Abstract
Bacterial biofilms are highly resistant to common antibacterial treatments, and several physiological explanations have been offered to explain the recalcitrant nature of bacterial biofilms. Herein, a biophysical aspect of biofilm recalcitrance is being reported on. While engineering structures are often overdesigned with a factor of safety (FOS) usually under 10, experimental measurements of biofilm cohesive strength suggest that the FOS is on the order of thousands. In other words, bacterial biofilms appear to be designed to withstand extreme forces rather than typical or average loads. In scenarios requiring the removal or control of unwanted biofilms, this emphasizes the importance of considering strategies for structurally weakening the biofilms in conjunction with bacterial inactivation.
Keywords: biofilm, factor of safety, cohesive strength, shear, extracellular polymeric substances, cystic fibrosis
Introduction
Biofilms are sessile communities of bacteria housed in a self-produced adhesive matrix consisting of extracellular polymeric substances (EPS), including polysaccharides, proteins, lipids, and DNA. Bacterial biofilms inhabit niches from water distribution system pipes to human lungs.1,2 Microorganisms in biofilms and biofilms themselves are highly persistent despite the efforts to eradicate them with antimicrobials (eg, antibiotics and chlorine) and physical removal (eg, brushing, scraping, flushing, and coughing).
Over the years, several physiological explanations have been offered to possibly explain the remarkable recalcitrance of bacterial biofilms. Some of the suggested mechanisms include diffusion limitation, microscale chemical gradients, existence of altered chemical microenvironments, and existence of recalcitrant bacterial phenotypes within the biofilm.3,4 Recently, some researchers have also attributed biofilm resistance to the presence of sack-like structures within the biofilm EPS.5 These unusual structures, made up of lipids, contain (or hide) several bacterial cells in a separate enclosure within the EPS. However, further work is needed to demonstrate the occurrence of these lipid sacks and to elucidate their specific antibiotic resistance characteristics, if any. In general, more specific investigations are needed to clearly understand the individual or collective role of these suggested physiological mechanisms in biofilm resistance and recalcitrance.
Surprisingly, apart from these physiological explanations, there has been very little focus on the biophysical aspects of biofilm persistence in natural and engineered environments. Shaw et al6 was perhaps the first to highlight biofilm visco-elastic properties as a biofilm survival mechanism. In a recent review article, Stewart7 also highlighted biofilm mechanical properties as a likely basis for the tenacity of biofilm-induced infections in the human body. Nevertheless, this particular aspect of biofilm recalcitrance has received very little attention, and the link between biophysical measurements and biofilm recalcitrance has not been widely explored. This is somewhat surprising given the considerable efforts and progress that have been made toward measurement and understanding of biofilm mechanical properties in the past decade.8,9 In this experimental study, a key biofilm mechanical property (cohesive strength) is being presented as a primary biophysical mechanism that enables the biofilms to withstand mechanical stresses and physical assaults, and thus, contributing toward overall biofilm recalcitrance.
Materials and Methods
Biofilm mechanical properties
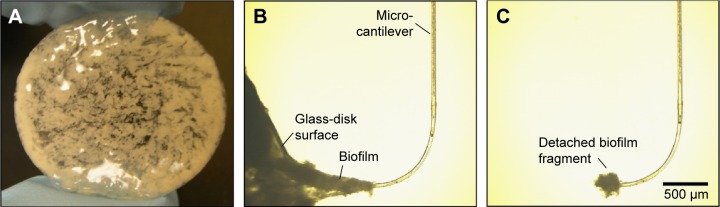
The biofilm mechanical property data for Pseudomonas aeruginosa and Staphylococcus epidermidis used here have been published previously.10,11 These single species biofilms were developed on 22 mm glass coupons (Fig. 1A) in a rotating disk reactor, and subsequently tested using the microcantilever method (Fig. 1B and C). Details of bacteria, inocula preparation, biofilm development, and mechanical testing using the microcantilever methods have also been provided in detail in the aforementioned publications. It is also noteworthy that the strength metric reported and discussed in the current manuscript is cohesive strength, which quantifies the strength of biofilm–biofilm linkages. Another related term is adhesive strength, which refers to the strength of linkages at the biofilm–substratum interface.
