Abstract
Pulmonary fibrosis is a severe lung disease characterized by sustained propagation of lung fibroblasts and relentless accumulation of extracellular matrix (ECM). Idiopathic pulmonary fibrosis (IPF) is the most severe chronic form of pulmonary fibrosis and results both in the gradual exchange of normal lung parenchyma with fibrotic tissue and in the irreversible impairment of gas exchange in the lung. Despite the urgency for novel therapies in IPF treatment, there is no effective and proven medical therapy available. Molecular mechanisms underlying IPF pathogenesis include aberrant ECM signaling through the canonical integrin/PI3K/Akt/mTORC1 signal transduction pathway. One important and well-characterized downstream effector of this pathway is the cellular protein synthesis machinery. Here we will review the recent advances in our understanding of the function of ECM and integrin receptor signaling in development of IPF and will present evidence indicating that the dysregulation of the eIF4F-mediated translational apparatus is an important factor in the development and progression of IPF and other fibrotic disorders. We further discuss the perspectives and challenges to curbing this deadly disease by targeting aberrant translation.
Keywords: IPF, Integrins, collagen, extracellular matrix, pulmonary fibrosis
Introduction
Pulmonary fibrosis can be a complication of a group of lung disorders called interstitial lung diseases (ILDs).1 In cases when pulmonary fibrosis develops within the lung in the absence of any known provocations, it is termed idiopathic pulmonary fibrosis (IPF), which is also known as cryptogenic fibrosing alveolitis (CFA). IPF is characterized by abnormal expansion of granulation tissue due to excessive production of ECM, hyperpropagation of stromal fibroblasts and vast scarring of normal lung parenchyma leading to a deficiency in gas exchange. IPF is usually fatal with life expectance within 2 to 6 y following diagnosis.2 Although there has been some progress in understanding the pathogenesis of IPF, there is still no proven effective therapy available and lung transplantation is the only viable intervention in end-stage disease. To address this limitation, recent efforts have focused on the elucidation of mechanisms responsible for conferring fibroblasts with the IPF phenotype that can be considered as the therapeutic targets to limit the progression of this deadly disease. Recent findings suggest that one such mechanism is the signaling network anchored by the cellular translational apparatus by which ECM signals regulation of major cellular functions including cell proliferation and viability. Deregulation of this network occurs in several diseases including cancer and other disorders associated with abnormal cell proliferation. In this review, we describe the known cellular and molecular processes implicated in pathogenesis of IPF and discuss how alteration of translational control can promote these processes.
Etiology and Pathogenesis of IPF
By definition, etiology and genesis of IPF is unknown and the mechanisms underlying its pathogenesis remain poorly understood. The current concept for the development of pulmonary fibrosis, including IPF, is that at least three physiologically balanced processes implicated in the maintenance of lung fibroblasts populations: proliferation, apoptosis of (myo) fibroblasts and production of ECM-are disturbed.3 Unlike normal tissue repair, where (myo)fibroblast proliferation is self-limited and cells are eliminated by apoptosis upon completion of repair, in IPF there is a relentless accumulation of fibroblasts and ECM due to evading apoptosis and sustained cell proliferation.4 Morphological studies have demonstrated that the sentinel morphological lesion of IPF is the sub-epithelial accumulation of fibroblasts termed the “fibroblastic focus”5-7. The origin of pathological fibroblast foci remains puzzling. Possibilities include abnormal self-renewal and differentiation of resident mesenchymal stem/progenitor cells, recruitment of circulating fibroblast progenitors and transdifferentiation of epithelial cells into the pathological fibroblast phenotypes (epithelial-to-mesenchymal transition, EMT). Ultrastructural analysis of the fibroblastic focus has further revealed that it is composed of myofibroblasts which express the specific marker α-smooth muscle actin and which are enmeshed in a matrix rich in polymerized type I collagen.8
IPF Cells Can Propagate on ECM that Does not Support Normal Lung Fibroblasts
Continuing propagation of myofibroblasts in fibroblastic foci and specific alterations in the ECM structure due to extensive deposition of type I collagen lead to permanently scarred and functionally disabled alveoli.9,10 ECM is composed of collagens, elastin, proteoglycans (including hyaluronan) and noncollagenous glycoproteins and forms a complex, three dimensional network among cells of different tissues in an organ-specific manner and reciprocally influences cellular function to modulate diverse fundamental aspects of cell biology.11,12 ECM components are classified as fiber forming and non-fiber forming (interfibrillar) molecules. ECM constitutes the cellular microenvironment for all cells outside the circulation and its composition profoundly affects cell proliferation, adhesion, migration, differentiation and viability.12,13 Several essential ECM components such as proteoglycans, fibronectin, elastin and fibrillins form its macromolecular structures. In animal tissues, the most abundant matrix components are the collagens that form a super-family of 27 different members which are