Abstract
Aims/Introduction
Some previous studies reported no significant association of consuming fruit or vegetables, or fruit and vegetables combined, with type 2 diabetes. Others reported that only a greater intake of green leafy vegetables reduced the risk of type 2 diabetes. To further investigate the relationship between them, we carried out a meta‐analysis to estimate the independent effects of the intake of fruit, vegetables and fiber on the risk of type 2 diabetes.
Materials and Methods
Searches of MEDLINE and EMBASE for reports of prospective cohort studies published from 1 January 1966 to 21 July 2014 were carried out, checking reference lists, hand‐searching journals and contacting experts.
Results
The primary analysis included a total of 23 (11 + 12) articles. The pooled maximum‐adjusted relative risk of type 2 diabetes for the highest intake vs the lowest intake were 0.91 (95% confidence interval [CI] 0.87–0.96) for total fruits, 0.75 (95% CI 0.66–0.84) for blueberries, 0.87 (95% CI 0.81–0.93) for green leafy vegetables, 0.72 (95% CI 0.57–0.90) for yellow vegetables, 0.82 (95% CI 0.67–0.99) for cruciferous vegetables and 0.93 (95% CI 0.88–0.99) for fruit fiber in these high‐quality studies in which scores were seven or greater, and 0.87 (95% CI 0.80–0.94) for vegetable fiber in studies with a follow‐up period of 10 years or more.
Conclusions
A higher intake of fruit, especially berries, and green leafy vegetables, yellow vegetables, cruciferous vegetables or their fiber is associated with a lower risk of type 2 diabetes.
Keywords: Meta‐analysis, Nutrition intake, Type 2 diabetes
Introduction
Diabetes has become a serious and increasing global health burden. An estimated 382 million people worldwide were affected by diabetes in 2013, and this number is expected to rise to 592 million by 20351. Consequently, diabetes is predicted to become the major cause of death and disability in the world by 20302, 3. Primary prevention of diabetes is clearly a major public health priority. Type 2 diabetes makes up >90% of all diabetes cases. Although the development of type 2 diabetes is complicated, dietary factors could play an important role in its pathogenesis. Dietary modification has been shown to delay or prevent the development of type 2 diabetes4, 5.
Intake of sufficient amounts of fruit and vegetables is recommended as a part of a healthy diet, though the individual contribution from different food sources remains largely unknown. Increasing fruit and vegetable consumption could reduce the risk of many chronic diseases, including cardiovascular diseases, cancers, stroke and type 2 diabetes6, 7, 8, 9, 10, 11, 12. These foods contain considerable protective constituents, including potassium, folate, vitamins, fiber, anti‐oxidant content and phenolic compounds13, 14, 15. However, the mechanisms by which fruit and vegetables reduce the risk of type 2 diabetes have not been precisely elucidated. To date, many epidemiological studies have examined the association between type 2 diabetes risk and fruit and vegetable intake12, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28. Findings from these studies have been surprisingly inconsistent. Some studies suggested a higher intake of fruit and vegetables, especially the intake of berries and green leafy vegetables, has the inverse associations with the risk of type 2 diabetes12, 16, 17, 18, 19, 20, 21, 22. Some studies have not shown such associations with the intake of fruit or vegetables23, 24, 25, 26, 27, 28. The discrepancy of these results could arise from the complicity of the disease itself and functional heterogeneity of the biological responses to various foods.
In the present study, we sought to carry out a meta‐analysis to investigate the relationship between fruit or vegetable intake, and the incidence of type 2 diabetes. Notably, several previously carried out meta‐analyses used relatively limited pooled results around 2008. They primarily focused on the relationship between the intake of fruit or vegetables and type 2 diabetes11, 16, 20, 27, 28, 29, 30. They reported no significant association of fruit or vegetables, or fruit and vegetables combined with type 2 diabetes11, 29, 30. Only a greater intake of green leafy vegetables reduced the risk of type 2 diabetes11, 29. Presently, we collected a more up‐to‐date and large number of prospective cohort relevant studies in the present meta‐analysis. Additionally, we included the relationship between the intake of fruit and vegetable fiber, and the risk of type 2 diabetes.
Materials and Methods
Search Strategy
Two authors (Ping‐Yu Wang and Jun‐Chao Fang) carried out the literature search. We systematically searched MEDLINE and EMBASE for studies published from 1 January 1966 to 21 July 2014. To ensure a broad range of relative issues, the search strategy included a combined text and the medical subject headings (type 2 diabetes, diabetes mellitus, prediabetes, impaired glucose tolerance, impaired fasting glucose, fruits, vegetables, fiber, fibre, follow‐up, and prospective studies). Furthermore, to search for more studies, we also sought expert opinion and additionally hand‐checked the reference lists of original publications and previous meta‐analyses or reviews. There were no language restrictions.
Study Selection
To be included, studies had to fulfil the following criteria: (i) prospective cohort studies with healthy participants at baseline; (ii) an individual measure of intake of fruits, vegetables, fruit and vegetables, or fruit and vegetable fiber; (iii) an assessment of the development of type 2 diabetes; (iv) multivariate adjusted relative risk (RR) or hazard ratios (HR) with their corresponding 95% confidence interval (CI). Studies that did not meet the inclusion criteria were excluded during the initial review. When uncertainty existed, we retrieved and assessed the full text article. Two reviewers (Ping‐Yu Wang and Zong‐Hua Gao) resolved any uncertainty through discussion. If duplicate reports from the same study cohort were identified, only the most recent publication with the most detailed information or the study with the largest population was included.
