifespan, also known as leaf longevity or leaf retention time, describes the number of years that a leaf is alive. Leaf lifespan was estimated using the ‘counting cohorts’ method, which involves counting the number of annual leaf cohorts present on an individual branch back to the point where less than 50% of the leaves produced during a given year still remain attached to the branch27. Species-level and genus-level statistical summaries of leaf lifespan are given in Tables 2 and 3.
Stand characterization
Geographic location
The latitude, longitude, and elevation are provided for each plot. Geographic coordinates are given in decimal degrees based on the WGS84 geographic datum and elevation is in meters above sea level. Geographic coordinates were determined using a hand-held global positioning system (GPS). Elevations were extracted from the Shuttle Radar Topography Mission version 2 digital elevation model based on the geographic coordinates.
Stand age
Stand age is defined in this case as the average age, given in years, of the oldest 10% of trees located in a stand or, if fewer than three trees fell into the oldest 10%, then the average age of all trees that were cored. Stand age was estimated based on tree cores collected from eight to 20 trees on each plot, with sample size dependent on project. Trees were cored to the pith at breast height using an increment borer. The tree cores were mounted on a wooden block and sanded to reveal the annual rings. The age of each tree was then determined by either examining the tree core under a microscope, or by scanning the tree core with a flatbed scanner and then ingesting the image into the WinDENDRO software (Regent Instruments Inc., Québec). For large trees where increment core samples did not reach the pith, the missing rings were determined from total number of rings within 5 cm distance of the inner end of core sample and estimates of the missing length by subtracting increment core length from the radius of the tree at breast height.
Species overstory composition
Species overstory composition described the relative dominance of overstory species on a plot and is provided for up to four species. Each species is denoted using a six-letter genus-species abbreviation. Species dominance is described as the percentage of stand basal area represented by the species. Basal area was computed for each tree based on measurement of DBH and then summed within species.
Average canopy height
Average canopy height, given in meters, of all trees located on a plot. Tree height was measured using a laser.
Leaf area index
Summer maximum leaf area index (LAI; m2 leaf m−2 ground) of the overstory canopy corrected for canopy and foliage clumping, as well as wood interception6,28. Optical measurements of the effective overstory canopy LAI were made at breast height (1.37 m) with a LAI-2000 or LAI-2200 Plant Canopy Analyzer (LI-COR Inc., Lincoln, NE) under diffusive light at 35–45 locations on each sample plot. Measurements were corrected for clumping and wood interception based on the method described in Law et al.26. The measurements were subject to post-collection processing to remove data points measured below the threshold of irradiance (<0.2 umol m−2 s−1) of the above canopy sensor. Species-specific values of the needle-to-shoot ratio for foliage clumping within shoot (gamma) were from published data of Law et al.26, Gower et al.29, and Frazer et al.30. Species with unknown gamma were assigned values from morphologically similar species. In mixed-species stands, the gamma-clumping corrections were weighted by the frequency distribution of stem counts of each species, or by the relative basal area (BA; m2 wood ha−1) of each species, depending on project. Elemental clumping index (omega), which quantifies the effect of foliage clumping at scales larger than the shoot, was determined from continuous measurements with a TRAC (3rd Wave Engineering, Ontario, Canada) optical device along three 100-m transects26 or by LAI-2200 measurements at each sampling point. Wood interception (W), defined here as half-surface area of stem and branches above breast-height (1.37 m), was computed as a function of stand basal area at breast height as
based on the strong relationship (r2=0.90) observed between W and basal area across 96 sites measured as part of the EPA project (Law, unpublished data). Overstory LAI was then computed by subtracting W from clumping-corrected LAI.
Tree aboveground biomass
Aboveground biomass of live tree wood (i.e., stem, branches, and bark) and tree foliage per square meter of ground (g C m−2 ground), as well as total (i.e., wood+foliage) aboveground biomass pool, were estimate for each plot. For each tree on a plot, wood component biomass was estimated based on measurements of tree DBH and height. Stem volume was estimated using species- and ecoregion-specific volume allometric equations; where species- or ecoregion-specific equations were not available, substitutions were made based on growth form and plant type4,31–33. Stem volume was then converted to mass based on species-specific wood density, with wood density either measured using tree cores from the plots or taken from regional34,35 or national36 technical reports prepared by the USDA Forest Service. Branch and bark mass were estimated using species-specific biomass equations, again substituting equations for similar species were necessary. Stem, branch and bark dry mass were assumed to be 51% carbon. Aboveground tree wood biomass was then derived by summing component carbon pools across trees on a subplot, dividing by subplot area, and then averaging across subplots. Tree foliage biomass was estimated for each subplot from overstory LAI and species biomass-weighted average leaf mass per unit area (LMA=1/SLA), converted to carbon mass based on plot-average leaf carbon concentrations, and then averaged across subplots. Total aboveground biomass on each subplot was computed as the sum of wood and foliage biomass.
