Abstract
Dysregulation of gluconeogenesis is a key pathological feature of type 2 diabetes. However, the molecular mechanisms underlying the regulation of gluconeogenesis remain unclear. Bhalla et al. recently reported that cyclin D1 suppresses hepatic gluconeogenesis through CDK4‐dependent phosphorylation of PGC1alpha and consequent inhibition of its activity. The cyclin D1–CDK4 might thus serve as an important link between the cell cycle and control of energy metabolism through modulation of PGC1alpha activity.
Hepatic gluconeogenesis is necessary for maintenance of an appropriate blood glucose level and for the supply of glucose as an energy source to other tissues. On the other hand, dysregulation of gluconeogenesis is a key pathological feature of type 2 diabetes. The molecular mechanisms underlying the regulation of gluconeogenesis have therefore been extensively studied. Peroxisome proliferator–activated receptor γ coactivator 1α (PGC1α) is a transcriptional coactivator that plays an important role in the regulation of gluconeogenesis by supporting the activity of transcription factors such as forkhead box protein O1 (FoxO1) and hepatocyte nuclear factor 4α (HNF4α). The activity of PGC1α is itself modulated both at the level of protein abundance and by posttranslational modification such as phosphorylation, acetylation, and ubiquitination1. Several kinases including adenosine monophosphate‐activated protein kinase (AMPK), p38 mitogen‐activated protein kinase (MAPK), and Akt have thus been found to regulate such activity by mediating the phosphorylation of PGC1α at various amino acid residues1.
Cyclin D1 is a regulator of the cell cycle that promotes the transition from G1 to S phase by activating cyclin‐dependent kinase 4 (CDK4) or CDK6. Although the function of cyclin D1 in the liver has mostly been studied in the context of liver damage, regeneration, or carcinogenesis2, Bhalla et al.3 recently revealed that cyclin D1 represses hepatic gluconeogenesis and oxidative phosphorylation through inhibition of PGC1α activity in a CDK4‐dependent manner (Figure 1). These authors first speculated that cyclin D1 might contribute to the regulation of glucose metabolism, given that it is highly expressed in the liver under basal physiological conditions. They found that its expression also declines during fasting and increases after feeding. Bhalla et al.3 examined the effects of genetic or pharmacological loss‐of‐function of cyclin D1 or CDK4 in the liver of cyclin D1−/− mice, mouse embryonic fibroblasts from CDK4−/− mice, cells depleted of CDK4 by ribonucleic acid (RNA) interference, and cells treated with a specific inhibitor of cyclin D1–CDK4, as well as of genetic gain‐of‐function of cyclin D1 in mice that express a cyclin D1 transgene specifically in the liver or that overexpress cyclin D1 in the liver as a result of infection with an adenovirus vector. They found that cyclin D1–CDK4 suppresses the expression of genes related to gluconeogenesis and oxidative phosphorylation in the liver and is thereby able to influence blood glucose levels. These effects were achieved through CDK4‐dependent phosphorylation of PGC1α and consequent inhibition of its activity. In silico analysis identified two highly conserved putative phosphorylation sites for CDK4 (threonine‐298 and serine‐312) in the transcriptional repression domain of mouse PGC1α. Mutation of both of these residues to alanine abolished the inhibitory effect of cyclin D1–CDK4 on PGC1α activity, suggesting that phosphorylation of these sites is required for this effect. CDK4 thus now joins the list of kinases known to phosphorylate PGC1α and thereby to control the activity of this master regulator of energy metabolism. These kinases, including AMPK, p38 MAPK, and Akt, serve as sensors and effectors for cellular energy status, stress, and insulin. The demonstration that the cyclin D1–CDK4 complex targets PGC1α for regulation of gluconeogenesis suggests that this complex may serve as an important link between the cell cycle and control of energy metabolism through modulation of PGC1α activity.
Figure 1.