Data Extraction and Validity Assessment
Data extraction was carried out independently by two authors (Ping‐Yu Wang and Jun‐Chao Fang), and any differences were resolved through discussion. From each study, the following details were extracted: the first author, publication year, country, number of participants, participants' age, sex, duration of follow up, number of events, methods used to measure exposure, outcome assessment, multivariate adjusted RR or HR of type 2 diabetes and corresponding 95% CI for the highest vs lowest level of intake with the greatest number of adjustments, and confounding factors in the statistical analysis.
For assessing the quality of an observational study, the Newcastle–Ottawa quality assessment Scale (NOS) is recommended31. Two authors (Can Zhang and Shu‐Yang Xie) independently assessed all studies for quality and validity using the NOS, and any discrepancies were resolved by discussion. For cohort studies, the NOS consisted of three dimensions of quality: selection (4 points), comparability (2 points) and outcome (3 points). It allowed a total score from 0 to 9 points, with a total score of 7 or greater reflecting high‐quality studies.
Statistical Analysis
HRs or RRs were used to measure the association between intake of fruit, vegetables or their fiber and risk of type 2 diabetes. We transformed these values by taking their natural logarithms and calculating their standard errors and corresponding 95% CIs. Then we generated pooled estimates to calculate summary hazard ratios and 95% CIs for the highest vs lowest level of intake.
For each outcome, tests of heterogeneity were carried out (using the χ2‐test of heterogeneity and I² statistic). If there was no heterogeneity, a fixed‐effect meta‐analysis was carried out. If there was substantial heterogeneity (I 2 greater than 50%), the review authors looked for a possible reason for this (e.g., participants). We carried out subgroup analysis based on the quality of the study (high quality vs lower quality), sex (men and women included vs women only), length of follow up (<10 years vs ≥10 years) and location (USA and Europe vs China), as these were thought to be possible sources of heterogeneity. If the heterogeneity could not be explained, we used a random‐effects model with appropriate cautious interpretation.
We also carried out sensitivity analysis to assess the stability of the results by excluding or including studies at high risk of bias (e.g., those follow‐up years <5 years) or using the trim and fill method. The effects of publication bias were assessed using funnel plots and Egger's regression test to measure funnel plot asymmetry32, 33. All statistical analyses were analyzed with Stata (version 12.0; StataCorp, College Station, TX, USA).
Results
Search Results
The systematic search identified 2,626 articles (Figure 1), of which 2,589 were excluded on the basis of titles and abstracts. We obtained 37 potentially relevant articles for full‐text assessment. Of these, several articles, which examined fruit and vegetable intake within dietary patterns only or were meta‐analyses, were not included. Two articles reported the same cohort data, so we excluded the article with the smaller population16, 29. One study, which examined plasma vitamin C level with the risk of incident of type 2 diabetes, was excluded for data presented as odds ratios22, but we added the data from the study to see if it significantly altered the observed associations in the sensitivity analysis. One additional article was included by checking the reference lists of identified reports24. A total of 22 articles, published between 1992 and 2014, were included in this meta‐analysis.
Figure 1.
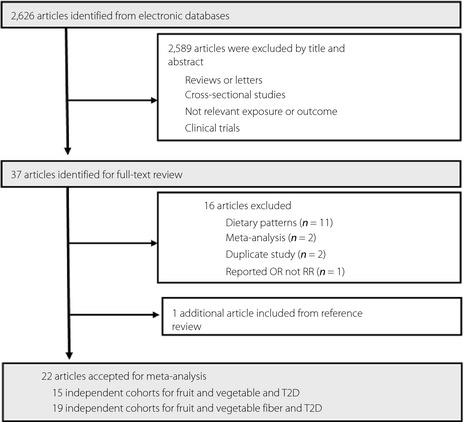
Process of study selection in the meta‐analysis. OR, odds ratio; RR, relative risk; T2D, type 2 diabetes.
Study Characteristics and Quality Assessment
There were 11 articles including 15 independent cohorts on fruit and vegetable intake and risk of type 2 diabetes (Table 1). In the publications of Muraki et al.20, Ford and Mokdad21, and Kurotani et al.28, the results, reporting for sex or independent cohorts separately, were treated as separate cohorts in the current meta‐analysis. The age of participants ranged from 25 to 79 years. Study duration of follow up ranged from 4 to 24 years. In most papers, some adjustments including age, sex, body mass index, energy intake, smoking, alcohol and family history were made for potential confounding factors. None of the papers met all of the criteria of the quality assessment tool, and the quality scores ranged from 5 to 8.
Table 1.