Tree annual aboveground net primary productivity
Annual aboveground net primary productivity of tree wood and tree foliage per square meter of ground per year (g C m−2 ground year−1), as well as total aboveground productivity, were estimate for each plot. For each tree on a plot wood component net productivity was estimated based on the difference in biomass at two points in time divided by the number of intervening years, generally 5- or 10-years depending on project. Current wood biomass was estimated as described above, while prior wood biomass was estimated by hindcasting tree DBH and height using tree core increment measurements and DBH-height relationships. Wood component productivity was summed across trees on a subplot, divided by subplot area, and then averaged across subplots. Tree foliage productivity was calculated for each subplot by dividing foliage carbon mass, as described above, by the species biomass-weighted average leaf longevity and then averaged across subplots.
Soil characterization
Soil depth
Effective soil depth (cm) measured with a steel tile probe to a maximum depth of 100 cm.
Soil profile minimum and maximum depth
Each soil characteristic described below was derived for a given portion of the mineral soil profile, defined by a minimum and maximum depth below the mineral soil surface, given in centimetres. Target depth in each soil profile included 0–20 cm, 20–50 cm, and 50–100 cm; however, the actual profile depths are noted for each layer.
Soil bulk density
Soil bulk density for a specified soil profile layer given in kilograms of rock-free soil per square meter of earth (kg m−2). Bulk density was determined using material <2 mm diameter based on air-dry weight and soil core volumes that excluded coarser fragments.
Soil carbon and nitrogen mass
Carbon or nitrogen mass for a specified mineral soil profile layer, given in grams of C or N per square meter of ground (e.g., g C m−2). The soil samples were air-dried using a ventilated drying system and then live vegetation and roots were removed. Samples were pulverized, then carbon and nitrogen content were measured with a LECO CNS2000 analyzer by Central Analytical Laboratory at Oregon State University. Carbon and nitrogen content in each mineral soil profile layer were then computed from elemental concentrations, soil bulk density, and sampling depth. See Sun et al.20 for more details.
Soil pH
Soil pH for a specified profile layer was determined using a ratio of soil mass to water of 1:2. The analysis was performed by Central Analytical Laboratory at Oregon State University.
Soil sand, silt, and clay content
Mass fraction of sand (0.05–2 mm diameter), silt (0.002–0.05 mm diameter), and clay (<0.002 mm diameter) for a specified profile layer, with values given as a percentage of total air-dry mass excluding fragments >2 mm diameter. The fraction of each particle size class was determined using air-dried soil samples following the specification hydrometer method described in Gee and Bauder37. This analysis was performed by Central Analytical Laboratory at Oregon State University.
Data Records
The dataset (NACP TERRA-PNW: Forest Plant Traits, NPP, Biomass, and Soil Properties, 1999–2014) is hosted with other contributions from the North American Carbon Program (NACP) by the Oak Ridge National Laboratory Distributed Active Archive Center for Biogeochemical Dynamics (Data Citation 1). The dataset includes three files in a comma-separated values format (.csv), where the first row below the column names stores the column units. Missing values in each file are denoted by −9999. The file containing leaf trait measurements (NACP_TERRA_PNW_leaf_trait_dataset.csv) is structured such that each non-header row (n=1296) contains all measurements from a single plant (tree or shrub), with columns (n=28) describing each plants sampling location, taxonomy, and trait characteristics (Table 5). The file containing measurements of stand composition, biomass, and productivity (NACP_TERRA_PNW_forest_biomass_productivity_dataset.csv) is structured such that each row (n=266) describes a sampling site, with columns (n=32) describing the sites location, species composition, aboveground biomass, and productivity (Table 6). The file structure largely conforms to the Biological, Ancillary, Disturbance and Metadata (BADM) protocol used by AmeriFlux and Fluxnet2. In contrast with the preceding two files, the file containing measurements of soil physical and chemical characteristics (NACP_TERRA_PNW_soil_dataset.csv) is structured in a long-format to accommodate multiple sampling depths at some sites (Table 7). In other words, each row (n=467) stores measurements from a site x sampling depth combination, while each column (n=25) describes the site location and various soil characteristics. All files include a PLOT_ID column that can be used to link measurements across the datasets. Additionally, some sites were sampled more than once as part of separate projects, in which case each resample is a separate entry. The column PLOT_ID_ALT in combination with latitude and longitude can be used to identify sites that were resampled.