divided into different subgroups. The fibrillar collagens, types I, III and V, the FACIT collagens, types XII, XIV and XVI, and collagen VI are all expressed in the collagen-rich dermis. About one-third of the 19 collagens—types I, II, III, V, and XI—are called fibrillar collagens, because they are found in tissues as long, highly ordered fibrils with a characteristic banding pattern readily detected by electron microscopy. Among them, type I collagen is the major component of ECM in skin, bone, ligamnents, etc, is composed of glycin- and proline rich two-α1 (I) and one-α2 (I) chains.53 α1 (I) and α2 (I) are produced from two genes. The pro-COL1A1 and COL1A2 polypeptide chains are synthesized by fibroblasts, osteoblasts, or odontoblasts. Normal structural and functional type I collagen production and deposition in ECM to make normal physiological connective tissue needs regulation at several steps. Studies showed abnormality in any step may cause hypo-, hyper-, or defective synthesis and accumulation of collagen in ECM, which in turn causes different diseases in humans such as osteogenesis imperfecta, scurby, scleroderma or systemic sclerosis, keloids, lung fibrosis, liver fibrosis, etc.54-57 Inactivation of the collagen α1(I) gene in mice results in embryonic lethality.58
ECM dynamics results from altered synthesis or degradation of one or more ECM components.13 Recent findings indicate that there is a sharp difference in proliferation profiles of human lung fibroblasts when they are cultured on 2D and 3D type 1 collagen matrices. Specifically, tissue culture plates coated with 2D monomeric collagen provide a proliferation-permissive environment for normal and IPF lung fibroblasts.14 3D matrix, which closely imitates the physiological forms of ECM, is composed of polymerized collagen (fibrillar collagen). In sharp contrast to the 2D matrix, 3D polymerized collagen does not support proliferation and viability of normal lung fibroblasts, whereas IPF fibroblasts survive and propagate.15,16 Furthermore, immunohistochemical analysis revealed that α-smooth muscle actin-expressing myofibroblasts are enmeshed in a type I collagen-rich 3D matrix. These findings show that the composition and architecture of ECM govern a category of signals required for viability and proliferation of lung fibroblasts and that IPF cells harbor an intrinsic program(s) allowing them to survive and propagate under the ECM conditions that do not support growth and viability of normal fibroblasts.
IPF Cells Manifest Sustained Activity of the PI3K/Akt/mTORC1 Signaling Pathway
Several cell surface receptors including integrins, discoidin domain receptors (DDRs), syndecans and CD44 have been identified as components of a complex system responsible for cell immobilization on normal ECM. Integrins are transmembrane molecules, which primarily mediate cell-to-cell and cell-to-ECM adhesion and are composed of non- covalently associated α and β chains which form heterodimeric receptor complexes.17 Eighteen α subunits and 8 β subunits of integrin have been identified that associate to form 24 known types of integrins. The α and β chains of integrins cooperate in a specific mode in which the extracellular portion of the α chain is responsible for the ligand-binding specificity of the complex whereas the intracellular domain of the β chain is associated with the induction of intracellular signaling cascades. Among them, β1 integrin is the major integrin subunit that mediates attachment of fibroblasts to ECM by associating with several α subunits (Table 1). For example, α2β1 integrin predominantly binds to collagen while α5β1 integrin binds to fibronectin. αvβ3, α6β1 are receptors known to bind to vitronectin, laminin, respectively.18,29,30
Table 1. αβ integrin subunit pairings for integrin receptors.
| β subunit | α subunit |
|---|---|
| β1 | α1 |
| α2 | |
| α3 | |
| α4 | |
| α5 | |
| α6 | |
| α7 | |
| α8 | |
| α9 | |
| α10 | |
| α11 | |
| αv | |
| β2 | αL |
| αM | |
| αx | |
| β3 | αv |
| αIIb | |
| β4 | α6 |
| β5 | αv |
| β6 | αv |
| β7 | α4 |
| αE | |
| β8 | αv |
Beyond these proximal events lie several critical intracellular decision points within cellular signaling cascades regulating cell viability and proliferation. Recent studies demonstrated that lung fibroblasts utilize the PTEN-regulated PI3K/Akt- dependent signaling pathway to curb apoptosis and stimulate cell proliferation.14,15 When normal fibroblasts attach to monomeric type I collagen, integrin receptors signal the downregulation of PTEN and activation of the PI3K/Akt protein phosphorylation cascade.18 In contrast, interactions with polymerized collagen do not signal decreasing PTEN and the activity of PI3K/Akt remains low. The integrin-mediated signaling is partly modulated by the membrane protein caveolin-1 (cav-1), which regulates integrin turnover and modifies a variety of cellular processes including cell proliferation and apoptosis operating mainly through the PI3K/Akt/mTORC1 signal pathway.34,40,51 Cav-1 can bind to PTEN using the consensus binding sequence (ɸXɸXXXXɸ corresponding to amino acids 271–278 (FHFWVNTF, ɸ = aromatic amino acid phenylalanine).32-34 Binding to cav-1 increases the ability of PTEN to antagonize PI3K activity. Normal lung fibroblasts express a relatively high level of cav-1, which interacts with PTEN to suppress PI3K/Akt/mTORC1 signaling on polymerized collagen. Conversely, IPF cells express aberrantly low levels of α2β1 integrin, cav-1 and PP2A.30,31 As a result, IPF fibroblasts reveal sustained PI3K/Akt/mTORC1 signaling and propagate under conditions when normal fibroblasts undergo apoptotic death.