Characteristics of included studies on fruit and vegetable intake and risk of type 2 diabetes
| Study | Country | Sex | Age (years) | Follow‐up (years) | Cases/size | Assessment of T2DM | Measure of intake | Adjustments | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Meyer (2000)26 | USA | F | 55–69 | 6 | 1,141/35,988 | Self‐report | FFQ | Age, BMI, smoking, alcohol, energy, WHR, physical activity, education | 6 |
| Ford (2001)21 | USA | M | 25–74 | 20 | 416/3,874 | Self‐report and HM | 24 h recall | Age, BMI, sex, smoking, alcohol, SBP, cholesterol, exercise, education | 6 |
| Ford (2001)21 | USA | F | 25–74 | 20 | 602/5,791 | Self‐report and HM | 24 h recall | Age, BMI, sex, smoking, alcohol, SBP, cholesterol, exercise, education | 6 |
| Liu (2004)23 | USA | F | ≥45 | 8.8 | 1,614/38,018 | Self‐report | FFQ | Age, BMI, smoking, alcohol, cholesterol, exercise, total calories, history | 6 |
| Hodge (2004)24 | Australia | M&F | 40–69 | 4 | 365/31,641 | Self‐report | FFQ | Age, sex, BMI, WHR, country of birth, physical activity, family history of diabetes, alcohol, education, weight change, energy | 5 |
| Montonen (2005)12 | Finland | M&F | 40–69 | 23 | 383/4,304 | HM and via Social Insurance Institution register | DHI (dietary history interview) | Age, sex, BMI, energy intake, smoking, family history of diabetes, and geographic area | 7 |
| Bazzano (2008)25 | USA | F | 38–63 | 18 | 4,529/71,346 | Self‐report and confirmed if met WHO criteria or ADA criteria | FFQ | BMI, physical activity, family history, postmenopausal hormone, alcohol, smoking, energy | 7 |
| Villegas (2008)18 | China | F | 40–70 | 4.6 | 1,608/64,191 | HM | FFQ | Age, energy, meat, BMI, WHR, smoking, alcohol, physical activity, income, education, occupational status, and hypertension | 6 |
| Cooper (2012)29 | Europe | M&F | 40–79 | 11 | 10,821/24,939 | Self‐report, linkage to primary‐care registers, secondary‐care registers, medication use, hospital admissions | FFQ and 24 h recall | Age, sex, education, BMI, physical activity, smoking, energy, alcohol | 8 |
| Kurotani (2013)28 | Japan | M | 45–75 | 5 | 530/21,269 | Self‐report | FFQ | Age, public health centre area, BMI, smoking, alcohol, leisure‐time activity, history of hypertension, family history of diabetes, coffee, Mg, Ca, energy intake | 7 |
| Kurotani (2013)28 | Japan | F | 45–75 | 5 | 366/27,168 | Self‐report | FFQ | Age, public health centre area, BMI, smoking, alcohol, leisure‐time activity, history of hypertension, family history of diabetes, coffee, Mg, Ca, energy intake | 7 |
| Muraki (2013)20 | USA (NHS) | F | 38–63 | 24 | 6,358/66,105 | Self‐report and supplementary questionnaires/medical records | FFQ | Age, ethnicity, BMI, smoking, multivitamin use, physical activity, family history of diabetes, energy intake and other factors | 6 |
| Muraki (2013)20 | USA (NHS II) | F | 26–46 | 18 | 3,153/85,104 | Self‐report and supplementary questionnaires/medical records | FFQ | Age, ethnicity, BMI, smoking, multivitamin use, physical activity, family history of diabetes, energy intake and other factors | 6 |
| Muraki (2013)20 | USA (HPFS) | M | 40–75 | 22 | 2,687/36,173 | Self‐report and supplementary questionnaires/medical records | FFQ | Age, ethnicity, BMI, smoking, multivitamin use, physical activity, family history of diabetes, energy intake and other factors | 7 |
| Mursu (2014)27 | Finland | M | 42–60 | 19.3 | 432/2,332 | A self‐administered questions for a physician‐set diagnosis | 4‐d food recording | Age, examination years, BMI, waist‐to‐hip ratio, smoking, alcohol, education, physical activity, family history of diabetes, and other factors | 7 |
There were 12 articles including 19 independent cohorts studying on intake of fruit and vegetable fiber and risk of type 2 diabetes (Table 2). In the publications of Sameron, Stevens and Hopping34, 35, 36, the results, reporting for independent cohorts separately, were treated as separate cohorts in the current meta‐analysis. The age of participants ranged from 26 to 79 years. Study duration of follow‐up ranged from 4.6 years to 14 years. All papers made some adjustments for potential confounding factors. The quality scores ranged from 5 to 8.
Table 2.
Characteristics of included studies on fruit and vegetable fiber intake and risk of type 2 diabetes
| Study | Country | Sex | Age (years) | Follow‐up (years) | Cases/size | Assessment of T2DM | Measure of intake | Adjustments | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Colditz (1992)37 | USA | F | 30–55 | 6 | 702/84,360 | Self‐report | FFQ | Age, BMI, alcohol, follow‐up period | 5 |
| Salmeron (1997)35 | USA | F | 40–65 | 6 | 915/65,173 | Self‐report | FFQ | Age, BMI, smoking, alcohol, energy, physical activity, family history of diabetes | 8 |
| Salmeron (1997)35 | USA | M | 40–75 | 6 | 523/42,759 | Self‐report | FFQ | Age, BMI, smoking, alcohol, energy, physical activity, family history of diabetes | 8 |
| Meyer (2000)26 | USA | F | 55–69 | 6 | 1,141/3,5988 | Self‐report | FFQ | Age, BMI, smoking, alcohol, energy, WHR, physical activity, education | 6 |
| Stevens (2002)36 White | USA | M&F | 45–64 | 9 | 971/9,529 | Self‐report | FFQ | Age, BMI, sex, smoking, physical activity, education, field center | 6 |
| Stevens (2002)36 Black | USA | M&F | 45–64 | 9 | 476/2,722 | Self‐report | FFQ | Age, BMI, sex, smoking, physical activity, education, field center | 6 |
