Abstract
The eukaryotic translation initiation factor (eIF) 4G functions as a scaffold protein that assembles components of the translation initiation complex required to recruit the 40S ribosomal subunit to an mRNA. Although many eukaryotes express two highly similar eIF4G isoforms, those in plants are highly divergent in size and sequence from one another and are referred to as eIF4G and eIFiso4G. Although the domain organization of eIFiso4G differs substantially from eIF4G orthologs in other species, the domain organization of plant eIF4G is largely unknown despite the fact that it is more similar in size and sequence to eIF4G of other eukaryotes. In this study, we show that eIF4G differs from eIFiso4G in that it contains two distinct interaction domains for the poly(A) binding protein (PABP) and eIF4B but is similar to eIFiso4G in having two eIF4A interaction domains. PABP and eIF4B bind the same N-terminal region of eIF4G as they do to a region C-proximal to the HEAT-1 domain in the middle domain of eIF4G, resulting in competitive binding between eIF4B and PABP to each site. eIF4G also differs from eIFiso4G in that no competitive binding was observed between PABP and eIF4A or between eIF4B and eIF4A to its HEAT-1-containing region. These results demonstrate that despite substantial differences in size, sequence, and domain organization, PABP and eIF4B bind to eIF4G and eIFiso4G competitively.
Keywords: protein synthesis, translation initiation, eIF4G, eIF4B, eIF4A, poly(A) binding protein
Introduction
During protein synthesis in eukaryotes, the recruitment of a 40 S ribosomal subunit to an mRNA requires the participation of several translation initiation factors that are also involved in the recognition of the initiation codon and the accompanying assembly of a translationally competent 80 S ribosome.1–3 Factors that promote 40 S subunit recruitment include eIF4E, eIF4G, eIF4A, eIF4B, eIF3, and the poly(A) binding protein (PABP). eIF4E binds to the cap structure at the 5′-end of an mRNA. eIF4G interacts with eIF4E and eIF4A to form eIF4F and serves as a scaffolding protein that also assembles additional factors important during translation initiation such as eIF4B, eIF3, and PABP, the latter of which binds the poly(A) tail that is present in almost all cellular mRNAs.4 Thus, through its interaction with eIF4E bound to the 5′-cap and PABP bound to the poly(A) tail, eIF4G serves to bridge the two ends of an mRNA which facilitates its circularization and promotes recruitment of the 40 S ribosomal subunit to the 5′-end of an mRNA.5, 6
Like most eukaryotes, plants express two eIF4G isoforms.7 In contrast to the high degree of similarity that is typical between the two eIF4G proteins in most species, plant eIF4G and eIFiso4G are highly divergent in size and sequence from one another and exhibit functional specialization in that some mRNAs preferentially use eIF4G for their translation whereas others preferentially use eIFiso4G.8 eIF4G in plants is 165 kDa whereas eIFiso4G is just 86 kDa which is smaller than any eIF4G protein in animals or yeast.8 Similarly, two eIF4E isoforms are present in plants which are referred to as eIF4E and eIFiso4E. eIF4E, eIF4G, and eIF4A comprise eIF4F whereas eIFiso4E, eIFiso4G, and eIF4A comprise eIFiso4F although eIF4A typically does not co-purify as part of either eIF4F or eIFiso4F.7 While eIF4E or eIFiso4E binds to the 5′-cap structure, eIF4A functions as an ATP-dependent RNA helicase to unwind any secondary structure in the 5′-untranslated region in order to facilitate scanning of the 40S subunit.2 The helicase activity of eIF4A is enhanced by eIF4B which interacts directly with eIF4A.9, 10 Binding of PABP to eIFiso4F stabilizes the binding of the latter to the 5′-cap structure11 while binding of eIFiso4F to PABP increases the affinity of PABP for poly(A).12, 13 PABP also interacts with eIF4B, an interaction that also increases the affinity of PABP for poly(A).12–14 Together, eIF4B and eIF4G (or eIFiso4G) synergistically increase the poly(A) binding affinity of PABP.12, 13 Because eIF4G and eIFiso4G interact with a number of proteins needed during translation initiation, determining how each eIF4G isoform accomplishes its scaffolding function and how the difference in their size might affect their domain organization would be important to understand the basis for their functional differences.
The domain organization of eIFiso4G was previously investigated.15 eIF4B and PABP bind to eIFiso4G within its HEAT-1 domain (Fig. 1) and these interaction domains overlap resulting in their competitive binding to eIFiso4G.15 The eIF4B and PABP interaction domains also overlap with the eIF4A binding domain within HEAT-1 (Fig. 1). eIF4B and PABP compete with eIF4A for binding to HEAT-1 of eIFiso4G in the absence of the C-terminal eIF4A/HEAT-2 domain but not in its presence.15 This suggests that the C-terminal eIF4A interaction domain stabilizes the binding of eIF4A to eIFiso4G in the presence of eIF4B or PABP. The interaction domains for eIF4B and eIFiso4G within the first RNA recognition motif (RRM) 1 of PABP also overlap, preventing them from binding the same PABP molecule simultaneously.16 Thus, the overlapping nature of the interaction domains on these factors suggests that eIFiso4G does not interact with eIF4B and PABP simultaneously but can bind either eIF4A and eIF4B or eIF4A and PABP.15
Figure 1. Sequence conservation between wheat eIF4G, eIFiso4G, and mammalian eIF4G. The sequence of wheat eIF4G (Ta eIF4G; accession number: ABO15893) was aligned with wheat eIFiso4G (Ta eIFiso4G; accession number: M95747) and human eIF4G (Hs eIF4G, accession number: NM_198241). Conservation of identical or similar residues is indicated by shading. Binding sites for select partner proteins of wheat eIFiso4G are indicated above the sequence as reported.15 Repeats of HEAT-1, HEAT-2, and HEAT-3 domains of human eIF4G are indicated below the sequence.
Although plant eIF4G is more similar in size to eIF4G in other eukaryotes than is eIFiso4G, little is known about its interactions with its partner proteins and whether its domain organization is similar to the much smaller eIFiso4G. Given their divergence in size and sequence, differences in the interactions of eIF4G and eIFiso4G with their partner proteins may help to explain why certain mRNAs are preferentially translated by one isoform over the other. In this study, the interactions of eIF4G with proteins and RNA have been examined. In addition to containing the conserved sequences involved in eIF4E and eIF3 binding, eIF4G contains interaction domains for eIF4A, eIF4B, and PABP. The two eIF4A interaction domains in eIF4G are conserved with those in eIFiso4G and in mammalian eIF4G orthologs, demonstrating that both plant eIF4G isoforms are more similar to animal eIF4G than to yeast eIF4G, the latter of which contains just one eIF4A/HEAT-1 domain. eIF4G differs from eIFiso4G in that it binds PABP and eIF4B in an N-terminal region that is absent from eIFiso4G. The N-terminal PABP interaction domain is similar in position to the single PABP interaction domain present in animal and yeast eIF4G. The N-terminal PABP and eIF4B interaction domains in plant eIF4G overlap resulting in their competitive binding to eIF4G at this domain. The interaction domains for PABP and eIF4B present in the eIF4G middle domain also differs from eIFiso4G in that they overlap the eIF4A binding/HEAT-1 domain only slightly in contrast to their more extensive overlap with the eIF4A binding/HEAT-1 domain in eIFiso4G. As a consequence, PABP and eIF4B do not compete with eIF4A in binding the HEAT-1-containing region of eIF4G unlike eIFiso4G. However, as with the N-terminal PABP and eIF4B interaction domains, the PABP and eIF4B interaction domains C-proximal to the HEAT-1 domain overlap with one another, resulting in their competitive binding to this region of eIF4G as they do to the N-terminal region.