Translational Control in Fibrotic Disease
The eIF4F-mediated translational apparatus is a key regulatory hub in the cancer signaling circuitry
The PI3K/Akt/mTORC1-mediated signaling pathway senses and integrates a variety of environmental cues to regulate cell homeostasis, viability and propagation.51 One important downstream effector of mTORC1 signaling is the translational apparatus. Translational regulation primarily occurs at the mRNA recognition step.19,20 In eukaryotes, this step is facilitated by the mRNA 5′ cap structure (7-methyl-G(5′)ppp(5′)N where N is any nucleotide). The heterotrimeric translation initiation complex eIF4F functions to recognize capped mRNA and recruit these transcripts to the 40S ribosome subunit with subsequent ribosome scanning toward the initiation codon. It consists of the translational factors eIF4E, eIF4G and eIF4A. A 25 kDa phosphoprotein eIF4E directly associates with the cap structure and is rate-limiting for translation initiation. eIF4GI and II serve a docking function, binding to eIF4E and eIF4A. The eIF4AI and eIF4AII isoforms function as an ATP-requiring helicase, which unwinds the 5′ region of the mRNA. The primary regulation of eIF4F integrity is exerted by three repressor proteins, designated 4E binding protein (4E-BP) 1, -BP2 and -BP3.21-23 When hypophosphorylated, 4E-BPs avidly bind with eIF4E thereby sequestering it into a translationally inactive complex. A wide variety of extracellular regulatory cues including growth factors, hormones and components of ECM stimulate eIF4F assembly and cap-dependent translation by signaling phosphorylation of the 4E-BPs on four serine/threonine sites important for their association with eIF4E through the PI3K/Akt/mTORC1 kinase cascade, which is positively regulated by Ras.24,25 Ras can activate eIF4F-driven translation operating through the canonical MAPK/ERK pathway leading to phosphorylation of eIF4E by protein kinases MNK1 and MNK2.26 Deregulation of cap-dependent translation consists of sustained, constitutive activation of eIF4F by a vast array of upregulated upstream signals including the Myc, Ras and PI3K/AKT/mTORC1 pathways as well as by genetic alterations in components of the translational apparatus.20 There is now strong support for the idea that eIF4F is an obligatory regulatory hub in most - if not all - human malignancies.19,27,50 More recently, gene analysis revealed derangement of translational control is also an important pathological mechanism of IPF.28
Translational control in IPF fibroblasts on ECM
Emerging pieces of evidence demonstrate that extracellular matrix greatly regulates PI3K/Akt/mTORC1 via the integrin/PTEN axis.14,15,18 Because aberrant translation is the ultimate step for producing proteins driving abnormal cell proliferation, motility and microenvironment in cancer and other proliferative disorders,35 it is reasonable to propose that translational control can be a crucial step in the interactions of fibroblasts with matrix constituents and that its dysregulation may be involved in the onset and progression of IPF. Indeed, fibroblast adhesion to the major matrix protein fibronectin activates eIF4F-dependent translation through a PI3K-mediated pathway in a manner that is independent on mTORC1 activity and that can be impaired by blocking the β1 integrin engagement.36 Integrin-induced activation of the translational machinery may be selective for changes in translational efficiency