Figure 1.
(A) 22 mm glass disk covered with a three-day-old S. epidermidis biofilm grown in a rotating disk reactor. (B) Selected frame from a microcantilever tensile test on a three-day-old S. epidermidis biofilm at the edge of glass substratum. (C) Completed tensile test showing detached biofilm fragment held by the microcantilever tip.
Because multispecies biofilms are frequently encountered in engineered systems (eg, drinking water treatment plants, distribution systems, and wastewater treatment plants) and various natural environments (rivers and streams), additional data for multispecies biofilms were obtained for this study. Mississippi river water (MRW) was selected as a convenient and natural source for a multispecies bacterial inoculum used to cultivate multispecies laboratory biofilms.
For the MRW biofilm, a water sample was collected from the Mississippi River in Minneapolis, MN, filtered (5 µm filter, Millipore) to remove algae and larger particles, and cultured in R2A media on a shaker table at 37°C for 48 hours to obtain optical density at 600 nm of 0.9. Biofilms were grown from this inoculum in a rotating disk reactor, as described previously.11 R2A medium was fed at a flow rate of 2.5 mL/minute, which resulted in a hydraulic residence time of 96 minutes, and biofilm-coated coupons were removed after 6 days of growth. Subsequently, mechanical testing was performed using the microcantilever method as described previously.10
Fluid shear calculations on rotating disks
The fluid shear stress (τ), acting on a clean biofilm coupon during growth, was estimated using the equation for a smooth disk rotating in an infinite fluid:
Here the shear stress (τ) is a function of viscosity of the surrounding fluid (ν), the density of the fluid (ρ), the rotational speed of the disk (ω), and radial distance from the center of the disk (r).12 The stress calculated here represents the stress faced by the bacteria in the biofilms, while the bacteria transitioned from the planktonic to a sessile phase on a clean coupon surface formed an agglomeration ultimately leading to a mature biofilm.
Results and Discussion
From the above experiments, average (mean ± standard error) cohesive strength values for P. aeruginosa biofilms, S. epidermidis biofilms, and MRW biofilms were 1,760 ± 400 Pa (n = 19), 1,470 ± 210 Pa (n = 47), and 27,510 ± 7,620 Pa (n = 13). In addition, the estimated shear stresses experienced by the three biofilms during growth were 0.07 Pa, 0.18 Pa, and 1.9 Pa, respectively. Thus, the cohesive strengths of the biofilms are four to five orders of magnitude greater than the shear stress experienced during growth (Fig. 2).
Figure 2.
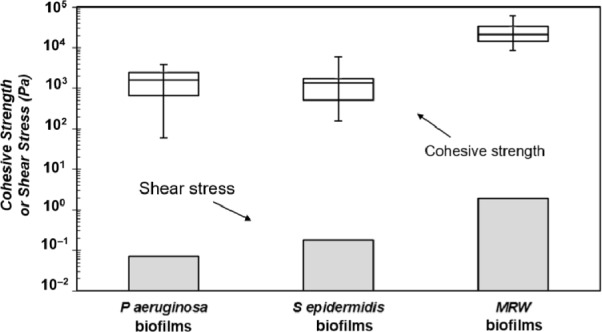
Box and whisker plots showing the minimum value, the 25th percentile, the median, the 75th percentile, and the maxima for cohesive strength of P. aeruginosa, S. epidermidis, and MRW biofilms. Bar plots denote the fluid shear stress on a clean coupon (during growth) for the three biofilms, respectively.