Table 5. Summary of each column included in the leaf trait dataset, including column number, column name, data format, data units, and a short description.
| Col. | Col. name | Format | Units | Range | Description |
|---|---|---|---|---|---|
| 1 | PROJECT | categorical | — | — | Project name |
| 2 | YEAR | numerical | year | 2000–2014 | Sampling year |
| 3 | MONTH | numerical | month | 5–10 | Sampling month |
| 4 | DAY | numerical | day | 1–31 | Sampling day |
| 5 | ECOREGION | categorical | — | — | EPA Level III Ecoregion |
| 6 | CLUSTER | categorical | — | — | General sampling area |
| 7 | PLOT_ID | numerical | — | 1–1,014 | Plot identification number |
| 8 | LATITIDE | numerical | decimal degree | 37.7844–45.9477 | Plot latitude (WGS84) |
| 9 | LONGITUDE | numerical | decimal degree | −123.9038–−117.1309 | Plot longitude (WGS84) |
| 10 | LAI_O | numerical | m2 m−2 | 0–14.7 | Overstory leaf area index |
| 11 | HEIGHTC | numerical | m | 1–54 | Plot-average canopy height |
| 12 | PFT | categorical | — | — | Plant function type |
| 13 | DIVISION | categorical | — | — | Angiosperm or gymnosperm |
| 14 | GENUS | categorical | — | — | Genus name |
| 15 | SPECIES | categorical | — | — | Species name |
| 16 | COMMON_NAME | categorical | — | — | Species common name |
| 17 | LEAF_PSA | numerical | cm2 | 1–557 | Leaf projected surface area (PSA) |
| 18 | PSA_to_HSA | numerical | — | 1.00–1.57 | Coefficient converting PSA to HSA |
| 19 | LEAF_HSA | numerical | cm2 | 2–557 | Leaf hemisurface area (HSA) |
| 20 | LEAF_DRY_WT | numerical | g | 0.01–3.88 | Leaf sample dry weight |
| 21 | LEAF_CARBON_WT | numerical | g C | 0.005–1.741 | Leaf sample carbon weight |
| 22 | SLA_PSA | numerical | cm2 PSA g−1 C | 27–1004 | Specific leaf area based on PSA |
| 23 | SLA_HSA | numerical | cm2 HSA g−1 C | 43–1,146 | Specific leaf area based on HSA |
| 24 | LEAF_CARBON | numerical | % | 38–57 | Leaf carbon mass fraction |
| 25 | LEAF_NITROGEN | numerical | % | 0.45–3.89 | Leaf nitrogen mass fraction |
| 26 | LEAF_CN | numerical | — | 12–112 | Leaf carbon to nitrogen mass ratio |
| 27 | LEAF_LIFE | numerical | year | 1–26 | Leaf lifespan |
| 28 | NOTES | categorical | — | — | General notes |
Table 6. Summary of each column included in the forest carbon cycling dataset, including column number, column name, data format, units, range of values, and a short description.
| Col. | Col. name | Format | Units | Range | Description |
|---|---|---|---|---|---|
| 1 | PROJECT | categorical | — | — | Project name |
| 2 | YEAR | numerical | year | 1999–2014 | Sampling year |
| 3 | MONTH | numerical | month | 5–10 | Sampling month |
| 4 | DAY | numerical | day | 1–31 | Sampling day |
| 5 | ECOREGION | categorical | — | — | EPA Level III Ecoregion |
| 6 | CLUSTER | categorical | — | — | General sampling area |
| 7 | PLOT_ID | numerical | — | 1–1,014 | Plot identification number |
| 8 | PLOT_ID_ALT | numerical | — | — | Alternative PLOT_ID is previously sampled |
| 9 | PLOT_ID_AMERIFLUX | categorical | — | — | AmeriFlux identification |
| 10 | LATITUDE | numerical | decimal degree | 37.7844–45.9477 | Plot latitude (WGS84) |
| 11 | LONGITUDE | numerical | decimal degree | −123.9038–−117.1309 | Plot longitude (WGS84) |
| 12 | ELEVATION | numerical | m | 138–2,758 | Elevation above sea level |
| 13 | MAT | numerical | °C | 2.43–13.53 | Mean annual temperature (1984–2013) |
| 14 | MAP | numerical | mm yr−1 | 248–2,839 | Mean annual precipitation (1984–2013) |
| 15 | SITE_DESC | categorical | — | — | General site description |
| 16 | ASA | numerical | year | 8–795 | Average stand age |
| 17 | SPP_O1_ ABBREV | categorical | — | — | Primary overstory species abbreviation |
| 18 | SPP_O1_BASAL_AREA_FRACTION | numerical | % | 33–100 | Primary overstory species basal area fraction |
| 19 | SPP_O2_ ABBREV | categorical | — | — | Secondary overstory species abbreviation |
| 20 | SPP_O2_ BASAL_AREA_FRACTION | numerical | % | 0–50 | Secondary overstory species basal area fraction |
| 21 | SPP_O3_ ABBREV | categorical | — | — | Tertiary overstory species abbreviation |
| 22 | SPP_O3_ BASAL_AREA_FRACTION | numerical | % | 0–30 | Tertiary overstory species basal area fraction |
| 23 | SPP_O4_ ABBREV | categorical | — | — | Quaternary overstory species abbreviation |
| 24 | SPP_O4_ BASAL_AREA_FRACTION | numerical | % | 0–17 | Quaternary overstory species basal area fraction |
| 25 | LAI_O | numerical | m2 m−2 | 0–14.7 | Stand overstory leaf area index |
| 26 | HEIGHTC | numerical | m | 1–54 | Average canopy height |
| 27 | AG_BIOMASS_TREE_WOOD_AS_CARBON | numerical | g C m−2 | 71–64,035 | Tree wood aboveground biomass |
| 28 | AG_BIOMASS_TREE_FOLIAGE_AS_CARBON | numerical | g C m−2 | 0–1,738 | Tree foliage aboveground biomass |
| 29 | AG_BIOMASS_TREE_TOTAL_AS_CARBON | numerical | g C m−2 | 53–65,151 | Tree total aboveground biomass |
| 30 | AG_PROD_TREE_WOOD_AS_CARBON | numerical | g C m−2 yr−1 | 0–800 | Tree wood aboveground productivity |
| 31 | AG_PROD_TREE_FOLIAGE_AS_CARBON | numerical | g C m−2 yr−1 | 0–388 | Tree foliage aboveground productivity |
| 32 | AG_PROD_TREE_TOTAL_AS_CARBON | numerical | g C m−2 yr−1 | 0–958 | Tree total aboveground productivity |
Table 7. Summary of each column included in the soil dataset, including column number, column name, data format, units, range of values, and a short description.