of some subsets of mRNAs. It has been shown that α6β4 integrin specifically stimulates recruitment of ribosomes to mRNAs encoding the Ras, ErbB2 and VEGF oncoproteins in breast carcinoma cells and that this activity is mediated through the Ras/MAPK/ERK signaling pathways leading to MNK1-mediated phosphorylation of eIF4E.37-39 In line with this, downregulation of the αVβ3 integrin-dependent PI3K/Akt/ mTORC1 signaling axis suppresses eIF4F-mediated translation and promotes apoptotic death of endothelial cells by stimulating the sequestration of eIF4E by hypophosphorylated 4E-BP1.40 In the course of interactions between normal lung fibroblasts and collagen matrix, mTORC1 kinase is upregulated as a result of signaling from the PI3K/Akt pathway, thereby affecting the activity of the translational machinery by hyperphosphorylation of 4E-BP1. Unlike the active propagation of normal lung fibroblast cultivated on monomeric type I collagen, their proliferation and viability on polymerized collagen is dramatically suppressed and their PI3K/Akt/mTORC1 signaling is downregulated.14,15
PP2A is an important phosphatase that regulates the activity of many proteins via dephosphorylation41 and is thought to regulate translational activity. Studies also showed that Src-dependent suppression of PP2A activity stimulates eIF4F-mediated translation in lung fibroblasts cultivated on monomeric collagen (2D matrix).42 These observations support the notion that the eIF4F integrity and its activity can be curbed by activation of PP2A.48 Moreover, active proliferation of fibroblasts signaled by β1 integrin receptors is associated with profound reduction of 4E-BP1 expression and subsequent dissociation of the translationally-inactive 4E-BP1/eIF4E complex.42 These studies suggest that permissive ECM signals proliferation and viability of normal fibroblasts through activation of multiple pathways leading to regulation of eIF4F. In sharp contrast, the rescue of IPF fibroblasts from apoptosis and the maintenance of their proliferation on 3D matrix is associated with sustained PI3K/Akt/mTORC1- and PP2A-mediated activation of eIF4F-mediated translation43 (Fig. 1).
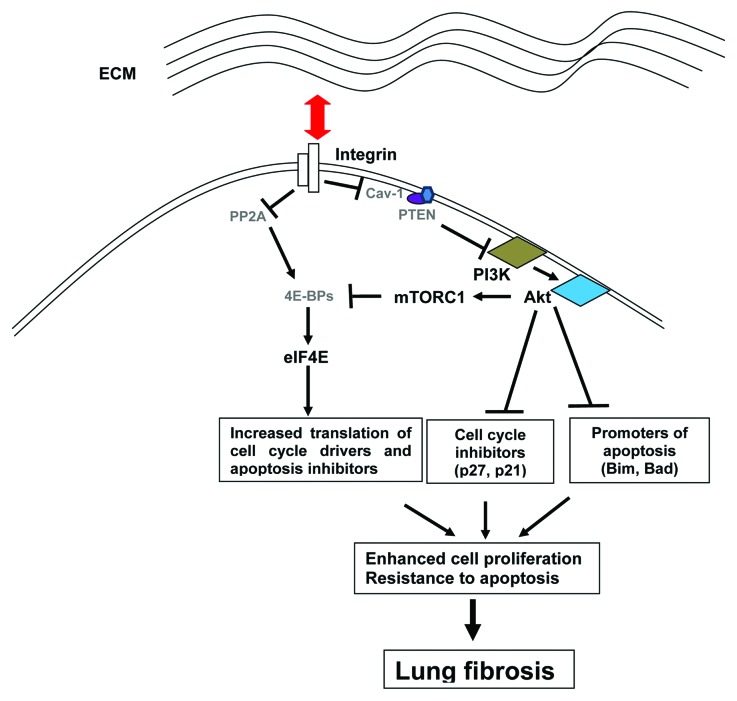
Figure 1. Hypothetical model for the rescue of IPF fibroblasts from apoptosis and the maintenance of their proliferation on 3D matrix. When IPF fibroblasts interact with collagen rich matrix, integrin receptors signal downregulation of PTEN and subsequent activation of the PI3K/Akt/mTORC1 phosphorylation cascade leading to phosphorylation of 4E-BP1 and intensification of eIF4F-driven translation. Integrins signal also increase phosphorylation of 4E-BP1 by suppressing activity of PP2A phosphatase. These up-stream signals profoundly reprogram the eIF4F-mediated translation. At the same time, inappropriately high Akt activity promotes fibroblast proliferation by inhibiting cell cycle inhibitor proteins such as p27 and p21. High Akt activity also suppresses apoptosis inducing proteins such as Bim and Bad, which confers fibroblasts with apoptosis resistant phenotype on ECM. The mRNAs most affected are those encoding proteins that regulate cell growth and viability. Thus cells like fibrotic fibroblasts acquire highly proliferative and apoptosis resistant phenotype. These finding show that aberrant activation of eIF4F-mediated translation can be causally linked to abnormal propagation of IPF fibroblasts and the progression of lung fibrosis.