Engineering designs of structures such as bridges and buildings incorporate a factor of safety (FOS).13 The FOS is defined as a ratio of the structural strength (eg, of the building or bridge) to that of the applied loads. A biofilm FOS was calculated by taking the ratio of the measured cohesive strength to the estimated fluid shear stress. From the data in this study, the biofilm FOS values ranged from 330 to 55,000. The ability to compare biofilm FOS values with those from other research groups is limited because there are few reports on biofilm strength in the literature, and the tested biofilms are not usually grown under defined shear conditions (thus hindering the calculation of shear stress during growth). However, our results are in agreement with a previous report by Möhle et al (FOS = 200–1,100) who used fluid dynamic gauging to determine biofilm cohesive strength.14
Typically, FOS values employed by engineers for the design of buildings and other structures are <10.13 Thus, these high biofilm FOS values are certainly surprising and seem counter-intuitive, because interspecies competition in nature dictates that organisms function at or near optimum efficiency to occupy a given niche.15 Increasing EPS density to increase cohesive strength diverts resources that could be used for growth (ie, reproduction) or energy storage.
On the other hand, it is also possible that the actual forces experienced by the biofilm during growth, such as the local forces resulting from the biofilm surface morphology (ie, roughness) or dynamic forces during reactor startup and maintenance activities, far exceed the shear stress estimated as described earlier. Specifically, as a biofilm structure protrudes off the substratum into the flow regime, viscous forces increase dramatically and pressure forces (which are zero on the clean substratum) also begin to appear, thus adding to the net local shear stress. For example, Manz et al16 reported that local shear stresses were up to an order of magnitude greater than the estimated average stress. Nevertheless, even with an order of magnitude correction to our estimated stresses, the FOS values are still quite high (33–5,500). Thus, it appears that bacterial biofilms are designed to withstand extreme forces and not just typical or average applied forces. However, more research is needed to elucidate the full range of stresses experienced by the biofilms grown for strength testing. Perhaps, experimental techniques such as particle image velocimetry or modeling approaches such as computational fluid dynamics could aid in these efforts.
Finally, it could also be argued that the strength of the biofilm matrix is not dictated by the applied fluid shear but is merely coincidental because the EPS composition and density are dictated by other purposes such as serving as a defense from biocides17 or as a cache of stored food.18,19 If this were the case, one would not expect the strength to increase with fluid shear. Nevertheless, correlations between strength and the fluid shear experienced by the biofilm during growth have been reported in the literature.20–22 This indicates that higher shear conditions actually select for stronger biofilms. If this is the case, then either the bacteria in the biofilm sense the increased shear and respond by changing EPS composition, increasing EPS density, or both or the weak EPS and the bacteria that secreted them are simply washed away leaving the stronger biofilm formers behind.22
Our findings offer a possible explanation for the persistence of biofilms in nature and medicine. For example, shear forces in the bronchial tubes of patients with cystic fibrosis (CF) need to exceed the strength of the resident P. aeruginosa biofilms in order to dislodge them. Unfortunately, the shear forces generated in bronchial tubes, even during peak airflow events such as coughing, are at most a few Pa (Table 1). Interestingly, one successful therapy for treating early biofilm development in patients with CF is to employ DNase to weaken the biofilm, as it appears extracellular DNA is an important structural component of these biofilms.23 Additionally, shear stress data in engineering scenarios (eg, open channel flows and membrane systems), where undesirable biofilms persist (Table 1), support the argument of biofilm persistence due to a mechanical advantage.
Table 1.
Shear stresses in natural/engineered systems where biofilms are routinely encountered.
| ENVIRONMENT | SHEAR STRESSES | REFERENCE |
|---|---|---|
| Open channel flows near a bridge | 0–1.6 Pa | Adhikary et al26 |
| Smooth rectangular channels | 0–20 Paa | Guo and Julien27 |
| Human bronchial airways | 0–0.06 Pab | Xia et al28 |
| 0–0.4 Pac | Nucci et al29 | |
| 19 Pad | Green30 | |
| 0.9 Pae | ||
| Hollow fiber membrane systems | 0–0.15 Paf | Nagaoka et al31 |
Notes:
Assuming a bed depth of 1 m, and slope = 2 × 10−3;
based on finite element based simulations;
baseline case with no constriction in the bronchial airway;
calculated maximum value for the case of 8 L/second coughing event;
calculated maximum for the case of 1 L/second coughing event;
calculated shear stress values based on water flow alone (excluding the effect of airflow and bubbles).