Results
eIF4G contains multiple regions that support RNA binding
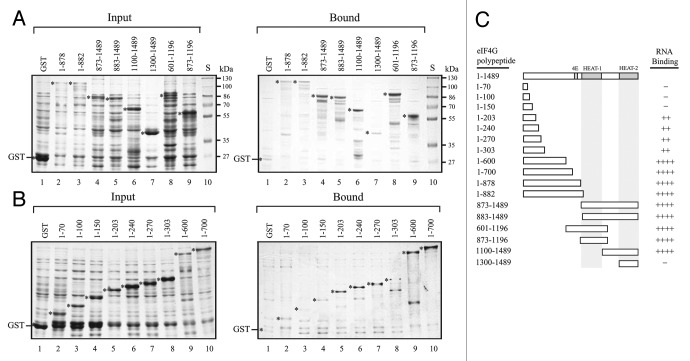
Similar to mammalian eIF4G which contains two RNA binding regions that span the middle domain containing the eIF4A/HEAT-1 domain,17, 18 eIFiso4G also exhibits RNA binding activity. The N-terminal 451 amino acid region of eIFiso4G exhibited strong binding to poly(G) as did the second RNA binding domain between residues 420 and 481.15 In addition to these two RNA binding domains, a third RNA binding domain was detected at the C-terminal end of the protein, corresponding to the C-terminal eIF4A/HEAT-2 domain which bound poly(G) but not poly(A) RNA.15 To determine whether these RNA binding domains are conserved in eIF4G, cDNAs representing separate regions of wheat eIF4G were introduced into pGEX-2TK and the GST-eIF4G polypeptides expressed in E. coli. Because eIFiso4G exhibited stronger binding to poly(G) RNA than to poly(A) RNA,15 each eIF4G polypeptide was tested for binding to poly(G) RNA. Full length eIF4G was not included as it is highly susceptible to proteolysis when expressed in E. coli and is difficult to isolate in the amounts needed for the assays. Crude extract containing an eIF4G polypeptide was incubated with poly(G) agarose resin, the resin washed extensively, and bound protein analyzed by SDS-PAGE. Relative to the molar amount of input protein, GST-eIF4G1–882 and GST-eIF4G883–1489, representing the N-terminal and C-terminal halves of eIF4G, respectively, bound to poly(G) RNA whereas GST alone did not (Fig. 2A, compare lanes 3 and 5 to lane 1). GST-eIF4G1–878 and GST-eIF4G873–1489 also bound to RNA (Fig. 2A, lanes 2 and 4, respectively). The N-terminal region exhibited little RNA binding activity as GST-eIF4G1–70, GST-eIF4G1–100, and GST-eIF4G1–150 did not bind RNA substantially above the level observed for GST (Fig. 2B, compare lanes 2–4 to lane 1). Weak binding was observed for GST-eIF4G1–203, GST-eIF4G1–240, GST-eIF4G1–270, and GST-eIF4G1–303 (Fig. 2B, lanes 5–8, respectively). Stronger RNA binding was observed for GST-eIF4G1–600 and GST-eIF4G1–700 (Fig. 2B, lanes 9 and 10, respectively), suggesting that the region between residues 303 and 600 may have significant poly(G) RNA binding activity. The region between residues 601 and 1196 or 873 and 1196 (i.e., GST-eIF4G601–1196 and GST-eIF4G873–1196, respectively) bound RNA (Fig. 2A, lanes 8 and 9, respectively). The region between residues 1100 and the C-terminus (i.e., GST-eIF4G1100–1489) also bound RNA (Fig. 2A, lane 6) but the region between residues 1300 and the C-terminus (i.e., GST-eIF4G1300–1489) exhibited little RNA binding activity (Fig. 2A, lane 7), suggesting that the C-proximal eIF4A/HEAT-2 domain does not exhibit poly(G) RNA binding activity unlike eIFiso4G.15 The lack of RNA binding by the C-proximal eIF4A/HEAT-2 domain may have been a result of poor folding of the eIF4G HEAT-2 domain although this is not supported by the binding of eIF4A to this same region (see below). These results suggest that, like eIFiso4G, multiple regions of eIF4G exhibit RNA binding activity. Other regions of eIF4G may exhibit RNA binding to sequences other than poly(G) RNA but these were not examined.
Figure 2. Identification of RNA binding domains in wheat eIF4G. In A and B, eIF4G polypeptides were expressed as GST fusion proteins in E. coli (Input protein) and RNA binding activity determined by the ability to bind to poly(G) agarose (Bound protein). Input and bound protein were analyzed by SDS-PAGE and detected using Coomassie staining. GST was employed as a negative control. The region of eIF4G included in each polypeptide is indicated numerically by the residues included. S, molecular weight standards. In C, summary of the RNA binding activity of eIF4G polypeptides. Strength of RNA binding is indicated by the number of pluses. -, Lack of RNA binding.
eIF4G contains two eIF4A binding domains
Mammalian eIF4G contains two eIF4A binding domains where the first corresponds to HEAT-1 and the second corresponds to HEAT-2.3 In contrast, yeast eIF4G proteins contain only the eIF4A/HEAT-1 domain.19 Despite its small size, wheat eIFiso4G contains eIF4A/HEAT-1 and eIF4A/HEAT-2 that are conserved in sequence and position with the respective domains in animal eIF4G (Fig. 1).15 Wheat eIF4G also contains HEAT-1 and HEAT-2 domains (Fig. 1). To investigate whether the wheat eIF4G HEAT domains bind eIF4A, the same pull-down approach used to map the eIF4A interaction domains in eIFiso4G was used to examine eIF4A binding in eIF4G. Little binding of eIF4A to GST-eIF4G1–882, which includes the region up to the first half of helix H1a of the HEAT-1 domain (Fig. 1), was observed (Fig. 3, lane 2). In contrast, substantial binding to GST-eIF4G883–1489, which contains the HEAT-1 and HEAT-2 domains, was observed (Fig. 3, lane 3). Inclusion of the entire helix H1a with the C-terminal half of eIF4G, i.e., GST-eIF4G873–1489, also bound eIF4A equally well (Fig. 3, lane 4). Interestingly, the eIF4G polypeptide representing the HEAT-1 domain itself, i.e., GST-eIF4G883–1106 or GST-eIF4G873–1121, did not support eIF4A binding (Fig. 3, lanes 5 and 8, respectively) but the inclusion of an additional C-proximal 75 amino acid sequence, i.e., GST-eIF4G873–1196, did (Fig. 3, lane 6). The HEAT-2 domain itself, i.e., GST-eIF4G1300–1489, also supported eIF4A binding (Fig. 3, lane 7). These results suggest that wheat eIF4G contains two eIF4A binding domains like wheat eIFiso4G and animal eIF4G. Wheat eIF4G does differ from eIFiso4G in that eIF4A binding to the HEAT-1 domain required additional C-proximal sequence whereas the HEAT-1 domain of eIFiso4G was sufficient for eIF4A binding.15 It is possible that this additional C-proximal region was required to stabilize the folding of the eIF4G HEAT-1 domain.
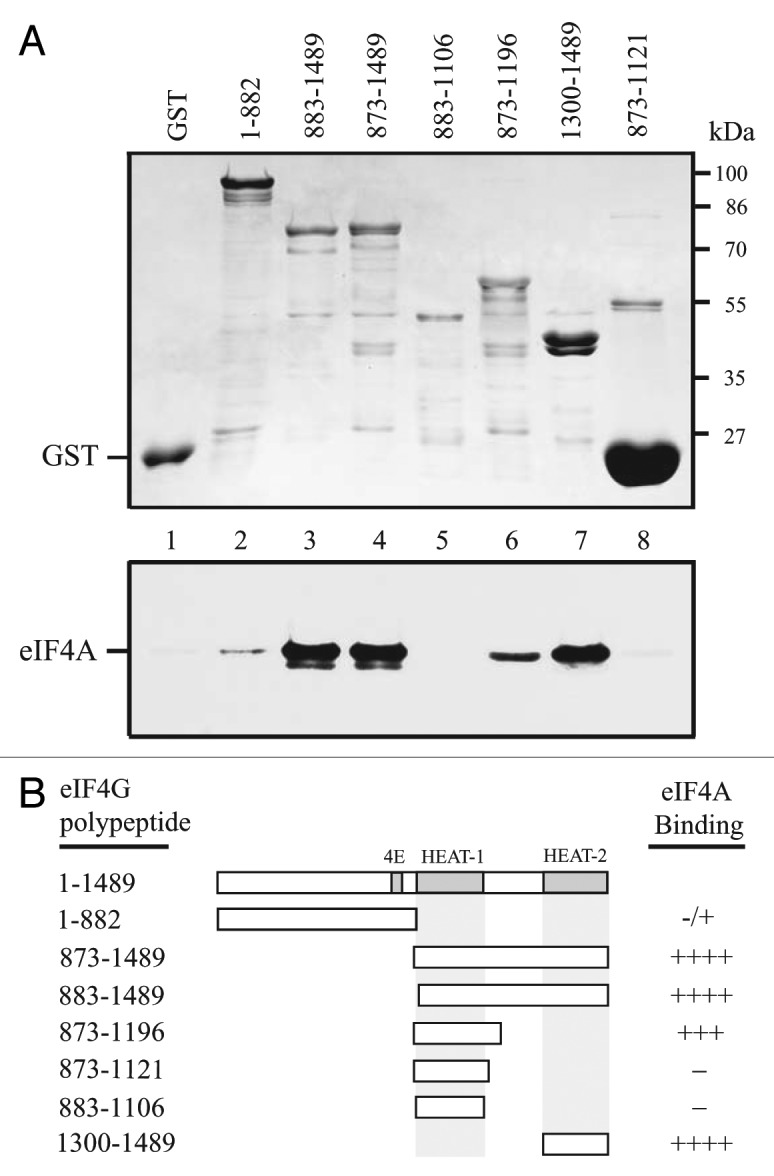
Figure 3. eIF4A binds eIF4G at two sites. In A, binding of full length eIF4A to the indicated eIF4G polypeptides in the Coomassie-stained SDS-PAGE gel (top panel) was determined following binding of the GST fusion proteins to glutathione Sepharose, resolution of bound eIF4A by SDS-PAGE, and its detection by Western analysis (bottom panel). GST was employed as a negative control. In B, summary of the eIF4A binding activity of eIF4G polypeptides. Strength of eIF4A binding is indicated by the number of pluses. -, Lack of eIF4A binding.