In response to injury, normal interstitial fibroblasts differentiate into myofibroblasts which synthesize and deposit type I collagen into the wound provisional matrix and contract this matrix, thereby facilitating wound closure.44-47 Under normal physiological conditions, contraction of matrix results in myofibroblast apoptosis to prevent hyperproduction of granulation tissue during the wound healing process.46 Analysis of the translational landscape in myofibroblasts growing on non-contractile or on contractile collagen matrices using microarray technology28 identified a substantial subset of mRNAs that exhibit differential patterns of ribosomal recruitment in IPF and normal myofibroblasts in a manner regulated by the state of the collagen matrix. Specifically, it was shown that mTORC1 and keratin 18 are translationally activated in IPF. The other mRNAs differentially recruited into and out of ribosomes are those encoding proteins involved in regulation of cell cycle transition, apoptosis, cytoskeleton and cell motility49,28. Pathological studies suggest an epithelial origin for IPF myofibroblasts through the epithelial to mesenchymal transition (EMT). In accordance with this, systems-level indications for TGF-β -driven EMT as one source of IPF myofibroblasts were found.49 Collectively, these findings strongly suggest that survival and propagation of IPF fibroblasts, under conditions that are non-permissive for viability of normal fibroblasts, are associated with an intrinsic capability to activate the integrin-dependent signaling cascades, leading to increased translational apparatus.
Conclusion and Future Challenges
IPF fibroblasts reveal high proliferative and anti-apoptotic capabilities under conditions that are non-permissive for viability and growth of normal fibroblasts. Available data show that these propensities are mediated by a constellation of signaling pathways leading to the eIF4F-driven translational apparatus. Although there has been significant progress in understanding the molecular mechanisms of translational control, particularly in cancer models, there are still gaps in understanding the function of translational control in the pathologic nature of IPF fibroblasts for fibrotic progression and the physiologic function of myofibroblasts for normal lung repair.
In order to find potential therapeutic targets that are effective to fibrotic diseases, the nature of cellular pathways by which collagen matrix signals normal or aberrant cell proliferation and viability must particularly be considered. Because patients come to medical attention with some degree of established fibrosis, the present therapeutic strategies predominantly serve to prevent the fibrotic obliteration of additional airspaces, but not to reverse what has already occurred. Rational therapy, however, could also target established fibrotic lesions, creating a state of vulnerability in fibrotic fibroblasts. The findings reviewed here suggest that the aberrant translational apparatus and the components of ECM regulating their activity can be a target for therapeutic interventions. Thus we propose that eIF4E target approach using small peptides or others may be more effective for limiting progression of IPF. This central integrative role of pathologically activated eIF4F in the oncogenic circuitry motivated the development of small molecule inhibitors of eIF4F as potential cancer therapies.27,61-64 For example, targeting eIF4E in eIF4F complex is a promising approach for the limiting fibrotic progression. This approach has already been validated in cancer models. Graff and his coworkers reported their findings that eIF4E targeting using eIF4E specific anti-sense oligonucleotide reduces tumor size without toxicity.59 Specifically this and other eIF4E targeting approaches might be effective in suppression of growth of breast cancer cells.60,65 We propose that similar approaches can be applied to normalize aberrant translation in IPF fibroblasts and can be considered as a potential therapeutic intervention to limit the progression of IPF.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank members of the Bitterman’s, Polunovsky’s and Henke’s research groups for the productive discussions. We specially appreciate the excellent assistance of Damien Tank during preparation of the manuscript and his highly productive remarks.