| Col. | Col. name | Format | Units | Range | Description |
|---|---|---|---|---|---|
| 1 | PROJECT | categorical | — | — | Project name |
| 2 | YEAR | numerical | year | 2001–2007 | Sampling year |
| 3 | MONTH | numerical | month | 6–10 | Sampling month |
| 4 | DAY | numerical | day | 1–31 | Sampling day |
| 5 | ECOREGION | categorical | — | — | EPA Level III Ecoregion |
| 6 | CLUSTER | categorical | — | — | General sampling area |
| 7 | PLOT_ID | numerical | — | 1–948 | Plot identification number |
| 8 | PLOT_ID_ALT | numerical | — | — | Alternative PLOT_ID is previously sampled |
| 9 | PLOT_ID_AMERIFLUX | categorical | — | — | AmeriFlux identification |
| 10 | LATITUDE | numerical | decimal degree | 37.784–45.948 | Plot latitude (WGS84) |
| 11 | LONGITUDE | numerical | decimal degree | −123.904–−117.1309 | Plot longitude (WGS84) |
| 12 | ELEVATION | numerical | m | 138–2758 | Elevation above sea level |
| 13 | MAT | numerical | °C | 2.43–13.53 | Mean annual temperature (1984–2013) |
| 14 | MAP | numerical | mm yr−1 | 414–2,839 | Mean annual precipitation (1984–2013) |
| 15 | SOIL_DEPTH | numerical | cm | 21–100 | Total soil depth |
| 16 | SOIL_LAYER | categorical | — | top/mid/bottom | Soil layer sampled |
| 17 | UPPER_DEPTH_OF_SOIL_LAYER | numerical | cm | 0–50 | Upper depth of the soil layer |
| 18 | LOWER_DEPTH_OF_SOIL_LAYER | numerical | cm | 3–103 | Lower depth of the soil layer |
| 19 | BULK_DENSITY_OF_SOIL_LAYER | numerical | kg m−2 | 2–884 | Bulk density of the soil layer |
| 20 | CARBON_CONTENT_OF_SOIL_LAYEL | numerical | g C m−2 | 10–24,480 | Carbon content of the soil layer |
| 21 | NITROGEN_CONTENT_OF_SOIL_LAYER | numerical | g N m−2 | 0–1330 | Nitrogen content of the soil layer |
| 22 | PH_OF_SOIL_LAYER | numerical | — | 3.87–7.45 | pH of the soil layer |
| 23 | VOLUME_FRACTION_OF_SAND_IN_SOIL_LAYER | numerical | % | 17–86 | Volume fraction of sand in the soil layer |
| 24 | VOLUME_FRACTION_OF_SILT_IN_SOIL_LAYER | numerical | % | 11–66 | Volume fraction of silt in the soil layer |
| 25 | VOLUME_FRACTION_OF_CLAY_IN_SOIL_LAYER | numerical | % | 1–50 | Volume fraction of clay in the soil layer |
This dataset represents over 15 years of intellectual investment. We request that the dataset is cited if used in a paper and, if incorporated into another dataset, that each data value/row includes a comment noting the dataset citation. Additionally, we would appreciate the opportunity to contribute intellectually and as co-authors to research projects that both incorporate this dataset and view it as a substantial contribution.
Technical Validation
Multiple steps were taken to ensure the technical quality of the dataset. Most importantly, consistent field and laboratory protocols2 were employed among projects. Exceptions did occur, such as Pinus leaf area estimated using callipers versus a LI-3100C when deriving SLA. The sampling intensity specified by the protocols was designed to achieve a coefficient of variation <20%. Repeat measurements of leaf area using the LI-3100C tended to vary by less than 3%. Additionally, all elemental analysis of leaf and soil carbon and nitrogen were performed by Central Analytical Laboratory, which incorporated periodic measurements of calibration samples and blanks to ensure accuracy. Furthermore, we took special care to standardize and define the units of SLA, which is very important given that there is no standard definition. Values reported in the literature are often ambiguously defined and can be derived from measurements of projected, one-sided, one-half total, and total surface area divided by either leaf dry mass or leaf carbon mass29.