eIF4G contains two eIF4B binding domains
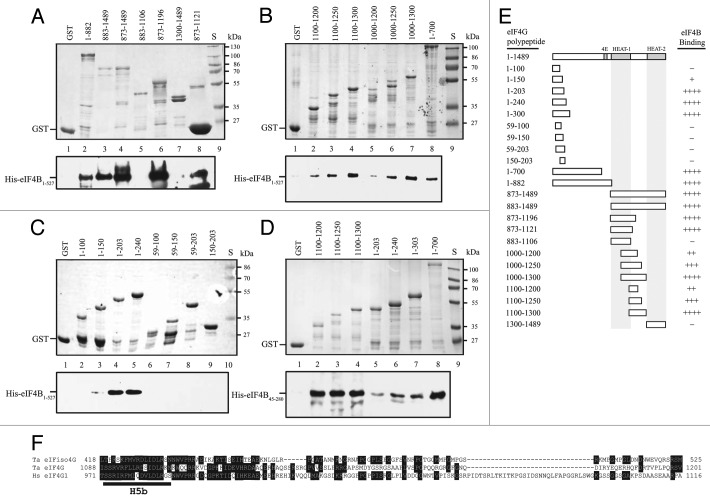
eIFiso4G contains a single eIF4B binding site that overlaps the C-terminal border of HEAT-1 as well as the PABP binding site (Fig. 1).15 To examine whether eIF4G is similar to eIFiso4G, eIF4G polypeptides were tested for their ability to bind full-length eIF4B. eIF4B bound GST-eIF4G1–882, GST-eIF4G883–1489, and GST-eIF4G873–1489 (Fig. 4A, lanes 2–4, respectively). eIF4B binding was not observed to the HEAT-1 domain itself, i.e., GST-eIF4G883–1106 (Fig. 4A, lane 5), but the inclusion of the C-proximal 15 amino acid region, i.e., GST-eIF4G873–1121, did bind eIF4B (Fig. 4A, lane 8) as did GST-eIF4G873–1196 (Fig. 4A, lane 6). The HEAT-2 domain itself, i.e., GST-eIF4G1300–1489, did not support eIF4B binding (Fig. 4A, lane 7).
Figure 4. eIF4B binds eIF4G at two sites. In A-D, binding of full-length eIF4B (i.e., residues 1–527) or the N-terminal half (i.e., residues 45–280) to the indicated eIF4G polypeptides in the Coomassie-stained SDS-PAGE gels (top panels) was determined following binding of the GST fusion proteins to glutathione Sepharose resin, resolution of bound eIF4B by SDS-PAGE, and its detection by Western analysis (bottom panels). GST was employed as a negative control. In E, summary of the eIF4B binding activity of eIF4G polypeptides. Strength of eIF4B binding is indicated by the number of pluses. -, Lack of eIF4B binding. In E, sequence comparison of the eIF4B binding domain of eIF4G to the corresponding region of wheat eIFiso4G and human eIF4G. The portion of each protein illustrated is indicated by residue numbers before and after each sequence.
To examine whether HEAT-1 was required for eIF4B binding, the region C-proximal to the HEAT-1 domain was investigated. eIF4B bound GST-eIF4G1100–1200, GST-eIF4G1100–1250, and GST-eIF4G1100–1300 (Fig. 4B, lanes 2–4, respectively) which do not include sequences of the HEAT-1 domain (Fig. 1). Similar results were obtained with GST-eIF4G1000–1200, GST-eIF4G1000–1250, and GST-eIF4G1000–1300 (Fig. 4B, lanes 5–7, respectively), indicating that inclusion of most of the HEAT-1 domain did not increase interaction with eIF4B. These results suggest that eIF4B binds to the region C-proximal to HEAT-1.
In addition to this site, eIF4B bound GST-eIF4G1–700 (Fig. 4B, lane 8), indicating the presence of a second eIF4B interaction domain in eIF4G. To delineate this second site, polypeptides representing the N-terminal end of eIF4G were examined for their ability to support eIF4B binding. Although GST-eIF4G1–100 did not bind eIF4B (Fig. 4C, lane 2), weak binding was observed to GST-eIF4G1–150 (Fig. 4C, lane 3) and stronger binding was observed toGST-eIF4G1–203 and GST-eIF4G1–240 (Fig. 4C, lanes 4 and 5, respectively). No binding was observed when the N-terminal 59 amino acids were deleted, as in GST-eIF4G59–150 or GST-eIF4G59–203 (Fig. 4D, lanes 7 and 8, respectively) or when the N-terminal 150 amino acids were deleted as in GST-eIF4G150–203 (Fig. 4C, lane 9).
Together, these data suggest that eIF4G contains two eIF4B binding sites: the first lies within the N-terminal 203 amino acids of eIF4G whereas the second corresponds to the single eIF4B binding site in eIFiso4G that lies C-proximal to HEAT-1.15 The N-terminal eIF4B binding site lies in a region of eIF4G not represented in eIFiso4G (Fig. 1). eIFiso4G binds eIF4B45–280 where residues 55–74 were important.14 To demonstrate that eIF4G binds eIF4B directly and to a similar region, binding of eIF4B45–280 to those eIF4G polypeptides identified as supporting eIF4B binding was examined. eIF4B45–280 bound GST-eIF4G1100–1200, GST-eIF4G1100–1250, and GST-eIF4G1100–1300 (Fig. 4D, lanes 2–4, respectively) as it did to GST-eIF4G1–203, GST-eIF4G1–240, GST-eIF4G1–300, and GST-eIF4G1–700 (Fig. 4D, lanes 5–8, respectively), demonstrating that eIF4G binds eIF4B directly and to a region similar to that bound by eIFiso4G.
PABP binds to two domains in eIF4G
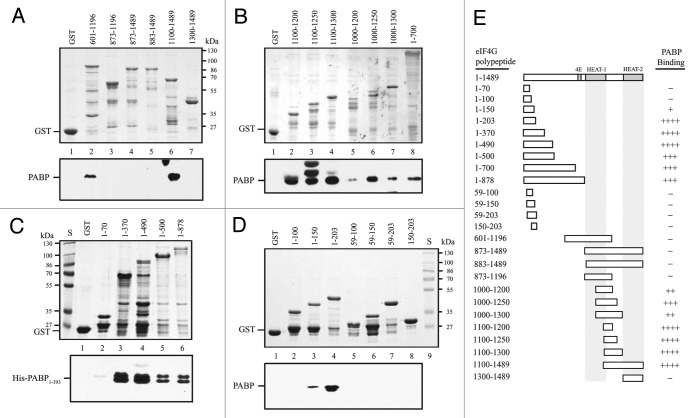
PABP binds to the HEAT-1 domain of eIFiso4G.15 To determine the conservation of domain organization, the binding of PABP to eIF4G was examined. PABP did not bind GST-eIF4G873–1196, GST-eIF4G873–1489, or GST-eIF4G883–1489 (Fig. 5A, lanes 3–5, respectively) but did bind GST-eIF4G601–1196 and GST-eIF4G1100–1489 (Fig. 5A, lanes 2 and 6, respectively), suggesting that polypeptides initiating at 873 or 883 may not have folded in a manner that permitted PABP binding. No binding to HEAT-2, i.e., GST-eIF4G1300–1489, was observed (Fig. 5A, lane 7). To examine whether the PABP interaction domain mapped to a similar region as that for eIF4B, the same eIF4G polypeptides used to map eIF4B binding were tested for binding PABP. PABP bound eIF4G1100–1200, GST-eIF4G1100–1250, and GST-eIF4G1100–1300 (Fig. 5B, lanes 2–4, respectively). PABP also bound GST-eIF4G1000–1200, GST-eIF4G1000–1250, and GST-eIF4G1000–1300 (Fig. 5B, lanes 5–7, respectively), but the binding was less strong supporting the notion the additional N-proximal sequence may affect binding strength. These results suggest that PABP binds to the same HEAT-1 C-proximal region that eIF4B binds. eIF4G, eIFiso4G, and eIF4B bind PABP within the first RRM and are known to compete in their binding to PABP.16
Figure 5. PABP binds eIF4G at two sites. In A-D, Binding of the N-terminal half of PABP containing the four RRMs (i.e., residues 1–393 which contains the eIF4G interaction domain) to the indicated eIF4G polypeptides in the Coomassie-stained SDS-PAGE gels (top panels) was determined following binding of the GST fusion proteins to glutathione Sepharose resin, resolution of bound PABP by SDS-PAGE, and its detection by Western analysis (bottom panels). GST was employed as a negative control. In E, summary of the PABP binding activity of eIF4G polypeptides. Strength of PABP binding is indicated by the number of pluses. -, Lack of PABP binding.