Reference
- 1.Sivakumar PS, Ntolios, P, Jenkins, G, Laurent, G. Current opinion 2012;18:1070-5287. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. . Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174:810 - 6; http://dx.doi.org/ 10.1164/rccm.200602-163OC; PMID: 16809633 [DOI] [PubMed] [Google Scholar]
- 3.Todd, NW, Luzina, IG, Atamas, SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis & Tissue repair 2012; 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polunovsky VA, Chen B, Henke C, Snover D, Wendt C, Ingbar DH, et al. . Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest 1993; 92:388 - 97; http://dx.doi.org/ 10.1172/JCI116578; PMID: 8326006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn C 3rd, Boldt J, King TE Jr., Crouch E, Vartio T, McDonald JA. . An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989; 140:1693 - 703; http://dx.doi.org/ 10.1164/ajrccm/140.6.1693; PMID: 2604297 [DOI] [PubMed] [Google Scholar]
- 6.Kuhn C, McDonald JA. . The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 1991; 138:1257 - 65; PMID: 2024710 [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda Y, Ishizaki M, Masuda Y, Kimura G, Kawanami O, Masugi Y. . The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol 1987; 126:171 - 82; PMID: 3812636 [PMC free article] [PubMed] [Google Scholar]
- 8.Gay SE, Kazerooni EA, Toews GB, Lynch JP 3rd, Gross BH, Cascade PN, et al. . Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med 1998; 157:1063 - 72; PMID: 9563720 [DOI] [PubMed] [Google Scholar]
- 9.Katzenstein AL, Myers JL. . Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998; 157:1301 - 15; PMID: 9563754 [DOI] [PubMed] [Google Scholar]
- 10.Wight TN, Potter-Perigo S. . The extracellular matrix: an active or passive player in fibrosis?. Am J Physiol Gastrointest Liver Physiol 2011; 301:G950 - 5; http://dx.doi.org/ 10.1152/ajpgi.00132.2011; PMID: 21512158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubmacher D, Apte SS. . The biology of the extracellular matrix: novel insights. Curr Opin Rheumatol 2013; 25:65 - 70; http://dx.doi.org/ 10.1097/BOR.0b013e32835b137b; PMID: 23143224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes RO. . The extracellular matrix: not just pretty fibrils. Science 2009; 326:1216 - 9; http://dx.doi.org/ 10.1126/science.1176009; PMID: 19965464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011; 1:3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, et al. . Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med 2008; 205:1659 - 72; http://dx.doi.org/ 10.1084/jem.20080001; PMID: 18541712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nho RS, Hergert P, Kahm J, Jessurun J, Henke C. . Pathological alteration of FoxO3a activity promotes idiopathic pulmonary fibrosis fibroblast proliferation on type i collagen matrix. Am J Pathol 2011; 179:2420 - 30; http://dx.doi.org/ 10.1016/j.ajpath.2011.07.020; PMID: 21893017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. . Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell 1996; 87:1069 - 78; http://dx.doi.org/ 10.1016/S0092-8674(00)81801-2; PMID: 8978611 [DOI] [PubMed] [Google Scholar]
- 17.Margadant C, Sonnenberg A. . Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 2010; 11:97 - 105; http://dx.doi.org/ 10.1038/embor.2009.276; PMID: 20075988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian B, Lessan K, Kahm J, Kleidon J, Henke C. . β 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem 2002; 277:24667 - 75; http://dx.doi.org/ 10.1074/jbc.M203565200; PMID: 11986332 [DOI] [PubMed] [Google Scholar]
- 19.Silvera D, Formenti SC, Schneider RJ. . Translational control in cancer. Nat Rev Cancer 2010; 10:254 - 66; http://dx.doi.org/ 10.1038/nrc2824; PMID: 20332778 [DOI] [PubMed] [Google Scholar]
- 20.Sonenberg N, Hinnebusch AG. . Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009; 136:731 - 45; http://dx.doi.org/ 10.1016/j.cell.2009.01.042; PMID: 19239892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Harris TE, Roth RA, Lawrence JC Jr.. . PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem 2007; 282:20036 - 44; http://dx.doi.org/ 10.1074/jbc.M702376200; PMID: 17510057 [DOI] [PubMed] [Google Scholar]
- 22.Pause A, Belsham GJ, Gingras AC, Donzé O, Lin TA, Lawrence JC Jr., et al. . Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 1994; 371:762 - 7; http://dx.doi.org/ 10.1038/371762a0; PMID: 7935836 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Li Y, Yang DQ. . Phosphorylation of eIF-4E positively regulates formation of the eIF-4F translation initiation complex following DNA damage. Biochem Biophys Res Commun 2008; 367:54 - 9; http://dx.doi.org/ 10.1016/j.bbrc.2007.12.118; PMID: 18164262 [DOI] [PubMed] [Google Scholar]
- 24.Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC Jr.. . Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci U S A 1998; 95:7772 - 7; http://dx.doi.org/ 10.1073/pnas.95.13.7772; PMID: 9636226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi B, Cai AL, Keiper BD, Minich WB, Mendez R, Beach CM, et al. . Phosphorylation of eukaryotic protein synthesis initiation factor 4E at Ser-209. J Biol Chem 1995; 270:14597 - 603; http://dx.doi.org/ 10.1074/jbc.270.24.14597; PMID: 7782323 [DOI] [PubMed] [Google Scholar]