| Montonen (2003)38 | Finland | M&F | 40–69 | 10 | 156/4316 | Social insurance institution register | Dietary history interview | Age, BMI, sex, smoking, energy, area | 7 |
| Schulze (2004)39 | USA | F | 26–46 | 8 | 741/91,249 | Self‐report | FFQ | Age, BMI, smoking, alcohol, physical activity, energy, family history, hormone, magnesium, caffeine | 7 |
| Hodge (2004)24 | Australia | M&F | 40–69 | 4 | 365/31,641 | Self‐report | FFQ | Age, sex, BMI, WHR, country of birth, physical activity, family history of diabetes, alcohol, education, weight change, energy | 5 |
| Schulze (2007)54 | Germany | M&F | 35–65 | 7 | 844/25,067 | Self‐report | FFQ | Age, sex, BMI, education, sports activity, smoking, alcohol, waist circumference, energy, carbohydrate intake, PUFA‐SFA ratio, MUFA‐SFAratio | 7 |
| Barclay (2007)40 | Australia | M&F | >49 | 10 | 138/1,833 | Fasting plasma glucose level/self‐report | FFQ | Age, sex, family history of diabetes, smoking, triglycerides, HDL cholesterol and other factors | 7 |
| Wannamethee (2009)41 | British | M | 60–79 | 7 | 162/3,428 | Self‐report | FFQ | Age, waist circumference, smoking, physical activity, social class, alcohol, total calorie intake and other factors | 6 |
| Hopping (2010)34 | Caucasian | M | 45–75 | 14 | 1,080/15,116 | Self‐report/medical records | FFQ | BMI, physical activity, education, and calories | 7 |
| Hopping (2010)34 | Caucasian | F | 45–75 | 14 | 715/1,4643 | Self‐report/medical records | FFQ | BMI, physical activity, education, and calories | 7 |
| Hopping (2010)34 | Japanese American | M | 45–75 | 14 | 2,677/16,572 | Self‐report/medical records | FFQ | BMI, physical activity, education, and calories | 7 |
| Hopping (2010)34 | Japanese American | F | 45–75 | 14 | 2,374/18,672 | Self‐report/medical records | FFQ | BMI, physical activity, education, and calories | 7 |
| Hopping (2010)34 | Native Hawaiian | M | 45–75 | 14 | 798/4,568 | Self‐report/medical records | FFQ | BMI, physical activity, education, and calories | 7 |
| Hopping (2010)34 | Native Hawaiian | F | 45–75 | 14 | 943/5,941 | Self‐report/medical records | FFQ | BMI, physical activity, education, and calories. | 7 |
| Weng (2012)42 | Taiwanese | M&F | ≥30 | 4.6 | 141/1,604 | Fasting plasma glucose concentration/self‐report | FFQ | Age, sex, caloric intake, family history of diabetes, BMI, education, smoking, drinking, hypertension, and other factors | 6 |
Fruit Only
The overall maximum‐adjusted pooled RR based on all available data for the highest intake of fruit vs the lowest intake with risk of type 2 diabetes was 0.91 (95% CI 0.87–0.96). The I 2 statistic for heterogeneity between studies was 11.2% and P = 0.33 for homogeneity, suggesting low between‐study heterogeneity. The meta‐analysis showed a significant reduction in the risk of type 2 diabetes incidence for consumption of fruits (Figure 2).
Figure 2.
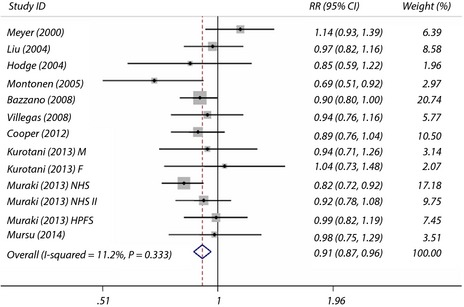
Maximum‐adjusted relative risk (RR) for type 2 diabetes, comparing highest vs lowest intake of fruit. Weights are from fixed effect analysis. CI, confidence interval.
Some cohort studies also examined the intake of citrus, strawberries and blueberries with the risk of type 2 diabetes. Eight cohort studies investigated the association between citrus intake and the risk of type 2 diabetes, the summary RR was 1.02 (95% CI 0.96–1.09) for the highest intake compared with the lowest intake, with no evidence of between‐study heterogeneity (I 2 = 0.0% and P = 0.67 for homogeneity). Five cohort studies investigated the association between strawberry intake and the risk of type 2 diabetes, the summary RR was 0.96 (95% CI 0.78–1.18) for the highest intake compared with the lowest intake. Four cohort studies investigated the association between blueberry intake and the risk of type 2 diabetes, the summary RR was 0.75 (95% CI 0.66–0.84) for the highest intake compared with the lowest intake, with a significant reduction in risk of type 2diabetes.
Vegetables Only
The overall maximum‐adjusted pooled RR based on all available data for highest intake vegetables vs lowest intake with risk of type 2 diabetes incidence was 0.91 (95% CI 0.82–1.01). The I 2 statistic for heterogeneity between studies was 57.2% and P = 0.01 for homogeneity, suggesting substantial between‐study heterogeneity.
Some cohort studies also examined intake of green leafy vegetables, yellow vegetables and cruciferous vegetables with the risk of type 2 diabetes. Seven cohort studies investigated the association between the intake of green leafy vegetables and the risk of type 2 diabetes, the summary maximum‐adjusted RR was 0.87 (95% CI 0.81–0.93) for the highest intake compared with the lowest intake, with no evidence of between‐study heterogeneity (I 2 = 0.0% and P = 0.50 for homogeneity; Figure 3). Three cohort studies investigated the association between yellow vegetables intake and the risk of type 2 diabetes, the summary RR was 0.72 (95% CI 0.57–0.90) for the highest intake compared with the lowest intake. Three cohort studies investigated the association between cruciferous vegetables intake and the risk of type 2 diabetes, the summary RR was 0.82 (95% CI 0.67–0.99) for the highest intake compared with the lowest intake.
Figure 3.
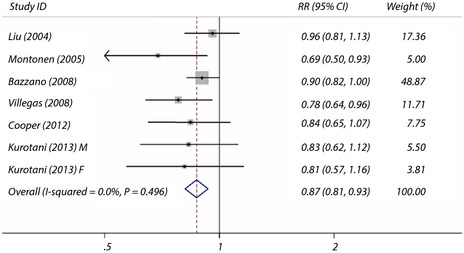
Maximum‐adjusted relative risk (RR) for type 2 diabetes, comparing highest vs lowest intake of green leafy vegetables. Weights are from fixed effect analysis. CI, confidence interval.