After compiling the dataset, we implemented several quality control measures on the leaf trait (SLA, C, N, and lifespan) measurements. We plotted each variable and combinations of variables to identify and correct errors in data entry, as well as to identify and remove potential erroneous measurements. After correcting obvious data entry errors, we then identified leaf trait measurements that exceeded the species-average by more than three s.d. Making two passes through the dataset, we chose to screening branch samples if any trait measurement exceeded this threshold; a criteria for inclusion that struck a balance between the need to remove erroneous measurements, while also maintain the full range of phenotypic plasticity.
To further evaluate the dataset, we then compared plant functional type-average and species-average leaf trait summaries against published trait estimates22,38–50 (Table 8). Our estimates of leaf lifespan, leaf nitrogen, and SLA for evergreen needleleaf trees differed, respectively, by +60%, −15%, and −6% in comparison to global estimates for this PFT from the TRY plant-trait data base45. Similarly, our estimates for deciduous broadleaf trees differed from TRY estimates by +95%, 0%, and +12% for the same traits. Some of this variation can be attributed to differences in species mixture between our regional dataset and the globally-oriented TRY dataset.
Table 8. Comparison of leaf trait measurements presented in this study (mean±1s.d.) against estimates drawn from the literature.
| Trait | Taxa | This study | Literature | % Diff. | Literature sources |
|---|---|---|---|---|---|
| Traits include leaf lifespan (years), leaf nitrogen (% of dry weight), and specific leaf area (cm2 HSA g C−1). Trait values drawn from the literature represented mean characteristics. We ordered the literature values and sources numerically for species x trait combinations with multiple literature estimates. |
|||||
| Estimates of specific leaf area derived from the literature were converted from projected surface area (PSA) per gram of leaf dry matter to hemisurface area (HSA) per gram of carbon using our species-specific measurements of leaf carbon content and the PSA to HSA conversion coefficients given in Table 4. | |||||
| LEAF_LIFE | Abies lasiocarpa | 10.1±5.0 | 8.0 | 23 | Reich et al.38 |
| Picea engelmannii | 7.2±2.5 | 7.5 | −4 | Reich et al.38 | |
| Pinus contorta | 8.0±4.1 | 2.0, 4.6 | 120, 67 | Pease40; Ewers & Schmid39 | |
| Pseudotsuga menziesii | 5.4±2.0 | 5.4 | 0 | Wright et al.41 | |
| Thuja plicata | 5.0 | 8.9 | −56 | Harlow et al.42 | |
| Tsuga heterophylla | 5.2±1.3 | 5.4, 5.5 | −4, −6 | Ishii et al.44; Pease40 | |
| Evergreen needleleaf tree | 6.1±3.0 | 3.3 | 60 | Kattge et al.45 | |
| Deciduous broadleaf tree | 1.4±1.2 | 0.5 | 95 | Kattge et al.45 | |
| LEAF N | Acer macrophyllum | 2.81±0.78 | 2.82 | 0 | Lei & Lechowicz50 |
| Abies grandis | 0.88±0.16 | 0.90 | −2 | Nippert et al.53 | |
| Larix occidentalis | 1.87±0.37 | 1.7, 2.0 | 10, −7 | Gower & Richards47; Gower et al.46 | |
| Pinus contorta | 1.06±0.21 | 1.2, 1.4 | −12, −28 | Gower & Richards47; Gower et al.46 | |
| Pseudotsuga menziesii | 1.08±0.19 | 0.99 | 9 | Nippert et al.53 | |
| Tsuga mertensiana | 0.89±0.21 | 1.2 | −30 | Gower & Richards47 | |
| Evergreen needleleaf tree | 1.04±0.21 | 1.21 | −15 | Kattge et al.45 | |
| Deciduous broadleaf tree | 2.12±0.74 | 2.13 | 0 | Kattge et al.45 | |
| SLA_HSA* | Alnus rubra | 326±136 | 209 | 44 | Matson et al.49 |
| Abies concolor | 102±18 | 86 | 17 | Laughlin et al.48 | |
| Abies grandis | 91±19 | 95, 112 | −4, −21 | Nippert et al.53; Gower & Richards47 | |
| Abies lasiocarpa | 98±13 | 88 | 11 | Laughlin et al.48 | |
| Juniperus occidentalis | 49±2 | 57 | −15 | Matson et al.49 | |
| Larix occidentalis | 309±33 | 201, 222 | 42, 33 | Gower & Richards47; Gower et al.46 | |
| Picea engelmannii | 94±30 | 76, 116 | 21, −21 | Laughlin et al.48; Barr et al. (2013) | |
| Pinus contorta | 80±10 | 96, 98, 103 | −18, −20, −25 | Barclay & Goodman22; Gower & Richards47; Gower et al.46 | |
| Pinus ponderosa | 81±11 | 85, 88 | −5, −8 | Matson et al.49; Laughlin et al.48 | |
| Pseudotsuga menziesii | 120±24 | 87, 104, 124, 128, 129 | 32, 14, −3, −6, −7 | Nippert et al.53; Gower et al.46; Barclay & Goodman22; Ishii et al.43; Matson et al.49 | |
| Thuja plicata | 95±18 | 167 | −55 | Barclay & Goodman22 | |