Conclusion
In conclusion, attempting to simply kill bacteria (ie, antimicrobial treatment) is often insufficient when dealing with biofilms. Weakening the biofilm to promote detachment followed by washout or subsequent biocidal inactivation of the detached biomass is another perhaps more effective approach to dealing with this problem that should be considered.24,25
Footnotes
ACADEMIC EDITOR: Raul Rivas, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 335 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by National Science Foundation grants (Project award numbers 0728550 and 0728621). In addition, author S. Aggarwal acknowledges support from Alaska EPSCoR NSF award #OIA-1208927 and the state of Alaska. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: SA and RMH. Analyzed the data and wrote the first draft of the manuscript: SA. Contributed to the writing of the manuscript: SA, RMH and PSS. Agree with manuscript results and conclusions: SA, RMH, PSS. Jointly developed the structure and arguments for the paper: SA, RMH and PSS. Made critical revisions and approved final version: SA, RMH and PSS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.September SM, Els FA, Venter SN, et al. Prevalence of bacterial pathogens in biofilms of drinking water distribution systems. J Water Health. 2007;5:219–228. [PubMed] [Google Scholar]
- 3.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert P, McBain A, Lindsay S. Biofilms, multi-resistance, and persistence. In: Amábile-Cuevas CF, editor. Antimicrobial Resistance in Bacteria. Norfolk: Taylor & Francis; 2007. pp. 77–98. [Google Scholar]
- 5.Hess DJ, Henry-Stanley MJ, Barnes AM, et al. Ultrastructure of a novel bacterial form located in Staphylococcus aureus in vitro and in vivo catheter-associated biofilms. J Histochem Cytochem. 2012;60(10):770–776. doi: 10.1369/0022155412457573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw T, Winston M, Rupp CJ, et al. Commonality of elastic relaxation times in biofilms. Phys Rev Lett. 2004;93(9):098102. doi: 10.1103/PhysRevLett.93.098102. [DOI] [PubMed] [Google Scholar]
- 7.Stewart PS. Biophysics of biofilm infection. Pathog Dis. 2014;70(3):212–218. doi: 10.1111/2049-632X.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guélon T, Mathias JD, Stoodley P. Advances in biofilm mechanics. In: Flemming HC, Wingender J, Szewzyk U, editors. Mechanics Biofilm Highlights. 5th ed. Berlin, Heidelberg: Springer; 2011. pp. 111–139. [Google Scholar]
- 9.Böl M, Ehret AE, Bolea Albero A, et al. Recent advances in mechanical characterisation of biofilm and their significance for material modelling. Crit Rev Biotechnol. 2013;33(2):145–171. doi: 10.3109/07388551.2012.679250. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S, Poppele EH, Hozalski RM. Development and testing of a novel microcantilever technique for measuring the cohesive strength of intact biofilms. Biotechnol Bioeng. 2010;105:924–934. doi: 10.1002/bit.22605. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S, Hozalski RM. Determination of biofilm mechanical properties from tensile tests performed using a micro-cantilever method. Biofouling. 2010;26:479–486. doi: 10.1080/08927011003793080. [DOI] [PubMed] [Google Scholar]
- 12.Benton ER. On the flow due to a rotating disk. J Fluid Mech. 1966;24(4):781–800. [Google Scholar]
- 13.Burr A, Cheatham J. Mechanical Design and Analysis, 2nd Edition, Section 5.2. Prentice-Hall; 1995. [Google Scholar]
- 14.Möhle RB, Langemann T, Haesner M, et al. Structure and shear strength of microbial biofilms as determined with confocal laser scanning microscopy and fluid dynamic gauging using a novel rotating disc biofilm reactor. Biotechnol Bioeng. 2007;98(4):747–755. doi: 10.1002/bit.21448. [DOI] [PubMed] [Google Scholar]