Fruit and Vegetables Combined
We identified nine cohort studies on the association between the intake of fruit and vegetables combined and the risk of type 2 diabetes. The summary maximum‐adjusted RR was 0.95 (95% CI 0.90–1.02) for the highest intake compared with the lowest intake, with marginal between‐study heterogeneity (I 2 = 34.4% and P = 0.14 for homogeneity).
Fruit Fiber
The overall maximum‐adjusted pooled RR based on all available data for the highest intake of fruit fiber vs the lowest intake with the risk of type 2 diabetes incidence was 1.00 (95% CI 0.99–1.02). The I 2 statistic for heterogeneity between studies was 1.5% and P = 0.44 for homogeneity, suggesting almost no between‐study heterogeneity. However, subgroup analysis results showed that a significant reduction in the risk of type 2 diabetes incidence for consumption of fruit fiber in these high‐quality studies in which scores were 7 or greater (Figure 4).
Figure 4.
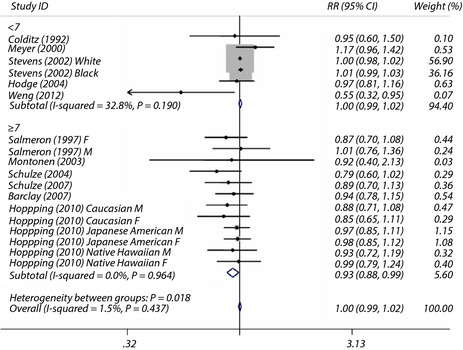
Maximum‐adjusted relative risk (RR) for type 2 diabetes, comparing highest vs lowest intake of fruit fiber. Weights are from fixed effect analysis. CI, confidence interval.
Vegetable Fiber
The overall maximum‐adjusted pooled RR based on all available data for the highest intake vegetable fiber vs the lowest intake with risk of type 2 diabetes was 0.94 (95% CI 0.86–1.03). The I 2 statistic for heterogeneity between studies was 53.4% and P = 0.005 for homogeneity, suggesting moderate between‐study heterogeneity. We then carried out subgroup analysis to investigate the potential sources of between‐study heterogeneity. Sex, quality score of studies, duration of follow up and location, as these were assumed to be potential sources of bias, were analyzed separately. Overall, there were significant associations between the risk of type 2 diabetes and the consumption of vegetables fiber in studies with a follow‐up time of 10 years or more (Figure 5).
Figure 5.
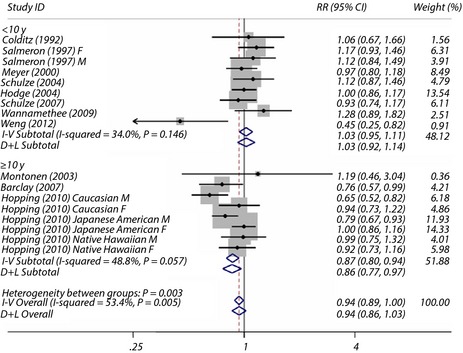
Maximum‐adjusted relative risk (RR) for type 2 diabetes, comparing highest vs lowest intake of vegetable fiber. Weights are from random effect analysis. CI, confidence interval.
Subgroup and Sensitivity Analyses
Table 3 shows the subgroup analysis results of intake of fruit, vegetables or their fiber and the risk of type 2 diabetes, comparing highest vs lowest intake. Sex, quality score of studies, duration of follow up and location were separately analyzed. In addition, we made sensitivity analysis by including studies that presented data as odds ratios, or excluding studies with follow‐up periods less than 5 years or that used the trim and fill method or both using a fixed and random model. The summary results did not greatly alter the associations.
Table 3.
Subgroup analyses of fruit and vegetable and their fiber intake and risk of type 2 diabetes, comparing highest vs lowest intake
| Fruit only | Vegetable only | Fruit and vegetable | Fruit fiber | Vegetable fiber | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohorts, n | RR (95% CI) | Cohorts, n | RR (95% CI) | Cohorts, n | RR (95% CI) | Cohorts, n | RR (95% CI) | Cohorts, n | RR (95% CI) | |
| All | 13 | 0.91 (0.87,0.96) | 10 | 0.91 (0.82,1.01) | 9 | 0.95 (0.90,1.02) | 18 | 1.00 (0.99,1.02) | 17 | 0.94 (0.86,1.03) |
| Sex | ||||||||||
| Male | 3 | 0.98 (0.85,1.12) | 2 | 0.81 (0.65,1.00) | 3 | 0.87 (0.71,1.06) | 4 | 0.95 (0.86,1.04) | 5 | 0.91 (0.73,1.14) |
| Female | 7 | 0.92 (0.86,0.97) | 5 | 0.95 (0.80,1.13) | 5 | 1.00 (0.92,1.08) | 7 | 0.96 (0.89,1.04) | 7 | 1.01 (0.93,1.10) |
| M&F | 3 | 0.84 (0.74,0.96) | 3 | 0.91 (0.83,1.01) | 1 | 0.90 (0.80,1.01) | 7 | 1.00 (0.99,1.02) | 5 | 0.86 (0.70,1.05) |
| Duration of follow‐up (year) | ||||||||||
| <10 | 6 | 0.99 (0.90,1.09) | 6 | 0.89 (0.75,1.07) | 4 | 1.03 (0.91,1.16) | 11 | 1.00 (0.99,1.02) | 9 | 1.03 (0.92,1.14) |
| ≥10 | 7 | 0.88 (0.83,0.94) | 4 | 0.94 (0.83,1.06) | 5 | 0.93 (0.86,1.00) | 7 | 0.94 (0.88,1.02) | 8 | 0.86 (0.77,0.97) |
| Location | ||||||||||
| Asia | 3 | 0.96 (0.82,1.12) | 3 | 0.77 (0.61,0.98) | 2 | 0.97 (0.75,1.25) | 1 | 0.55 (0.32,0.95) | 1 | 0.45 (0.25,0.82) |
| Non‐Asia | 10 | 0.91 (0.86,0.96) | 7 | 0.97 (0.90,1.05) | 7 | 0.95 (0.89,1.02) | 17 | 1.00 (0.99,1.02) | 16 | 0.95 (0.88,1.04) |
| Study quality | ||||||||||
| <7 | 5 | 0.91 (0.84,0.98) | 3 | 0.85 (0.60,1.19) | 4 | 0.98 (0.87,1.12) | 6 | 1.00 (0.99,1.02) | 5 | 0.97 (0.80,1.18) |
| ≥7 | 8 | 0.92 (0.86,0.98) | 7 | 0.96 (0.88,1.04) | 5 | 0.94 (0.88,1.02) | 12 | 0.93 (0.88,0.99) | 12 | 0.93 (0.84,1.03) |
Publication Bias
There was no evidence of substantial publication bias by using funnel plots and Egger's regression test (P = 0.50 fruit, P =0.15 vegetables, respectively; Figure 6).