As with eIF4B, GST-eIF4G1–700 supported PABP binding (Fig. 5B, lane 8), consequently, the N-terminal region was examined in greater detail. Although GST-eIF4G1–70 did not bind PABP (Fig. 5C, lane 2), strong binding was observed to GST-eIF4G1–370 and GST-eIF4G1–490 (Fig. 5C, lanes 3 and 4, respectively). Binding was also observed to GST-eIF4G1–500 and GST-eIF4G1–873 (Fig. 5C, lanes 5 and 6, respectively) although it appeared less strong.
Further delineation of the N-terminal region demonstrated that PABP did not bind GST-eIF4G1–100 (Fig. 5D, lane 2), but bound GST-eIF4G1–150 weakly (Fig. 5D, lane 3), and bound GST-eIF4G1–203 strongly (Fig. 5D, lane 4). No binding was observed when the N-terminal 59 amino acids were deleted as in GST-eIF4G59–100, GST-eIF4G59–150, or GST-eIF4G59–203 (Fig. 5D, lanes 5–7, respectively) or when the N-terminal 150 amino acids were deleted as in GST-eIF4G150–203 (Fig. 5D, lane 8).
Together, these data suggest that eIF4G contains two PABP binding sites. The first corresponds to the eIF4B binding site present within the N-terminal 203 amino acids of eIF4G. The second site is C-proximal to the HEAT-1 domain similar to the eIF4B binding site in this same region. As with eIF4B, the N-terminal PABP binding site lies in a region of eIF4G that is not represented in eIFiso4G (Fig. 1). Therefore, eIF4G contains two distinct PABP binding sites as it does for eIF4B.
PABP and eIF4B compete for binding to eIF4G
The overlapping nature of the PABP and eIF4B binding sites in eIFiso4G resulted in their competitive binding.15 As PABP and eIF4B appear to bind similar regions within eIF4G, this raised the possibility that they also bind competitively to eIF4G. To examine this using the same competitive binding assay used to examine the mutually exclusive binding of PABP and eIF4B to eIFiso4G,15 GST-eIF4G1–203, containing the N-terminal region required for interaction with eIF4B and PABP, was used in pull-down assays with eIF4B and PABP. Full-length 4B, i.e., His-eIF4B1–627, and His-eIF4B45–280, which lacks the interaction domain for PABP14 but contains the interaction domain for eIF4G (Fig. 4D), were tested for their ability to compete with PABP for binding eIF4G. His-PABP1–393, which contains the interaction domains for eIF4G and eIF4B, was added to the binding reaction at an equal molar amount to GST-eIF4G1–203. His-eIF4B1–627 was added in increasing molar amounts to examine its ability to compete with PABP in binding GST-eIF4G1–203. PABP bound GST-eIF4G1–203 in the absence of eIF4B (Fig. 6A, lane 1). However, PABP binding to eIF4G was substantially reduced by the presence of eIF4B at a 1:0.3 molar ratio (Fig. 6A, lane 4), with further reductions observed when higher molar ratios of eIF4B were used (Fig. 6A, lanes 5 and 6). Addition of His-eIF4B45–280 failed to compete with PABP for binding eIF4G at any molar ratio (Fig. 6B), suggesting a reduction in its affinity for this binding site in eIF4G.
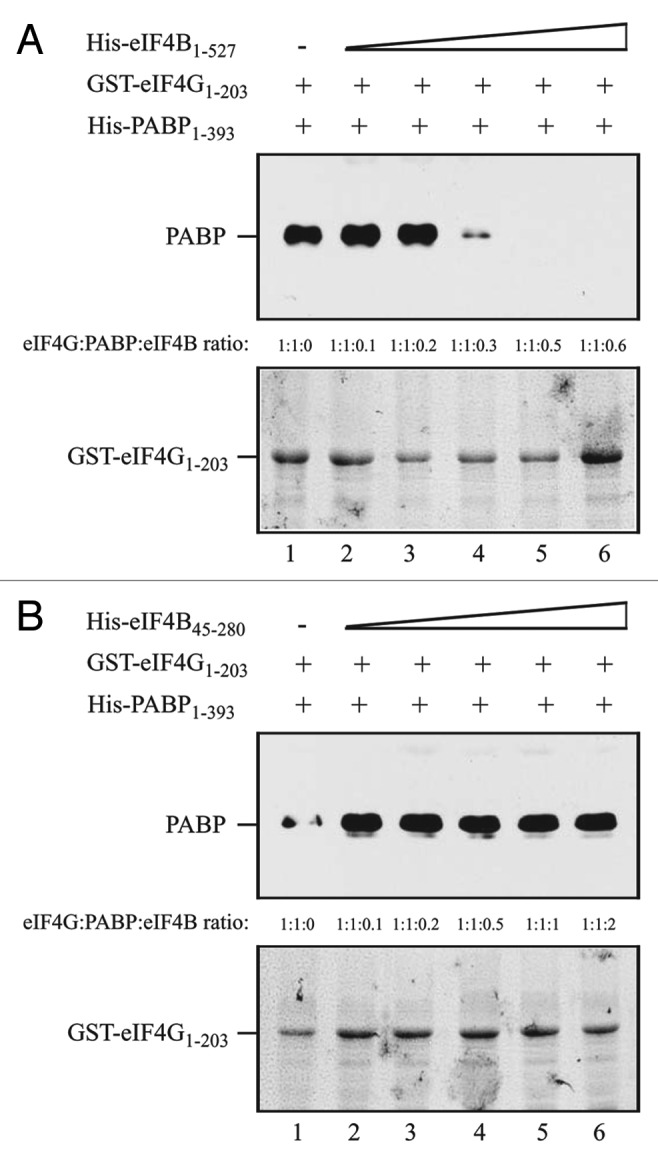
Figure 6. PABP and eIF4B compete for binding to the eIF4G N-terminus. In A and B, the amount of GST-eIF4G1–203, which contains the overlapping interaction domains for eIF4B and PABP, bound to glutathione Sepharose for each pull-down assay is shown in the Coomassie-stained gels (bottom panels). His-PABP1–393, which contains the interaction domains for eIF4G and eIF4B was added to each reaction in a 1:1 molar ratio to GST-eIF4G1–203. (A) Full-length eIF4B (i.e., residues 1–527) or (B) His-eIF4B45–280, which contains the interaction domain for eIF4G but lacks the interaction domains for PABP, was added in increasing amounts to the binding reactions. The amount of PABP bound to GST-eIF4G1–203 was detected by Western analysis (top panels).
To examine if PABP and eIF4B compete for binding to the HEAT-1 C-proximal region where each interacts with eIF4G, GST-eIF4G1100–1489, which contains this region and binds PABP and eIF4B, was used in competition assays with these partner proteins. PABP1–393 bound eIF4G1100–1489 in the absence of eIF4B (Fig. 7A, lane 1). The addition of full-length eIF4B reduced the amount of PABP bound to eIF44G even when eIF4B was added at a 1:0.06 molar ratio (Fig. 7A, lane 2). Further reductions in PABP binding were observed when the molar ratio of eIF4B was increased (Fig. 7A, lanes 3–6) with complete loss in PABP binding achieved at a 1:0.5 molar ratio of eIF4B. His-eIF4B45–280 also competed with PABP in binding eIF4G1100–1489 (Fig. 7B, lanes 2–6) although less efficiently than full-length eIF4B, again suggesting a reduction in its affinity for this binding site in eIF4G. Together, these results indicate that PABP and eIF4B compete for binding eIF4G at both interaction sites and confirm the mapping of the PABP and eIF4B binding sites in eIF4G.
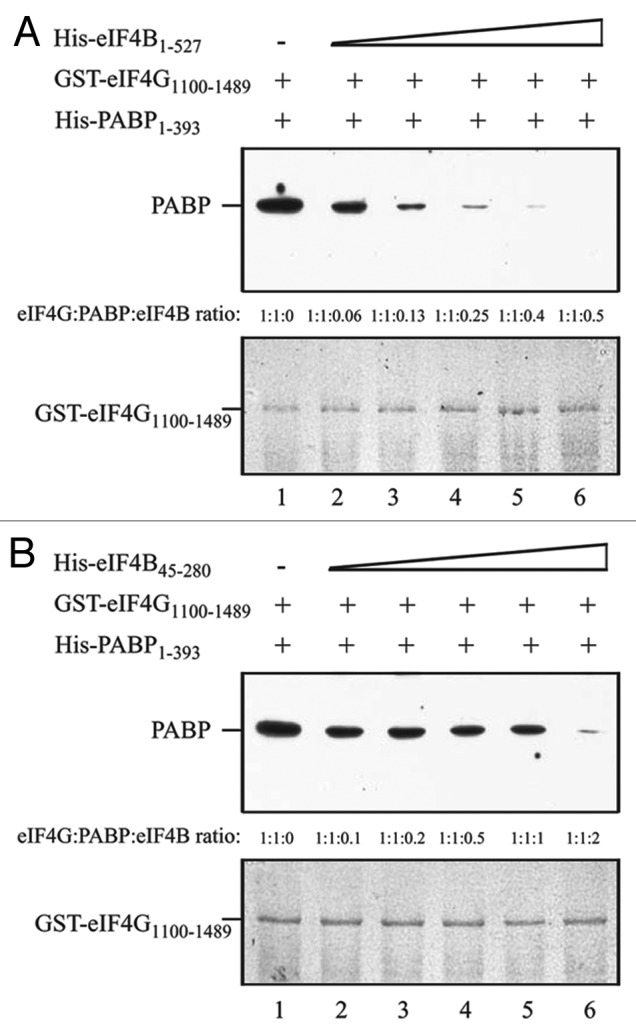
Figure 7. PABP and eIF4B compete for binding to the eIF4G central region. In A and B, the amount of GST-eIF4G1100–1489, which contains the interaction domain for eIF4B and PABP, bound to glutathione Sepharose for each pull-down assay is shown in the Coomassie-stained gels (bottom panels). His-PABP1–393 was added to each reaction in a 1:1 molar ratio to GST-eIF4G1100–1489. (A) Full-length eIF4B (i.e., residues 1–527) or (B) His-eIF4B45–280 was added in increasing amounts to the binding reactions. The amount of PABP bound to GST-eIF4G1100–1489 was detected by Western analysis (top panels).