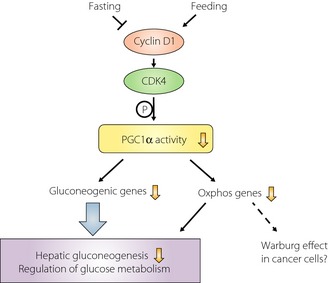
Regulation of glucose metabolism by cyclin D1–CDK4 via modulation of PGC1α activity. The expression of cyclin D1 in the liver is decreased during fasting and increased after feeding. Cyclin D1 inhibits the activity of the transcriptional coactivator PGC1α by promoting its CDK4‐dependent phosphorylation, resulting in down‐regulation of the expression of genes related to gluconeogenesis and oxidative phosphorylation (Oxphos). These effects on gene expression lead to suppression of hepatic gluconeogenesis and may contribute to the Warburg effect in hepatocellular carcinoma cells, respectively. CDK4; cyclin‐dependent kinase 4, PGC1α; peroxisome proliferator–activated receptor γ coactivator 1α.
Consistent with this theme of gluconeogenesis control by a cell cycle regulator, Bantubungi et al.4 recently showed that the CDK inhibitor p16INK4A also contributes to the regulation of hepatic gluconeogenesis. This protein and p14ARF are products of the CDKN2A and CDKN2B loci, respectively. Although genome‐wide association studies have identified a strong association between these loci and type 2 diabetes in various ethnic groups, how they affect glucose metabolism has been largely unclear. Bantubungi et al.4 found that genetic ablation of p16INK4A in mouse liver increased PGC1α expression and thereby promoted gluconeogenesis through activation of the protein kinase A (PKA)–cAMP response element binding protein (CREB) pathway. This finding contrasts with the results of Bhalla et al.3, however, in that it demonstrates repression of gluconeogenesis by a cell cycle inhibitor (p16INK4A) rather than by a cell cycle activator (cyclin D1–CDK4). It nevertheless provides another example of a molecular link between the cell cycle and regulation of energy metabolism via PGC1α.
The liver‐specific cyclin D1 transgenic mice studied by Bhalla and coworkers3 develop hepatocellular carcinoma by an age of ~15 months. These researchers suggest that the regulation of PGC1α activity by cyclin D1–CDK4 might contribute to the molecular mechanism of the Warburg effect—the metabolism of glucose in cancer cells predominantly via glycolysis rather than via oxidative phosphorylation even in the presence of oxygen5. Down‐regulation of the expression of genes related to oxidative phosphorylation as a result of the inhibition of PGC1α activity by cyclin D1–CDK4 might thus explain, at least in part, the Warburg effect apparent in the hepatocellular carcinoma of the cyclin D1 transgenic mice (Figure 1).
The mechanism responsible for the changes in the hepatic expression of cyclin D1 in response to fasting and refeeding observed by Bhalla et al.3 remains unclear. Given that insulin and glucagon are the major regulators of glucose metabolism in the liver during fasting and refeeding, determination of the effects of these two hormones on cyclin D1 expression in the liver should add to our understanding of the control of glucose metabolism in this organ. These authors also showed that hepatic expression of cyclin D1 is down‐regulated in individuals with diabetes compared with nondiabetic subjects, implicating cyclin D1 in the pathogenesis of diabetes. Whether attenuation of cyclin D1–CDK4 activity might ameliorate the defects in glucose metabolism in animal models of diabetes warrants investigation.
The study of Bhalla et al.3 thus identifies cyclin D1–CDK4 as a potential target for the treatment of type 2 diabetes, although close attention will need to be paid to the potential carcinogenic effects of this cell cycle regulatory complex. Further investigations may provide a firmer basis for the development of novel antidiabetes drugs that target the control of PGC1α activity by cell cycle regulators.
Disclosure
The authors declare no conflict of interest.
References
- 1. Fernandez‐Marcos PJ, Auwerx J. Regulation of PGC‐1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 2011; 93(Suppl): 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Musgrove EA, Caldon CE, Barraclough J, et al Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 2011; 11: 558–572. [DOI] [PubMed] [Google Scholar]
- 3. Bhalla K, Liu WJ, Thompson K, et al Cyclin D1 represses gluconeogenesis via inhibition of the transcriptional coactivator PGC1α. Diabetes 2014; 63: 3266–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bantubungi K, Hannou SA, Caron‐Houde S, et al Cdkn2a/p16Ink4a regulates fasting‐induced hepatic gluconeogenesis through the PKA‐CREB‐PGC1α pathway. Diabetes 2014; 63: 3199–3209. [DOI] [PubMed] [Google Scholar]
- 5. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11: 85–95. [DOI] [PubMed] [Google Scholar]