Figure 6.
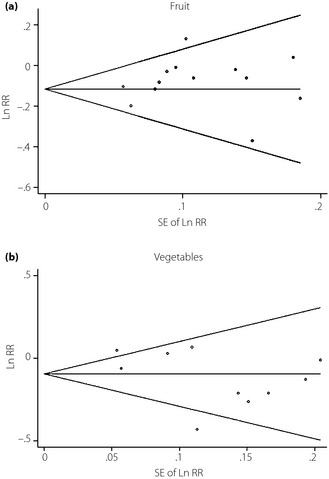
Publication bias was analyzed using funnel plot. (a) Fruit. (b) Vegetables. SE of Ln RR, standard error or natural logarithm of relative risk.
Discussion
The present meta‐analysis has quantitatively assessed the relationship between the intake of fruit, vegetables and their fiber, and the risk of type 2 diabetes. The results show that an increased consumption of fruit, especially berries, is associated with a reduced risk of type 2 diabetes. Increasing intake of green leafy vegetables, yellow leafy vegetables or cruciferous vegetables could help to reduce the risk of type 2 diabetes. In addition, there are significant associations between the risk of type 2 diabetes and the consumption of vegetable fiber in studies with follow‐up times of 10 years or more, and also a significant reduction in the risk of type 2 diabetes for the consumption of fruit fiber in these high‐quality studies in which the scores are seven or greater. These findings provide strong support for the recommendations encouraging the public to consume more fruit and vegetables. An important point is that different types of fruit and vegetables could have different effects on the risk of type 2 diabetes. Berries, green leafy vegetables, yellow leafy vegetables or cruciferous vegetables might be good choices for reducing of the risk of type 2 diabetes.
Fruit and vegetables are rich sources of fiber, flavonoids and anti‐oxidant compounds (carotenoids, vitamin C and E), folate, and potassium, which could explain the protective effects of fruit and vegetables on type 2 diabetes. Dietary fiber was associated with insulin sensitivity, and improved the ability to delay the absorption of carbohydrates and secrete insulin adequately to overcome insulin resistance, resulting in lower postprandial blood glucose and insulin levels12, 43. A high intake of dietary fiber can promote the feeling of fullness and reduce the intake of energy‐dense foods, resulting in a reduced risk of overweight/obesity, which is an established risk factor for type 2 diabetes22, 44, 45. The aforementioned studies supported our main finding that consumption of fruit or vegetable fiber decreased the risk of type 2 diabetes.
Fruits and vegetables also contain polyphenols, including flavonoids and anti‐oxidant compounds (carotenoids, vitamin C and E) that could also decrease the risk of type 2 diabetes by mitigating the oxidative stress that interferes with the glucose uptake by cells46. Intake of anti‐oxidants has reportedly improved insulin sensitivity and lowered the risk of incident type 2 diabetes30, 47. Berries with high amounts of anthocyanins, thus, could have beneficial effects on glucose metabolism and bodyweight regulation19, 48, 49. Green leafy vegetables and yellow vegetables also contain polyphenols, such as vitamin C and carotenoids (a‐carotene, β‐carotene and lutein), and cruciferous vegetables contain substantial amounts of glucocinolates, which are known for their anti‐oxidant properties, in addition to vitamins A and K, folate, fatty acid, and magnesium content50, 51, 52, which further supports the intake of fruits, especially berries, and vegetables, as they are associated with a lower risk of type 2 diabetes in our research.
The previous epidemiological studies, or systematic reviews and meta‐analyses have generated somewhat mixed results for the association between the intake of fruit only, vegetables only, fruit and vegetables combined, fruit fiber, vegetable fiber or dietary fiber and the risk of type 2 diabetes. To our knowledge, no other systematic review and meta‐analysis involving only prospective studies has been carried out to examine the combined effects of fruit and vegetable intake as well as their fiber with the risk of type 2 diabetes.
Three previous independent meta‐analyses investigated the association between fruit and vegetable intake and the risk of incident type 2 diabetes11, 29, 30. Two meta‐analyses investigated the association between fiber intake and the risk of incident type 2 diabetes53, 54. However, compared with this current updated meta‐analysis, some results limited their superiority.