PABP and eIF4B do not compete with eIF4A for binding eIF4G
Because the PABP and eIF4B interaction domains overlap with the eIF4A/HEAT-1 domain in eIFiso4G, eIF4B and PABP compete with eIF4A for binding to this domain.15 Although the second PABP and eIF4B interaction domains in eIF4G are C-proximal to the HEAT-1 domain, they partially overlap the region identified as required for eIF4A binding (Fig. 3). To determine whether PABP and eIF4B compete with eIF4A for binding to the HEAT-1-containing region of eIF4G, the same competition binding assays used for eIFiso4G were used to examine competitive binding between PABP and eIF4A or between eIF4B and eIF4A.
GST-eIF4G601–1196, which contains the eIF4A/HEAT-1 domain and the C-proximal region to which PABP binds, was used to investigate the competition between PABP and eIF4A. Full-length eIF4A was present at an equal molar amount to GST-eIF4G601–1196 and His-PABP1–393 was added in increasing amounts. eIF4A bound GST-eIF4G601–1196 in the absence of PABP (Fig. 8A, lane 1). The addition of PABP did not reduce eIF4A binding (Fig. 8A, lanes 2–6). Similar results were obtained when PABP1–393 was present at an equal molar amount to GST-eIF4G601–1196 and increasing amounts full-length eIF4A were added. PABP1–393 bound GST-eIF4G601–1196 in the absence of eIF4A (Fig. 8B, lane 1). Addition of eIF4A did not reduce PABP binding (Fig. 8B, lanes 2–6).
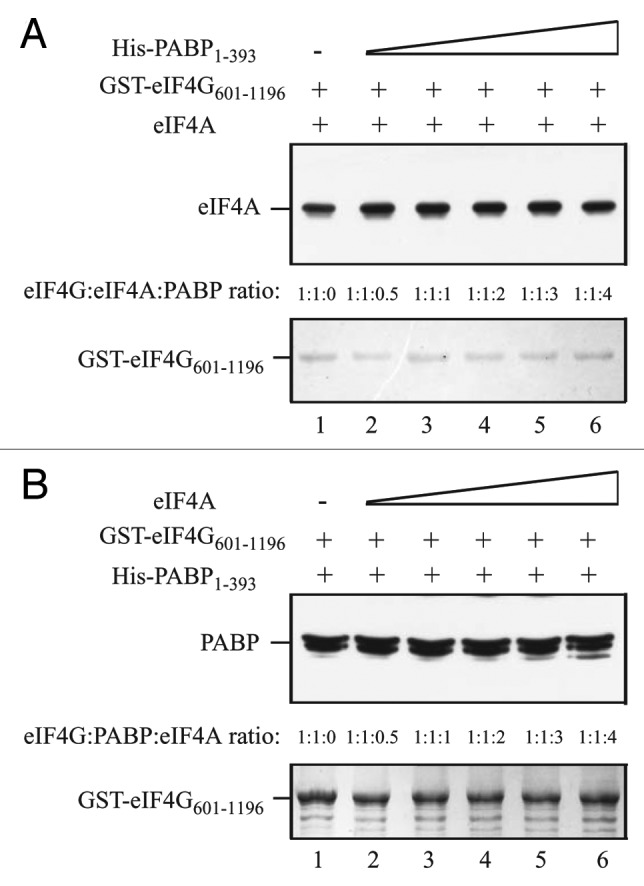
Figure 8. PABP does not compete with eIF4A for binding to the eIF4G central region. In A and B, the amount of GST-eIF4G601–1106, which contains the interaction domains for eIF4A and PABP, bound to glutathione Sepharose for each pull-down assay is shown in the Coomassie-stained gels (bottom panels). (A) Full-length eIF4A was added to each reaction in a 1:1 molar ratio to GST-eIF4G601–1106 and His-PABP1–393 was added in increasing amounts to the binding reactions. (B) His-PABP1–393 was added to each reaction in a 1:1 molar ratio to GST-eIF4G601–1106 and full-length eIF4A was added in increasing amounts to the binding reactions. The amount of (A) eIF4A or (B) PABP bound to GST-eIF4G601–1106 was detected by Western analysis (top panels).
Competition between eIF4B and eIF4A was also examined. Full-length eIF4A, present at an equal molar amount to eIF4G, bound GST-eIF4G601–1196 in the absence of His-eIF4B45–280 (Fig. 9A, lane 1). The addition of increasing amounts of His-eIF4B45–280, which lacks its two eIF4A binding sites,14 did not reduce eIF4A binding (Fig. 9A, lanes 2–6). Similar results were obtained when His-eIF4B45–280 was present at an equal molar amount to GST-eIF4G601–1196 and increasing amounts full-length eIF4A were added. His-eIF4B45–280 bound GST-eIF4G601–1196 in the absence of eIF4A (Fig. 9B, lane 1), confirming that this region of eIF4B contains the binding site for eIF4G as it does for eIFiso4G.14 The addition of eIF4A did not reduce eIF4B binding (Fig. 9B, lanes 2–6). Together, these results suggest that PABP and eIF4B do not compete with eIF4A in binding the HEAT-1-containing region of eIF4G in contrast to their ability to do so with the corresponding HEAT-1 region of eIFiso4G.15
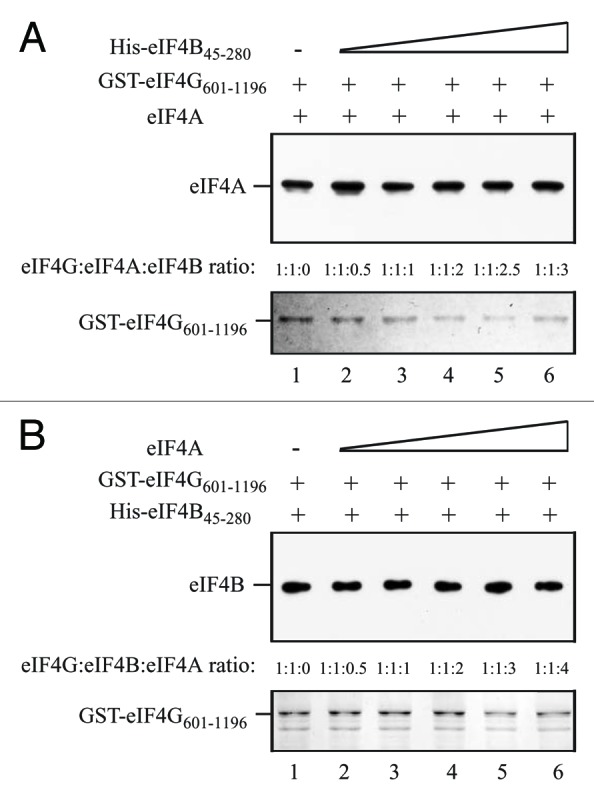
Figure 9. eIF4B does not compete with eIF4A for binding to the eIF4G central region. In A and B, the amount of GST-eIF4G601–1106, which contains the interaction domains for eIF4A and eIF4B, bound to glutathione Sepharose for each pull-down assay is shown in the Coomassie-stained gels (bottom panels). (A) Full-length eIF4A was added to each reaction in a 1:1 molar ratio to GST-eIF4G601–1106 and His-eIF4B45–280, which lacks the eIF4A interaction domains, was added in increasing amounts to the binding reactions. (B) His-eIF4B45–280 was added to each reaction in a 1:1 molar ratio to GST-eIF4G601–1106 and full-length eIF4A was added in increasing amounts to the binding reactions. The amount of (A) eIF4A or (B) eIF4B bound to GST-eIF4G601–1106 was detected by Western analysis (top panels).