| Tsuga heterophylla | 175±46 | 132, 316 | 28, −57 | Barclay & Goodman22; Ishii et al.44 | |
| Tsuga mertensiana | 193±61 | 68, 104 | 60 | Matson et al.49; Gower & Richards47 | |
| Evergreen needleleaf tree | 102±36 | 108 | −6 | Kattge et al.45 | |
| Deciduous broadleaf tree | 366±211 | 324 | 12 | Kattge et al.45 |
The species-specific comparisons also yielded a range in agreement. Across six species, our estimates of leaf lifespan differed by 0 to 120% in comparison to literature values, with five out of eight comparisons falling within 25% of each other. The smallest discrepancy was for Pseudotsuga menziesii and the largest discrepancy for Pinus contorta, which exhibited high geographic variability in leaf lifespan (2–17 years). Our estimates of leaf nitrogen content differed from literature values by 0 to 30% among six species, with five of the eight estimates differing by 10% or less. Lastly, across 13 species our estimates of SLA (HSA) differed by 4 to 60% from published values, with 18 of 27 estimates falling within 25%. Differences in species-specific leaf trait estimates between our study and other studies could be due to (1) trait variation along resource gradients51,52; (2) differences in plant exposure to short-term stress (e.g., leaf shedding due to drought-stress); (3) differences in the seasonality of sample collection49,53; (4) differences in sampling location within the canopy53,54; or (5) differences in sample processing methods (e.g., SLA estimated with or without the petiole)27.
Additional Information
How to cite this article: Berner, L. T. & Law, B. E. Plant traits, productivity, biomass and soil properties from forest sites in the Pacific Northwest, 1999–2014. Sci. Data 3:160002 doi: 10.1038/sdata.2016.2 (2016).
Supplementary Material
Acknowledgments
We acknowledge data collection by many students at Oregon State University over the years, and compilation of datasets from various projects by Steve Van Tuyl, Osbert Sun, John Campbell, Garrett Meigs, and Tara Hudiburg. We thank Robert Cook and Les Hook for curating our dataset at the ORNL DAAC. Additionally, we thank two anonymous reviewers for constructive feedback on earlier versions of the manuscript and dataset. We acknowledge support from the US EPA NCER EPASTAR program (Grant #R-82830901-0), NASA (Grant # NAG5–11231), the US Department of Agriculture National Institute of Food and Agriculture (Grant # 2013–67003–20652), and the US Department of Energy BER Terrestrial Carbon Program (Grant # DE-FG0300ER63014, DE-FC03–90ER61010, DE-FG02–04ER63917). This work was also supported by NASA Headquarters under the NASA Earth and Space Science Fellowship Program (Grant # NNX14AN65H) and by the ARCS Foundation Scholar program.
Data Citations
- Law B. E., Berner L. T. 2015. Oak Ridge National Laboratory Distributed Active Archive Center. http://dx.doi.org/10.3334/ORNLDAAC/1292
References
- Law B. E. Regional analysis of drought and heat impacts on forests: current and future science directions. Global Change Biology 20, 3595–3599 (2014). [DOI] [PubMed] [Google Scholar]
- Law B. E. et al. Terrestrial carbon observations: Protocols for vegetation sampling and data submission (Food and Agriculture Organization of United Nations, 2008). [Google Scholar]
- Treuhaft R. N., Law B. E. & Asner G. P. Forest attributes from radar interferometric structure and its fusion with optical remote sensing. BioScience 54, 561–571 (2004). [Google Scholar]
- Law B. E., Thornton P. E., Irvine J., Anthoni P. M. & Van Tuyl S. Carbon storage and fluxes in ponderosa pine forests at different developmental stages. Global Change Biology 7, 755–777 (2001). [Google Scholar]
- Treuhaft R. N., Asner G. P., Law B. E. & Van Tuyl S. Forest leaf area density profiles from the quantitative fusion of radar and hyperspectral data. Journal of Geophysical Research: Atmospheres (1984–2012) 107, 4568 (2002). [Google Scholar]
- Law B. E., Cescatti A. & Baldocchi D. D. Leaf area distribution and radiative transfer in open-canopy forests: implications for mass and energy exchange. Tree Physiology 21, 777–787 (2001). [DOI] [PubMed] [Google Scholar]
- Law B. E. et al. Disturbance and climate effects on carbon stocks and fluxes across Western Oregon USA. Global Change Biology 10, 1429–1444 (2004). [Google Scholar]
- Schwarz P. et al. Climatic versus biotic constraints on carbon and water fluxes in seasonally drought-affected ponderosa pine ecosystems. Global Biogeochemical Cycles 18, GB4007 (2004). [Google Scholar]