Our meta‐analysis showed that an increased consumption of fruit is associated with a reduced risk of type 2 diabetes. Not similar to the previous independent meta‐analyses, which were based on a limited number of studies and significant between‐study heterogeneity, the present results were similar to subgroup analysis results according to duration of follow up by Cooper29. In contrast to total fruit consumption, we also analyzed fruit type with the risk of type 2 diabetes, and found that the intake of berries in particular was inversely associated with the risk of type 2 diabetes. Furthermore, using the data from these high‐quality studies with scores of 7 or above, we further found that high fruit fiber intake reduced the risk of type 2 diabetes.
Similar to the previous independent meta‐analyses, we also found that there was no significant association between total vegetable intake and the risk of type 2 diabetes11, 29, 30. However, we discovered that increasing the intake of green leafy vegetables, yellow vegetables or cruciferous vegetables could help to reduce the risk of type 2 diabetes. Furthermore, we also found that high vegetable fiber intake reduced the risk of type 2 diabetes using the data from these studies with follow‐up period of 10 years or more.
Our meta‐analysis had some strengths. The present study included a large number of prospective cohort studies on the intake of fruit, vegetables and their fiber, and the risk of type 2 diabetes, which should eliminate selection bias and recall bias, and allowed for further subgroup analysis. Furthermore, all cohort studies were assessed for quality and validity using NOS, and most studies had high‐quality scores, with a large sample size and long duration of follow up, and maximum‐adjusted RR for risk of type 2 diabetes.
However, several limitations should also be considered. First, measurement errors were inevitable in the assessment of dietary intake and type 2 diabetes. The potential misclassification of individuals with undiagnosed type 2 diabetes might also have attenuated the present findings. Different studies used different methods of dietary assessment, such as 24‐h recall, dietary assessment interviews or Food Frequency Questionnaire. Different studies split dietary intake into different fractions – either thirds, quarters or fifths. Different studies also used different units of measurement. However, we only collected the data based on the highest vs lowest level of intake, and did not make further dose–response analysis or a standard for dietary measurement in future nutritional studies. Second, although we used the data based on the maximum‐adjusted RR for risk of type 2 diabetes, not all authors of the original studies made the same adjustments, in addition, residual or unknown confounding cannot be ruled out. Third, the possibility of publication bias is of a concern. Although there was no evidence of substantial publication bias by using funnel plots and Egger's regression test, it is noteworthy that there were more female cohorts than male cohorts in the original study populations included in our meta‐analysis. Further studies in male cohorts are required in future.
In conclusion, findings from the current meta‐analysis suggest that a higher intake of fruit, especially berries, as well as green leafy vegetables, yellow vegetables, cruciferous vegetables or their fiber is associated with a lower risk of type 2 diabetes. These results support recommendations on increasing consumption of fruit and vegetables for the primary prevention of many chronic diseases, including type 2 diabetes. However, large randomized controlled trials are required to confirm these findings, and further studies are required to explore potential mechanisms underlying the observed associations.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation (Nos. 81200601, 31371321, 31440061).
J Diabetes Investig 2016; 7: 56–69
References
- 1. Guariguata L, Whiting DR, Hambleton I, et al Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137–149. [DOI] [PubMed] [Google Scholar]
- 2. Whiting DR, Guariguata L, Weil C, et al IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94: 311–321. [DOI] [PubMed] [Google Scholar]
- 3. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14. [DOI] [PubMed] [Google Scholar]
- 4. Steyn NP, Mann J, Bennett PH, et al Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr 2004; 7: 147–165. [DOI] [PubMed] [Google Scholar]
- 5. Gillies CL, Abrams KR, Lambert PC, et al Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta‐analysis. BMJ 2007; 334: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pomerleau J, Lock K, McKee M. The burden of cardiovascular disease and cancer attributable to low fruit and vegetable intake in the European Union: differences between old and new Member States. Public Health Nutr 2006; 9: 575–583. [DOI] [PubMed] [Google Scholar]
- 7. van't Veer P, Jansen MC, Klerk M, et al Fruits and vegetables in the prevention of cancer and cardiovascular disease. Public Health Nutr 2000; 3: 103–107. [DOI] [PubMed] [Google Scholar]
- 8. Liu S, Lee IM, Ajani U, et al Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: The Physicians' Health Study. Int J Epidemiol 2001; 30: 130–135. [DOI] [PubMed] [Google Scholar]
- 9. Wang Q, Chen Y, Wang X, et al Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta‐analysis of cohort studies. Eur J Cancer 2014; 50: 1498–1509. [DOI] [PubMed] [Google Scholar]
- 10. Lock K, Pomerleau J, Causer L, et al The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ 2005; 83: 100–108. [PMC free article] [PubMed] [Google Scholar]
- 11. Carter P, Gray LJ, Troughton J, et al Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta‐analysis. BMJ 2010; 341: c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montonen J, Jarvinen R, Heliovaara M, et al Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 2005; 59: 441–448. [DOI] [PubMed] [Google Scholar]
- 13. Van Duyn MA, Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. J Am Diet Assoc 2000; 100: 1511–1521. [DOI] [PubMed] [Google Scholar]
- 14. Hofe CR, Feng L, Zephyr D, et al Fruit and vegetable intake, as reflected by serum carotenoid concentrations, predicts reduced probability of polychlorinated biphenyl‐associated risk for type 2 diabetes: National Health and Nutrition Examination Survey 2003‐2004. Nutr Res 2014; 34: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene 2004; 23: 6349–6364. [DOI] [PubMed] [Google Scholar]