Although PABP and eIF4B compete with eIF4A for binding to the eIF4A/HEAT-1 domain in eIFiso4G, they do not do so when the eIF4A/HEAT-2 domain is included.15 To investigate whether the inclusion of the eIF4A/HEAT-2 domain of eIF4G affects the binding of PABP or eIF4B to the HEAT-1-containing region of eIF4G, binding of His-PABP1–393 or His-eIF4B45–280 to GST-eIF4G601–1489, which contains the HEAT-1 and HEAT-2 domains, was examined in the presence of increasing amounts of eIF4A. PABP1–393 bound GST-eIF4G601–1489 in the absence of eIF4A (Fig. 10A, lane 1). The addition of eIF4A did not reduce PABP binding and slightly increased PABP binding at higher amounts of eIF4A (Fig. 10A, lanes 2–6). Similar results were obtained when eIF4B was examined. eIF4B45–280 bound GST-eIF4G601–1489 in the absence of eIF4A (Fig. 10B, lane 1). The addition of eIF4A slightly reduced eIF4B binding at a subsaturating amount of eIF4A but did not reduce eIF4B binding at higher amounts of eIF4A (Fig. 10B, lanes 2–6).
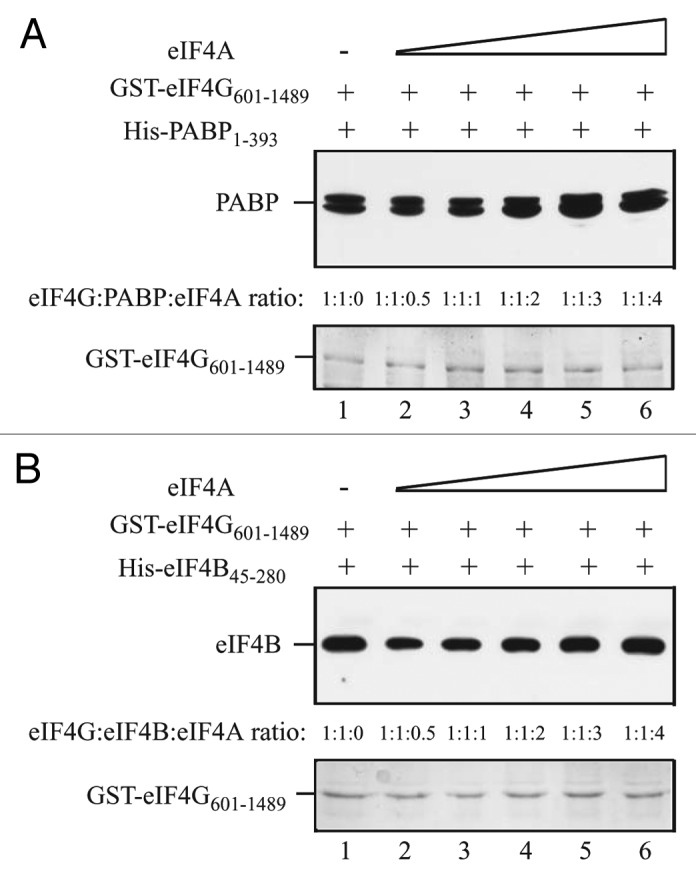
Figure 10. PABP and eIF4B do not compete with eIF4A for binding to the eIF4G region containing the HEAT-1 and HEAT-2 domains. In A and B, the amount of GST-eIF4G601–1489, which contains the interaction domains for PABP, eIF4B and eIF4A, bound to glutathione Sepharose for each pull-down assay is shown in the Coomassie-stained gels (bottom panels). (A) Full-length His-PABP1–393 was added to each reaction in a 1:1 molar ratio to GST-eIF4G601–1489 and full-length eIF4A was added in increasing amounts to the binding reactions. (B) His-eIF4B45–280 was added to each reaction in a 1:1 molar ratio to GST-eIF4G601–1489 and full-length eIF4A was added in increasing amounts to the binding reactions. The amount of (A) PABP or (B) eIF4B bound to GST-eIF4G601–1489 was detected by Western analysis (top panels).
Discussion
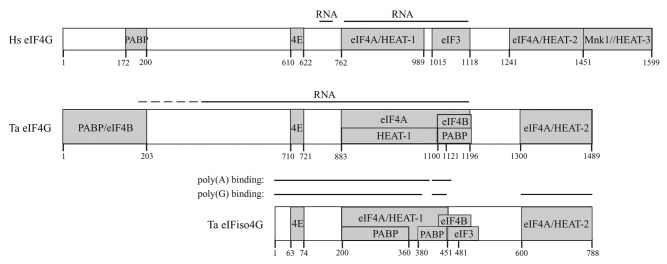
Although the domain organization of wheat eIF4G shares some similarities with eIFiso4G, it also differs in several important respects. eIF4G is similar to eIFiso4G and human eIF4GI in that its HEAT-1 domain exhibits RNA binding activity15, 17 and, in wheat eIF4G and human eIF4GI, this RNA binding domain includes the region immediately C-proximal to the HEAT-1 domain (Fig. 11). The HEAT-2 domain of eIF4G in wheat and humans appears to lack RNA binding activity, unlike this same domain in eIFiso4G.15, 17, 18 The region N-proximal to HEAT-1 of wheat eIF4G also exhibited RNA binding activity (Fig. 11).
Figure 11. Partner protein interactions of wheat eIF4G. The domain organization of the RNA and protein binding domains in wheat eIF4G, eIFiso4G, and human eIF4G is shown.
Animal eIF4G contains two eIF4A binding domains whereas yeast eIF4G contains just one.19, 20 eIF4A binds two of the three HEAT repeat domains (i.e., HEAT-1 and HEAT-2) in human eIF4G as well as the linker region between these two domains.20–23 HEAT-1 stimulates the helicase activity of eIF4A while HEAT-2 performs a modulatory role24 and each contacts separate surfaces of eIF4A. The absence of HEAT-2 (and HEAT-3) in yeast eIF4G accounts for its single eIF4A binding domain.
Despite its smaller size, eIFiso4G contains two eIF4A interaction domains corresponding to HEAT-1 and HEAT-2 (Fig. 11).15 eIF4G is similar to eIFiso4G and animal eIF4G in that it contains two eIF4A binding domains corresponding to HEAT-1 and HEAT-2. However, whereas HEAT-1 of eIFiso4G was sufficient to bind eIF4A, the region immediately C-proximal to the wheat eIF4G HEAT-1 domain was required for eIF4A binding, perhaps needed for stable folding of the eIF4A/HEAT-1 domain. Because eIF4G and eIFiso4G contain two eIF4A binding domains, they are more similar to animal eIF4G than to yeast eIF4G, which only contains the eIF4A/HEAT-1 domain. The binding of eIF4A to the same HEAT domains present in animal eIF4G confirmed our mapping of the eIF4A interaction domains in plant eIF4G.
PABP binds eIFiso4G within HEAT-1 at two nearly contiguous sites that overlap extensively with the eIF4A binding domain (Fig. 11).15 Sequence within the second PABP binding domain of eIFiso4G shares homology with the two PABP binding domains present in eIF4B.14 eIFiso4G binds PABP at two sites with one site that encompasses the C-terminal end of RRM1 and the adjacent linker sequence and the second site requiring RRM3-4.16 As these two sites share little homology, these may contact the two regions within the HEAT-1 domain of eIFiso4G. Because of the extensive overlap of the PABP and eIF4A binding domains in eIFiso4G, PABP competes with eIF4A for binding HEAT-1 in the absence of the eIF4A/HEAT-2 domain but not in its presence.15
Like eIFiso4G, wheat eIF4G contains a PABP binding site in its central region but its lies immediately C-proximal to HEAT-1 and thus overlaps just the C-terminal end of the eIF4A binding domain that includes HEAT-1 (Fig. 11). No competition was observed between PABP and eIF4A in binding the region containing HEAT-1 and the adjacent C-proximal sequence, suggesting that the PABP interaction domain does not overlap sufficiently to prevent eIF4A binding. Thus, eIF4G differs slightly from eIFiso4G in the proximity of the PABP and eIF4A/HEAT-1 domains that substantially affects the interaction of PABP and eIF4A to this domain. eIF4G also differs from eIFiso4G in that it contains another PABP interaction domain near its N-terminus, a region not present in eIFiso4G (Fig. 11). This N-terminal site is similar to the single PABP binding site reported for animal and yeast eIF4G which is also present near the N-terminal end of each protein.25–27
eIF4B binds eIFiso4G at a single site that partially overlaps the C-terminal end of the eIF4A/HEAT-1 domain and PABP interaction domain (Fig. 11).15 eIF4B competes with eIF4A for binding to eIFiso4G but only when the eIF4A/HEAT-2 domain is absent. As with PABP, eIF4B binds wheat eIF4G within the region that lies C-proximal to HEAT-1 (Fig. 11). No competition was observed between eIF4B and eIF4A in binding the HEAT-1-containing region. Inclusion of the eIF4A/HEAT-2 domain did not substantially affect the binding of either eIF4B or PABP to eIF4G, demonstrating that no competition exists between eIF4A and eIF4B or PABP, even when both eIF4A/HEAT domains are present. This indicates that the binding domains for eIF4B and PABP do not overlap sufficiently with that of eIF4A to cause competition in their binding to the HEAT-1-containing region. Thus, eIF4G differs from eIFiso4G in that no competition was observed between eIF4B and eIF4A or between PABP and eIF4A in binding eIF4G whereas the C-terminal eIF4A/HEAT-2 domain is needed to stabilize the binding of eIF4A to eIFiso4G in the presence of either PABP or eIF4B. Nevertheless, when both eIF4A/HEAT domains are present, neither eIF4B nor PABP compete with eIF4A in binding eIFiso4G,15 resulting in no difference with eIF4G in this regard.