- Law B. E. et al. in Scaling and Uncertainty Analysis in Ecology 167–190 (Springer, 2006). [Google Scholar]
- Hudiburg T. et al. Carbon dynamics of Oregon and Northern California forests and potential land-based carbon storage. Ecological Applications 19, 163–180 (2009). [DOI] [PubMed] [Google Scholar]
- Meigs G. W., Donato D. C., Campbell J. L., Martin J. G. & Law B. E. Forest fire impacts on carbon uptake, storage, and emission: the role of burn severity in the Eastern Cascades, Oregon. Ecosystems 12, 1246–1267 (2009). [Google Scholar]
- Berner L. T. & Law B. E. Water limitations on forest carbon cycling and conifer traits along a steep climatic gradient in the Cascade Mountains, Oregon. Biogeosciences 12, 6617–6635 (2015). [Google Scholar]
- Turner D. P. et al. Decadal trends in net ecosystem production and net ecosystem carbon balance for a regional socioecological system. Forest Ecology and Management 262, 1318–1325 (2011). [Google Scholar]
- Hudiburg T. W., Law B. E. & Thornton P. E. Evaluation and improvement of the Community Land Model (CLM4) in Oregon forests. Biogeosciences 10, 453–470 (2013). [Google Scholar]
- Xiao J. et al. Data-driven diagnostics of terrestrial carbon dynamics over North America. Agricultural and Forest Meteorology 197, 142–157 (2014). [Google Scholar]
- Luyssaert S. et al. Toward a consistency cross-check of eddy covariance flux–based and biometric estimates of ecosystem carbon balance. Global Biogeochemical Cycles 23, GB3009 (2009). [Google Scholar]
- Hudiburg T. W., Luyssaert S., Thornton P. E. & Law B. E. Interactive Effects of Environmental Change and Management Strategies on Regional Forest Carbon Emissions. Environmental science & technology 47, 13132–13140 (2013). [DOI] [PubMed] [Google Scholar]
- Hudiburg T. W., Law B. E., Wirth C. & Luyssaert S. Regional carbon dioxide implications of forest bioenergy production. Nature Climate Change 1, 419–423 (2011). [Google Scholar]
- Law B. E., Hudiburg T. W. & Luyssaert S. Thinning effects on forest productivity: consequences of preserving old forests and mitigating impacts of fire and drought. Plant Ecology & Diversity 6, 73–85 (2013). [Google Scholar]
- Sun O. J., Campbell J., Law B. E. & Wolf V. Dynamics of carbon stocks in soils and detritus across chronosequences of different forest types in the Pacific Northwest, USA. Global Change Biology 10, 1470–1481 (2004). [Google Scholar]
- Duursma R. A., Marshall J. D., Nippert J. B., Chambers C. C. & Robinson A. P. Estimating leaf-level parameters for ecosystem process models: a study in mixed conifer canopies on complex terrain. Tree Physiology 25, 1347–1359 (2005). [DOI] [PubMed] [Google Scholar]
- Barclay H. J. & Goodman D. Conversion of total to projected leaf area index in conifers. Canadian Journal of Botany 78, 447–454 (2000). [Google Scholar]
- Smith W., Schoettle A. & Cui M. Importance of the method of leaf area measurement to the interpretation of gas exchange of complex shoots. Tree physiology 8, 121–127 (1991). [DOI] [PubMed] [Google Scholar]
- Hicks R. & Dugas W. Estimating ashe juniper leaf area from tree and stem characteristics. Journal of Range Management Archives 51, 633–637 (1998). [Google Scholar]
- Gower S. T. & Norman J. M. Rapid estimation of leaf area index in conifer and broad-leaf plantations. Ecology 72, 1896–1900 (1991). [Google Scholar]
- Law B. E., Van Tuyl S., Cescatti A. & Baldocchi D. D. Estimation of leaf area index in open-canopy ponderosa pine forests at different successional stages and management regimes in Oregon. Agricultural and Forest Meteorology 108, 1–14 (2001). [Google Scholar]
- Pérez-Harguindeguy N. et al. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61, 167–234 (2013). [Google Scholar]
- Chen J. M. Optically-based methods for measuring seasonal variation of leaf area index in boreal conifer stands. Agricultural and Forest Meteorology 80, 135–163 (1996). [Google Scholar]
- Gower S. T., Kucharik C. J. & Norman J. M. Direct and indirect estimation of leaf area index, fapar, and net primary production of terrestrial ecosystems. Remote Sensing of Environment 70, 29–51 (1999). [Google Scholar]
- Frazer G. W., Trofymow J. & Lertzman K. P. Canopy openness and leaf area in chronosequences of coastal temperate rainforests. Canadian Journal of Forest Research 30, 239–256 (2000). [Google Scholar]