- 16. Cooper AJ, Sharp SJ, Lentjes MA, et al A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care 2012; 35: 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knekt P, Kumpulainen J, Jarvinen R, et al Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002; 76: 560–568. [DOI] [PubMed] [Google Scholar]
- 18. Villegas R, Shu XO, Gao YT, et al Vegetable but not fruit consumption reduces the risk of type 2 diabetes in Chinese women. J Nutr 2008; 138: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wedick NM, Pan A, Cassidy A, et al Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012; 95: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muraki I, Imamura F, Manson JE, et al Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ 2013; 347: f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ford ES, Mokdad AH. Fruit and vegetable consumption and diabetes mellitus incidence among U.S. adults. Prev Med 2001; 32: 33–39. [DOI] [PubMed] [Google Scholar]
- 22. Harding AH, Wareham NJ, Bingham SA, et al Plasma vitamin C level, fruit and vegetable consumption, and the risk of new‐onset type 2 diabetes mellitus: the European prospective investigation of cancer–Norfolk prospective study. Arch Intern Med 2008; 168: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 23. Liu S, Serdula M, Janket SJ, et al A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care 2004; 27: 2993–2996. [DOI] [PubMed] [Google Scholar]
- 24. Hodge AM, English DR, O'Dea K, et al Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004; 27: 2701–2706. [DOI] [PubMed] [Google Scholar]
- 25. Bazzano LA, Li TY, Joshipura KJ, et al Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008; 31: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyer KA, Kushi LH, Jacobs DR, et al Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000; 71: 921–930. [DOI] [PubMed] [Google Scholar]
- 27. Mursu J, Virtanen JK, Tuomainen TP, et al Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2014; 99: 328–333. [DOI] [PubMed] [Google Scholar]
- 28. Kurotani K, Nanri A, Goto A, et al Vegetable and fruit intake and risk of type 2 diabetes: Japan Public Health Center‐based Prospective Study. Br J Nutr 2013; 109: 709–717. [DOI] [PubMed] [Google Scholar]
- 29. Cooper AJ, Forouhi NG, Ye Z, et al Fruit and vegetable intake and type 2 diabetes: EPIC‐InterAct prospective study and meta‐analysis. Eur J Clin Nutr 2012; 66: 1082–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta‐analysis. J Hypertens 2007; 25: 2361–2369. [DOI] [PubMed] [Google Scholar]
- 31. Chaiyakunapruk N, Saokaew S, Sruamsiri R, et al Systematic review and network meta‐analysis in health technology assessment. J Med Assoc Thai 2014; 97(Suppl 5): S33–S42. [PubMed] [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, et al Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001; 323: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hopping BN, Erber E, Grandinetti A, et al Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr 2010; 140: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salmeron J, Manson JE, Stampfer MJ, et al Dietary fiber, glycemic load, and risk of non‐insulin‐dependent diabetes mellitus in women. JAMA 1997; 277: 472–477. [DOI] [PubMed] [Google Scholar]
- 36. Stevens J, Ahn K, Juhaeri, et al Dietary fiber intake and glycemic index and incidence of diabetes in African‐American and white adults: the ARIC study. Diabetes Care 2002; 25: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 37. Colditz GA, Manson JE, Stampfer MJ, et al Diet and risk of clinical diabetes in women. Am J Clin Nutr 1992; 55: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 38. Montonen J, Knekt P, Järvinen R, et al Whole‐grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr 2003; 77: 622–629. [DOI] [PubMed] [Google Scholar]
- 39. Schulze MB, Liu S, Rimm EB, et al Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle‐aged women. Am J Clin Nutr 2004; 80: 348–356. [DOI] [PubMed] [Google Scholar]
- 40. Barclay AW, Flood VM, Rochtchina E, et al Glycemic index, dietary fiber, and risk of type 2 diabetes in a cohort of older Australians. Diabetes Care 2007; 30: 2811–2813. [DOI] [PubMed] [Google Scholar]
- 41. Wannamethee SG, Whincup PH, Thomas MC, et al Associations between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 2009; 32: 1823–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weng LC, Lee NJ, Yeh WT, et al Lower intake of magnesium and dietary fiber increases the incidence of type 2 diabetes in Taiwanese. J Formos Med Assoc 2012; 111: 651–659. [DOI] [PubMed] [Google Scholar]
- 43. Liese AD, Roach AK, Sparks KC, et al Whole‐grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr 2003; 78: 965–971. [DOI] [PubMed] [Google Scholar]
- 44. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients 2010; 2: 1266–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu S, Willett WC, Manson JE, et al Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle‐aged women. Am J Clin Nutr 2003; 78: 920–927. [DOI] [PubMed] [Google Scholar]
- 46. Gordon M. Dietary antioxidants in disease prevention. Nat Prod Rep 1996; 13: 265–273. [DOI] [PubMed] [Google Scholar]
- 47. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 2004; 24: 816–823. [DOI] [PubMed] [Google Scholar]
- 48. Salas‐Salvado J, Martinez‐Gonzalez MA, Bullo M, et al The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis 2011; 21(Suppl 2): B32–B48. [DOI] [PubMed] [Google Scholar]
- 49. Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav 2009; 97: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tarwadi K, Agte V. Potential of commonly consumed green leafy vegetables for their antioxidant capacity and its linkage with the micronutrient profile. Int J Food Sci Nutr 2003; 54: 417–425. [DOI] [PubMed] [Google Scholar]
- 51. McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr 2003; 90: 687–697. [DOI] [PubMed] [Google Scholar]
- 52. Hulbert AJ, Turner N, Storlien LH, et al Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc 2005; 80: 155–169. [DOI] [PubMed] [Google Scholar]
- 53. Yao B, Fang H, Xu W, et al Dietary fiber intake and risk of type 2 diabetes: a dose‐response analysis of prospective studies. Eur J Epidemiol 2014; 29: 79–88. [DOI] [PubMed] [Google Scholar]
- 54. Schulze MB, Schulz M, Heidemann C, et al Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta‐analysis. Arch Intern Med 2007; 167: 956–965. [DOI] [PubMed] [Google Scholar]