- 26.Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. . Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol 1999; 19:1871 - 80; PMID: 10022874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitterman PB, Polunovsky VA. . Attacking a nexus of the oncogenic circuitry by reversing aberrant eIF4F-mediated translation. Mol Cancer Ther 2012; 11:1051 - 61; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0530; PMID: 22572598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, et al. . Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS One 2008; 3:e3220; http://dx.doi.org/ 10.1371/journal.pone.0003220; PMID: 18795102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potts AJ, Little CD. . Beta 1 integrins isolated from embryonic chicken fibroblasts bind to monomers and polymers of type I collagen. J Cell Physiol 1992; 152:558 - 67; http://dx.doi.org/ 10.1002/jcp.1041520316; PMID: 1380513 [DOI] [PubMed] [Google Scholar]
- 30.Xia H, Seeman J, Hong J, Hergert P, Bodem V, Jessurun J, et al. . Low α(2)β(1) integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the β-catenin pathway. Am J Pathol 2012; 181:222 - 33; http://dx.doi.org/ 10.1016/j.ajpath.2012.03.034; PMID: 22642910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, et al. . Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 2006; 203:2895 - 906; http://dx.doi.org/ 10.1084/jem.20061536; PMID: 17178917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi F, Sottile J. . Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci 2008; 121:2360 - 71; http://dx.doi.org/ 10.1242/jcs.014977; PMID: 18577581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. . Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol 2003; 23:9389 - 404; http://dx.doi.org/ 10.1128/MCB.23.24.9389-9404.2003; PMID: 14645548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia H, Khalil W, Kahm J, Jessurun J, Kleidon J, Henke CA. . Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol 2010; 176:2626 - 37; http://dx.doi.org/ 10.2353/ajpath.2010.091117; PMID: 20395445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggero D, Sonenberg N. . The Akt of translational control. Oncogene 2005; 24:7426 - 34; http://dx.doi.org/ 10.1038/sj.onc.1209098; PMID: 16288289 [DOI] [PubMed] [Google Scholar]
- 36.Gorrini C, Loreni F, Gandin V, Sala LA, Sonenberg N, Marchisio PC, et al. . Fibronectin controls cap-dependent translation through beta1 integrin and eukaryotic initiation factors 4 and 2 coordinated pathways. Proc Natl Acad Sci U S A 2005; 102:9200 - 5; http://dx.doi.org/ 10.1073/pnas.0409513102; PMID: 15961545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. . Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol 2002; 158:165 - 74; http://dx.doi.org/ 10.1083/jcb.200112015; PMID: 12105188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon SO, Shin S, Lipscomb EA. . A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: the alpha6beta4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res 2006; 66:2732 - 9; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2941; PMID: 16510594 [DOI] [PubMed] [Google Scholar]
- 39.Korneeva NL, Soung YH, Kim HI, Giordano A, Rhoads RE, Gram H, et al. . Mnk mediates integrin α6β4-dependent eIF4E phosphorylation and translation of VEGF mRNA. Mol Cancer Res 2010; 8:1571 - 8; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0091; PMID: 21047768 [DOI] [PubMed] [Google Scholar]
- 40.Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR, et al. . Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science 2002; 295:140 - 3; http://dx.doi.org/ 10.1126/science.1065298; PMID: 11778052 [DOI] [PubMed] [Google Scholar]
- 41.Klumpp S, Krieglstein J. . Serine/threonine protein phosphatases in apoptosis. Curr Opin Pharmacol 2002; 2:458 - 62; http://dx.doi.org/ 10.1016/S1471-4892(02)00176-5; PMID: 12127881 [DOI] [PubMed] [Google Scholar]
- 42.Nho RS, Peterson M. . Eukaryotic translation initiation factor 4E binding protein 1 (4EBP-1) function is suppressed by Src and protein phosphatase 2A (PP2A) on extracellular matrix. J Biol Chem 2011; 286:31953 - 65; http://dx.doi.org/ 10.1074/jbc.M111.222299; PMID: 21784851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia H, Seeman J, Hong J, Hergert P, Bodem V, Jessurun J, et al. . Low α(2)β(1) integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the β-catenin pathway. Am J Pathol 2012; 181:222 - 33; http://dx.doi.org/ 10.1016/j.ajpath.2012.03.034; PMID: 22642910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. . Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001; 12:2730 - 41; PMID: 11553712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desmoulière A, Chaponnier C, Gabbiani G. . Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 2005; 13:7 - 12; http://dx.doi.org/ 10.1111/j.1067-1927.2005.130102.x; PMID: 15659031 [DOI] [PubMed] [Google Scholar]