Just as eIF4G contains a second PABP interaction domain near its N-terminus, a second eIF4B interaction domain is also present in the same region (Fig. 11). Thus, eIF4G differs from eIFiso4G in that it contains two eIF4B and PABP binding sites compared with one each in eIFiso4G. The overlapping nature of the eIF4B and PABP interaction domains in eIF4G suggested possible competition in their binding. Competition between PABP and eIF4B for binding eIF4G was observed for the N-terminal PABP/eIF4B binding domain as well as for the PABP/eIF4B binding domain in the HEAT-1-containing region, suggesting that the binding of PABP and eIF4B to these sites is mutually exclusive. The ability of eIF4B to compete with PABP at substoichiometric concentrations might indicate that eIF4B binds eIF4G more tightly than PABP. The competition between PABP and eIF4B supports the extensive overlap of their binding domains in both regions of eIF4G and serves as confirmation that their interaction domains had been correctly mapped by our pull-down approach. eIF4B and PABP also compete for binding eIFiso4G,15 indicating that PABP and eIF4B bind each interaction region in eIF4G and eIFiso4G in a mutually exclusive manner. Because PABP and eIF4B bind eIF4G at two sites, it is possible that eIF4G could bind PABP at one site while eIF4B binds at the second site thus allowing both partner proteins to interact with the same molecule of eIF4G. If so, this would be a significant difference from eIFiso4G in which PABP and eIF4B compete to bind the single interaction site present in that isoform. Interestingly, the sequence representing the PABP binding sites in the HEAT-1 C-proximal region in eIF4G and eIFiso4G shares some similarity with the two repeated PABP binding sites in eIF4B,14 raising the possibility that they may be important for the interaction of PABP with these proteins.
As a poly(A) tail would be expected to bind multiple molecules of PABP, the competing nature of PABP and eIF4B to eIFiso4G would dictate that eIFiso4G binds either PABP or eIF4B but not both at the same time. PABP exists as differentially phosphorylated species28 and its phosphorylation state determines which partner protein it binds.13 eIF4B binds phosphorylated PABP whereas eIFiso4G exhibits little preference13 such that separate molecules of PABP could bind eIF4B and eIFiso4G. In contrast, the two binding sites for PABP and eIF4B in eIF4G might allow both of these partner proteins to potentially bind simultaneously. Thus, how eIF4G interacts with PABP bound to a poly(A) tail may differ from how eIFiso4G interacts with the PABP/poly(A) tail complex. Given that eIF4B also interacts with PABP, eIF4A, eIFiso4G,14 and eIF4G (this study), the interaction of these initiation factors at the 5′-end of an mRNA with multiple molecules of PABP bound to a poly(A) tail ensures a greater degree of stability than a simple PABP-eIF4G interaction. This is supported by the observation that eIF4B and eIF4G or eIF4B and eIFiso4G synergistically increase multimeric binding of PABP to poly(A) RNA.13 The observation that eIF4B dimerizes29 suggests two possible interaction scenarios. eIF4G (or eIFiso4G) and eIF4B may interact with separate molecules of PABP on a poly(A) tail therefore providing two separate complexes to contribute to the stability of the interaction of the termini. Alternatively, an eIF4B dimer might interact with eIF4G (or eIFiso4G) and PABP simultaneously, thus bridging the two proteins and increasing the stability of the complex even further.
Material and Methods
Plasmid construction and protein expression
Fragments representing regions of eIF4G were obtained by PCR from a full-length cDNA of eIF4G (a generous gift from Dr. Karen Browning) using the primers in Table S1. Constructs for the expression of eIF4B, eIF4A, and PABP were described previously.14, 15 All constructs were confirmed by sequencing. All constructs expressing recombinant proteins were introduced into E. coli (BL21 DE3), grown at 37°C in LB medium overnight. Fresh LB was inoculated with the overnight culture in a ratio of 1:50 and incubated at 37°C for 3 h. Large-scale cultures were inoculated with the fresh culture at a ratio of 1:100 and incubated for 2 h. Expression of recombinant proteins was induced following the addition of 1 mM IPTG and incubation of the culture for 4 h. Bacterial cells were collected by centrifugation and stored at -80°C.
RNA binding assay
E. coli cells expressing recombinant protein were broken by sonication in Buffer B-100 (25 mM HEPES, 100 mM KCl, 10% glycerol, 0.5 mM EDTA, 2 mM DTT) supplemented with protease inhibitor cocktail (Sigma). The cell debris was removed by centrifugation twice at 4°C. Prior to the binding reaction, crude extracts containing recombinant eIF4G polypeptides were normalized by SDS-PAGE followed by staining with Coomassie brilliant blue. Poly(G) agarose resin (Sigma) was equilibrated in washing buffer (Buffer B-100 with 0.1% Triton X-100). Approximately 5 μg of recombinant protein was added to poly(G) agarose resin in Buffer B-100 and incubated with shaking for 20 min at 4°C with shaking. The resin was collected by centrifugation and was washed 4 times with washing buffer. Bound protein was released by adding an equal volume of 2x SDS/6 M urea sample buffer with heating 70°C for 10 min and analyzed by SDS-PAGE followed by staining with Coomassie brilliant blue. E. coli expressing GST alone was used a negative control. The degree of RNA binding was determined as a comparison of the molar amount of bait protein bound to RNA (Bound) relative to the molar amount of bait protein added to the assay (Input).
Protein interaction assay
Protein interactions were analyzed as described previously14, 15 with some modification. Prior to the binding reaction, crude extracts containing bait and prey proteins were normalized by SDS-PAGE followed by staining with Coomassie brilliant blue. Approximately 5 μg of GST fusion protein was added to glutathione Sepharose 4B resin (GE Healthcare) that had been washed three times with pre-cooled Buffer B-100 (supplemented with 0.1% Triton X-100). Following incubation with shaking at 4°C for 1 h, the resin was collected by centrifugation and the supernatant removed. An equal molar amount of prey protein was added to the binding reaction. For competition assays, an equal molar amount of one prey protein and increasing molar amounts of the second prey protein were added. To eliminate apparent protein interaction resulting from RNA tethering, RNase A was also added and the binding reactions were incubated overnight at 4°C. The resin was collected by centrifugation and was washed 4 times with washing buffer. Bound protein was released by adding an equal volume of 2x SDS sample buffer/6 M urea followed by heating at 100°C for 5 min. Following centrifugation, equal amounts of bound bait protein among the reactions of a single experiment were confirmed by SDS-PAGE analysis prior to detecting the presence of the prey protein. Only if an equal amount of bound bait protein among the reactions of a single experiment was confirmed was the prey protein then resolved by SDS-PAGE and detected by Western analysis. E. coli expressing GST alone was used a negative control. The relative strength of binding was determined by the molar amount of prey protein bound to a given molar amount of bait protein.
Western analysis
Protein was transferred to 0.22 mm polyvinylidene difluoride membrane by electroblotting. The membranes were blocked in 5% milk, 1% NaN3 in TPBS followed by incubation with antiserum in TPBS with 1% milk for 1.5 h. Membranes were washed twice with Tween phosphate-buffered saline (TPBS: 0.1% Tween 20, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.4 mM KH2PO4) and incubated with goat anti-rabbit horseradish peroxidase-conjugated antibodies (Southern Biotechnology Associates) diluted to 1:10,000 for 1 h. The blots were washed twice with TPBS and the signal detected typically between 1 to 15 min using chemiluminescence (Pierce) with X-ray film. Anti-His antiserum (Santa Cruz Biochemical Inc.) was used at a 1:200 dilution as recommended by the manufacturer. eIF4A antiserum (a generous gift of Dr. Karen Browning) was used at a 1:1000 dilution. Anti-eIF4B antiserum was generated using recombinant eIF4B and was used at a 1:1000 dilution.
Supplementary Material
Glossary
Abbreviations:
- DTT
dithiothreitol
- eIF
translation initiation factor
- GST
glutathione-S-transferase
- HEAT
Huntington, Elongation Factor 3, PR65/A, TOR
- His
histidine
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- PABP
poly(A) binding protein
- RRM
RNA recognition motif
Supplemental Material
Supplemental material may be found here: www.landesbioscience.com/journals/translation/article/24038
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by grants from the National Science Foundation (DBI-0820047) and the University of California Agricultural Experiment Station. We thank Dr. Karen Browning for the eIF4G cDNA and for eIF4A antisera.