- Means J. E., Krankina O. N., Jiang H. & Li H. Estimating live fuels for shrubs and herbs with BIOPAK 21 (US Department of Agriculture, Forest Service, Pacific Northwest Research Station, 1996). [Google Scholar]
- Means J. E., Hansen H. A., Koerper G. J., Alaback P. B. & Klopsch M. W. Software for computing plant biomass--BIOPAK users guide 184 (US Department of Agriculture, Forest Service, Pacific Northwest Research Station, 1994). [Google Scholar]
- Gholz H. L., Grier C., Campbell A. & Brown A. Equations for estimating biomass and leaf area of plants in the Pacific Northwest (Oregon State University, School of Forestry, Forest Research Lab, 1979). [Google Scholar]
- USDA Forest Service. Western Wood Density Survey: Report No. 1, Vol. FPL-27 (USDA Forest Service, 1965). [Google Scholar]
- USDA Forest Service. Western Wood Density Survey: Report No. 2, Vol. FPL-183 (USDA Forest Service, 1972). [Google Scholar]
- Forest Products Laboratory. Would Handbook: Wood as an engineering material 72 (Forest Products Laboratory, 1974). [Google Scholar]
- Gee G. W., Bauder J. M. in Methods of Soil Analysis, Part 1- Physical and Mineralogical Methods, Vol. Agronomy Monograpy 9 (ed. Klute A.) 383–411 (Soil Science Society of America, 1986). [Google Scholar]
- Reich P. B. et al. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia 114, 471–482 (1998). [DOI] [PubMed] [Google Scholar]
- Ewers F. W. & Schmid R. Longevity of needle fascicles of Pinus longaeva (bristlecone pine) and other North American pines. Oecologia 51, 107–115 (1981). [DOI] [PubMed] [Google Scholar]
- Pease V. A. Duration of Leaves in Evergreens. American Journal of Botany 4, 145–160 (1917). [Google Scholar]
- Wright I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004). [DOI] [PubMed] [Google Scholar]
- Harlow B. A., Duursma R. A. & Marshall J. D. Leaf longevity of western red cedar (Thuja plicata) increases with depth in the canopy. Tree physiology 25, 557–562 (2005). [DOI] [PubMed] [Google Scholar]
- Ishii H. et al. Variation in specific needle area of old-growth Douglas-fir in relation to needle age, within-crown position and epicormic shoot production. Tree Physiology 22, 31–40 (2002). [DOI] [PubMed] [Google Scholar]
- Ishii H., Yoshimura K.-I. & Mori A. Convergence of leaf display and photosynthetic characteristics of understory Abies amabilis and Tsuga heterophylla in an old-growth forest in southwestern Washington State, USA. Tree physiology 29, 989– 998 (2009). [DOI] [PubMed] [Google Scholar]
- Kattge J. et al. TRY-a global database of plant traits. Global Change Biology 17, 2905–2935 (2011). [Google Scholar]
- Gower S. T., Grier C. C. & Vogt K. A. Aboveground production and N and P use by Larix occidentalis and Pinus contorta in the Washington Cascades, USA. Tree physiology 5, 1–11 (1989). [DOI] [PubMed] [Google Scholar]
- Gower S. T. & Richards J. H. Larches: Deciduous Conifers in an Evergreen World. BioScience 40, 818– 826 (1990). [Google Scholar]
- Laughlin D. C., Fule P. Z., Huffman D. W., Crouse J. & Laliberte E. Climatic constraints on trait‐based forest assembly. Journal of Ecology 99, 1489–1499 (2011). [Google Scholar]
- Matson P., Johnson L., Billow C., Miller J. & Pu R. Seasonal patterns and remote spectral estimation of canopy chemistry across the Oregon transect. Ecological Applications 4, 280–298 (1994). [Google Scholar]
- Lei T. & Lechowicz M. Functional responses of Acer species to two simulated forest gap environments: leaf-level properties and photosynthesis. Photosynthetica 33, 277–289 (1997). [Google Scholar]
- Dwyer J. M., Hobbs R. J. & Mayfield M. M. Specific leaf area responses to environmental gradients through space and time. Ecology 95, 399–410 (2014). [DOI] [PubMed] [Google Scholar]
- Guerin G. R., Wen H. & Lowe A. J. Leaf morphology shift linked to climate change. Biology letters 8, 882–886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nippert J. B. & Marshall J. D. Sources of variation in ecophysiological parameters in Douglas-fir and grand fir canopies. Tree Physiology 23, 591–601 (2003). [DOI] [PubMed] [Google Scholar]
- Marshall J. D. & Monserud R. A. Foliage height influences specific leaf area of three conifer species. Canadian Journal of Forest Research 33, 164–170 (2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Law B. E., Berner L. T. 2015. Oak Ridge National Laboratory Distributed Active Archive Center. http://dx.doi.org/10.3334/ORNLDAAC/1292