- 46.Desmoulière A, Redard M, Darby I, Gabbiani G. . Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995; 146:56 - 66; PMID: 7856739 [PMC free article] [PubMed] [Google Scholar]
- 47.Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich HP. . Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr Surg 1998; 102:124 - 31, discussion 132-4; http://dx.doi.org/ 10.1097/00006534-199807000-00019; PMID: 9655417 [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Yue P, Deng X, Ueda T, Fukunaga R, Khuri FR, et al. . Protein phosphatase 2A negatively regulates eukaryotic initiation factor 4E phosphorylation and eIF4F assembly through direct dephosphorylation of Mnk and eIF4E. Neoplasia 2010; 12:848 - 55; PMID: 20927323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adeli K. . Translational control mechanisms in metabolic regulation: critical role of RNA binding proteins, microRNAs, and cytoplasmic RNA granules. Am J Physiol Endocrinol Metab 2011; 301:E1051 - 64; http://dx.doi.org/ 10.1152/ajpendo.00399.2011; PMID: 21971522 [DOI] [PubMed] [Google Scholar]
- 50.Laplante M, Sabatini DM. . mTOR signaling in growth control and disease. Cell 2012; 149:274 - 93; http://dx.doi.org/ 10.1016/j.cell.2012.03.017; PMID: 22500797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee DY, Li YS, Chang SF, Zhou J, Ho HM, Chiu JJ, et al. . Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by alphavbeta3 and beta1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. J Biol Chem 2010; 285:30 - 42; http://dx.doi.org/ 10.1074/jbc.M109.010512; PMID: 19889638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuivaniemi H, Tromp G, Prockop DJ. . Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat 1997; 9:300 - 15; http://dx.doi.org/; PMID: 9101290 [DOI] [PubMed] [Google Scholar]
- 53.Ghosh AK. . Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 2002; 227:301 - 14; PMID: 11976400 [DOI] [PubMed] [Google Scholar]
- 54.Byers PH. . Collagens: building blocks at the end of the development line. Clin Genet 2000; 58:270 - 9; http://dx.doi.org/ 10.1034/j.1399-0004.2000.580404.x; PMID: 11076051 [DOI] [PubMed] [Google Scholar]
- 55.Uitto J, Chu M-L. Regulation of collagen gene expression in human skin fibroblasts and its alterations in diseases. In: Olsen BR, Nimni ME, Eds. Collagen. Boca Raton, FL: CRC Press, 1989; pp110–124. [Google Scholar]
- 56.Widom RL. . Regulation of matrix biosynthesis and degradation in systemic sclerosis. Curr Opin Rheumatol 2000; 12:534 - 9; http://dx.doi.org/ 10.1097/00002281-200011000-00010; PMID: 11092204 [DOI] [PubMed] [Google Scholar]
- 57.Uitto J, Kouba D. . Cytokine modulation of extracellular matrix gene expression: relevance to fibrotic skin diseases. J Dermatol Sci 2000; 24:Suppl 1 S60 - 9; http://dx.doi.org/ 10.1016/S0923-1811(00)00143-2; PMID: 11137398 [DOI] [PubMed] [Google Scholar]
- 58.Gullberg DE, Lundgren-Akerlund E. . Collagen-binding I domain integrins--what do they do?. Prog Histochem Cytochem 2002; 37:3 - 54; http://dx.doi.org/ 10.1016/S0079-6336(02)80008-0; PMID: 11876085 [DOI] [PubMed] [Google Scholar]
- 59.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, et al. . Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest 2007; 117:2638 - 48; http://dx.doi.org/ 10.1172/JCI32044; PMID: 17786246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettersson F, Yau C, Dobocan MC, Culjkovic-Kraljacic B, Retrouvey H, Puckett R, et al. . Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin Cancer Res 2011; 17:2874 - 84; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2334; PMID: 21415224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malina A, Cencic R, Pelletier J. . Targeting translation dependence in cancer. Oncotarget 2011; 2:76 - 88; PMID: 21378410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grzmil M, Hemmings BA. . Translation regulation as a therapeutic target in cancer. Cancer Res 2012; 72:3891 - 900; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0026; PMID: 22850420 [DOI] [PubMed] [Google Scholar]
- 63.Yan Jia,1 Vitaly Polunovsky,2 Peter B. Bitterman,2 and Carston R. Wagner 1,2 Cap-Dependent Translation Initiation Factor eIF4E: an emerging anticancer drug target medicinal research reviews 2012; 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsieh AC, Truitt ML, Ruggero D. . Oncogenic AKTivation of translation as a therapeutic target. Br J Cancer 2011; 105:329 - 36; http://dx.doi.org/ 10.1038/bjc.2011.241; PMID: 21772331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S, Jia Y, Jacobson BA, McCauley J, Kratzke R, Bitterman PB, et al. . Treatment of breast and lung cancer cells with a N-7 Benzyl guanosine monophosphate tryptamine phosphoramidate pronucleotide (4Ei-1) results in chemosensitization to gemcitabine and induced eIF4E proteasomal degradation. Mol Pharm 2013; 10:523 - 31; http://dx.doi.org/ 10.1021/mp300699d; PMID: 23289910 [DOI] [PMC free article] [PubMed] [Google Scholar]