References
- 1.Preiss T, W Hentze M. . Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays 2003; 25:1201 - 11; http://dx.doi.org/ 10.1002/bies.10362; PMID: 14635255 [DOI] [PubMed] [Google Scholar]
- 2.Kapp LD, Lorsch JR. . The molecular mechanics of eukaryotic translation. Annu Rev Biochem 2004; 73:657 - 704; http://dx.doi.org/ 10.1146/annurev.biochem.73.030403.080419; PMID: 15189156 [DOI] [PubMed] [Google Scholar]
- 3.Pestova TV, Lorsch JR, Hellen CUT. The mechanism of translation initiation in eukaryotes. In: Mathews M.B., Sonenberg N., Hershey J.W.B. ed. Translational Control in Biology and Medicine. Cold Spring Harbor Laboratory Press. 2007:87-128. [Google Scholar]
- 4.Gallie DR. . Protein-protein interactions required during translation. Plant Mol Biol 2002; 50:949 - 70; http://dx.doi.org/ 10.1023/A:1021220910664; PMID: 12516864 [DOI] [PubMed] [Google Scholar]
- 5.Wells SE, Hillner PE, Vale RD, Sachs AB. . Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell 1998; 2:135 - 40; http://dx.doi.org/ 10.1016/S1097-2765(00)80122-7; PMID: 9702200 [DOI] [PubMed] [Google Scholar]
- 6.Tarun SZ Jr., Sachs AB. . A common function for mRNA 5′ and 3′ ends in translation initiation in yeast. Genes Dev 1995; 9:2997 - 3007; http://dx.doi.org/ 10.1101/gad.9.23.2997; PMID: 7498795 [DOI] [PubMed] [Google Scholar]
- 7.Browning KS. . The plant translational apparatus. Plant Mol Biol 1996; 32:107 - 44; http://dx.doi.org/ 10.1007/BF00039380; PMID: 8980477 [DOI] [PubMed] [Google Scholar]
- 8.Gallie DR, Browning KS. . eIF4G functionally differs from eIFiso4G in promoting internal initiation, cap-independent translation, and translation of structured mRNAs. J Biol Chem 2001; 276:36951 - 60; http://dx.doi.org/ 10.1074/jbc.M103869200; PMID: 11483601 [DOI] [PubMed] [Google Scholar]
- 9.Rogers GW Jr., Richter NJ, Lima WF, Merrick WC. . Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J Biol Chem 2001; 276:30914 - 22; http://dx.doi.org/ 10.1074/jbc.M100157200; PMID: 11418588 [DOI] [PubMed] [Google Scholar]
- 10.Bi XP, Ren JH, Goss DJ. . Wheat germ translation initiation factor eIF4B affects eIF4A and eIFiso4F helicase activity by increasing the ATP binding affinity of eIF4A. Biochemistry 2000; 39:5758 - 65; http://dx.doi.org/ 10.1021/bi992322p; PMID: 10801326 [DOI] [PubMed] [Google Scholar]
- 11.Wei C-C, Balasta ML, Ren J, Goss DJ. . Wheat germ poly(A) binding protein enhances the binding affinity of eukaryotic initiation factor 4F and (iso)4F for cap analogues. Biochemistry 1998; 37:1910 - 6; http://dx.doi.org/ 10.1021/bi9724570; PMID: 9485317 [DOI] [PubMed] [Google Scholar]
- 12.Le H, Tanguay RL, Balasta ML, Wei C-C, Browning KS, Metz AM, et al. . Wheat germ poly(A) binding protein enhances the binding affinity of eukaryotic initiation factor 4F and (iso)4F for cap analogues. J Biol Chem 1997; 272:16247 - 55; http://dx.doi.org/ 10.1074/jbc.272.26.16247; PMID: 9195926 [DOI] [PubMed] [Google Scholar]
- 13.Le H, Browning KS, Gallie DR. . The phosphorylation state of poly(A)-binding protein specifies its binding to poly(A) RNA and its interaction with eukaryotic initiation factor (eIF) 4F, eIFiso4F, and eIF4B. J Biol Chem 2000; 275:17452 - 62; http://dx.doi.org/ 10.1074/jbc.M001186200; PMID: 10747998 [DOI] [PubMed] [Google Scholar]
- 14.Cheng S, Gallie DR. . Wheat eukaryotic initiation factor 4B organizes assembly of RNA and eIFiso4G, eIF4A, and poly(A)-binding protein. J Biol Chem 2006; 281:24351 - 64; http://dx.doi.org/ 10.1074/jbc.M605404200; PMID: 16803875 [DOI] [PubMed] [Google Scholar]
- 15.Cheng S, Gallie DR. . Competitive and noncompetitive binding of eIF4B, eIF4A, and the poly(A) binding protein to wheat translation initiation factor eIFiso4G. Biochemistry 2010; 49:8251 - 65; http://dx.doi.org/ 10.1021/bi1008529; PMID: 20795652 [DOI] [PubMed] [Google Scholar]
- 16.Cheng S, Gallie DR. . eIF4G, eIFiso4G, and eIF4B bind the poly(A)-binding protein through overlapping sites within the RNA recognition motif domains. J Biol Chem 2007; 282:25247 - 58; http://dx.doi.org/ 10.1074/jbc.M702193200; PMID: 17606619 [DOI] [PubMed] [Google Scholar]
- 17.Lomakin IB, Hellen CU, Pestova TV. . Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol Cell Biol 2000; 20:6019 - 29; http://dx.doi.org/ 10.1128/MCB.20.16.6019-6029.2000; PMID: 10913184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prévôt D, Décimo D, Herbreteau CH, Roux F, Garin J, Darlix JL, et al. . Characterization of a novel RNA-binding region of eIF4GI critical for ribosomal scanning. EMBO J 2003; 22:1909 - 21; http://dx.doi.org/ 10.1093/emboj/cdg175; PMID: 12682023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez D, Kislig E, Altmann M, Trachsel H. . Structural and functional similarities between the central eukaryotic initiation factor (eIF)4A-binding domain of mammalian eIF4G and the eIF4A-binding domain of yeast eIF4G. Biochem J 2001; 355:223 - 30; http://dx.doi.org/ 10.1042/0264-6021:3550223; PMID: 11256967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imataka H, Sonenberg N. . Human eukaryotic translation initiation factor 4G (eIF4G) possesses two separate and independent binding sites for eIF4A. Mol Cell Biol 1997; 17:6940 - 7; PMID: 9372926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamphear BJ, Kirchweger R, Skern T, Rhoads RE. . Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornaviral proteases. Implications for cap-dependent and cap-independent translational initiation. J Biol Chem 1995; 270:21975 - 83; http://dx.doi.org/ 10.1074/jbc.270.37.21975; PMID: 7665619 [DOI] [PubMed] [Google Scholar]
- 22.Bellsolell L, Cho-Park PF, Poulin F, Sonenberg N, Burley SK. . Two structurally atypical HEAT domains in the C-terminal portion of human eIF4G support binding to eIF4A and Mnk1. Structure 2006; 14:913 - 23; http://dx.doi.org/ 10.1016/j.str.2006.03.012; PMID: 16698552 [DOI] [PubMed] [Google Scholar]
- 23.Marcotrigiano J, Lomakin IB, Sonenberg N, Pestova TV, Hellen CU, Burley SK. . A conserved HEAT domain within eIF4G directs assembly of the translation initiation machinery. Mol Cell 2001; 7:193 - 203; http://dx.doi.org/ 10.1016/S1097-2765(01)00167-8; PMID: 11172724 [DOI] [PubMed] [Google Scholar]
- 24.Marintchev A, Wagner G. . Translation initiation: structures, mechanisms and evolution. Q Rev Biophys 2004; 37:197 - 284; http://dx.doi.org/ 10.1017/S0033583505004026; PMID: 16194295 [DOI] [PubMed] [Google Scholar]
- 25.Imataka H, Gradi A, Sonenberg N. . A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J 1998; 17:7480 - 9; http://dx.doi.org/ 10.1093/emboj/17.24.7480; PMID: 9857202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piron M, Vende P, Cohen J, Poncet D. . Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J 1998; 17:5811 - 21; http://dx.doi.org/ 10.1093/emboj/17.19.5811; PMID: 9755181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarun SZ Jr., Sachs AB. . Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J 1996; 15:7168 - 77; PMID: 9003792 [PMC free article] [PubMed] [Google Scholar]
- 28.Gallie DR, Le H, Caldwell C, Tanguay RL, Hoang NX, Browning KS. . The phosphorylation state of translation initiation factors is regulated developmentally and following heat shock in wheat. J Biol Chem 1997; 272:1046 - 53; http://dx.doi.org/ 10.1074/jbc.272.2.1046; PMID: 8995401 [DOI] [PubMed] [Google Scholar]
- 29.Cheng S, Sultana S, Goss DJ, Gallie DR. . Translation initiation factor 4B homodimerization, RNA binding, and interaction with Poly(A)-binding protein are enhanced by zinc. J Biol Chem 2008; 283:36140 - 53; http://dx.doi.org/ 10.1074/jbc.M807716200; PMID: 18977752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.