- 15.Darwin CR. The Origin of Species by Means of Natural Selection (Reprinted by the Modern Library) New York, NY: Random House; 1859. [Google Scholar]
- 16.Manz B, Volke F, Goll D, et al. Measuring local flow velocities and biofilm structure in biofilm systems with magnetic resonance imaging (MRI) Biotechnol Bioeng. 2003;84:424–432. doi: 10.1002/bit.10782. [DOI] [PubMed] [Google Scholar]
- 17.Campanac C, Pineau L, Payard A, et al. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob Agents Chemother. 2002;46:1469–1474. doi: 10.1128/AAC.46.5.1469-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JR, Liu CT, Edwards EA, et al. Effect of phosphorus limitation on microbial floc structure and gene expression in activated sludge. Water Sci Technol. 2006;54(1):247–255. doi: 10.2166/wst.2006.393. [DOI] [PubMed] [Google Scholar]
- 19.Hoa PT, Nair L, Visvanathan C. The effect of nutrients on extracellular polymeric substances production and its influence on sludge properties. Water SA. 2003;29:437–442. [Google Scholar]
- 20.Chen MJ, Zhang Z, Bott TR. Effects of operating conditions on the adhesive strength of Pseudomonas flurorescens biofilms in tubes. Colloids Surf B Biointerfaces. 2005;43:61–71. doi: 10.1016/j.colsurfb.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Stoodley P, Jacobsen A, Dunsmore BC, et al. The influence of fluid shear and AlCl3 on the material properties of Pseudomonas aeruginosa PAO1and Desulfo-vibrio sp.EX265 biofilms. Water Sci Technol. 2001;43:113–120. [PubMed] [Google Scholar]
- 22.Stoodley P, Cargo R, Rupp CJ, et al. Biofilm material properties as related to shear-induced deformation and detachment phenomena. J Ind Microbiol Biotechnol. 2002;29:361–367. doi: 10.1038/sj.jim.7000282. [DOI] [PubMed] [Google Scholar]
- 23.Whitchurch CB, Tolker-Nielsen T, Ragas PC, et al. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487–1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 24.Davies DG, Marques CNH. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol. 2009;191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers SA, Huigens Iii RW, Melander C. A 2-aminobenzimidazole that inhibits and disperses gram-positive biofilms through a zinc-dependent mechanism. J Am Chem Soc. 2009;131(29):9868–9869. doi: 10.1021/ja9024676. [DOI] [PubMed] [Google Scholar]
- 26.Adhikary BD, Majumdar P, Kostic M. CFD simulation of open channel flooding flows and scouring around bridge structures. Proceedings of the 6th WSEAS International Conference on Fluid Mechanics; Zhejiang, China: 2009. [Google Scholar]
- 27.Guo J, Julien PY. Shear stress in smooth rectangular open-channel flows. J Hydraul Eng. 2005;131(1):30–37. [Google Scholar]
- 28.Xia G, Tawhai MH, Hoffman EA, et al. Airway wall stiffening increases peak wall shear stress: a fluid–structure interaction study in rigid and compliant airways. Ann Biomed Eng. 2010;38(5):1836–1853. doi: 10.1007/s10439-010-9956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nucci G, Suki B, Lutchen K. Modeling airflow-related shear stress during heterogeneous constriction and mechanical ventilation. J Appl Physiol. 2003;95(1):348–356. doi: 10.1152/japplphysiol.01179.2001. [DOI] [PubMed] [Google Scholar]
- 30.Green AS. Modelling of peak-flow wall shear stress in major airways of the lung. J Biomech. 2004;37:661–667. doi: 10.1016/j.jbiomech.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Nagaoka H, Kurosaka M, Shibata N, et al. Effect of bubble flow velocity on drag-force and shear stress working on submerged hollow fibre membrane. Water Sci Technol. 2006;54(10):185–192. doi: 10.2166/wst.2006.818. [DOI] [PubMed] [Google Scholar]