Abstract
Introduction
To examine the long‐term efficacy and safety of duloxetine in the treatment of Japanese patients with diabetic neuropathic pain, we carried out a 52‐week, randomized, open‐label extension of a 12‐week, double‐blind, placebo‐controlled study.
Materials and Methods
Japanese adults with diabetic neuropathic pain who completed the double‐blind study were eligible for this long‐term study, carried out at 71 sites in Japan (March 2008 to March 2010). Participants (n = 258) were re‐randomized (1:1) to 40 mg/day or 60 mg/day duloxetine. Pain (Brief Pain Inventory severity and interference), quality of life (Patient's Global Impression of Improvement), and safety (primary outcome; adverse events, vital signs, metabolic measures) were measured.
Results
Significant (P < 0.0001) and sustained improvements (change ± standard deviation; n = 257) were observed in Brief Pain Inventory severity (average pain score −2.1 ± 1.7). Improvements were also seen in Brief Pain Inventory interference (mean of subscores −0.96 ± 1.52) and Patient's Global Impression of Improvement (−0.9 ± 1.1) scores; these scores decreased significantly (P < 0.0001) during the long‐term study. Frequently reported adverse events included somnolence (13.6%), constipation (13.2%) and nausea (10.5%). Increases were observed in plasma glucose, glycosylated hemoglobin and total cholesterol levels, and in bodyweight and heart rate; however, none of these were clinically meaningful. Overall, there were no clinically significant safety concerns.
Conclusions
This is the first publication of a long‐term study carried out in Asia with an entirely Japanese patient population to suggest that long‐term duloxetine therapy for diabetic neuropathic pain is effective and has an acceptable safety profile.
Keywords: Diabetes‐related complications, Diabetic neuropathy (painful), Duloxetine
Introduction
Diabetic peripheral neuropathy is a nerve disorder complication of diabetes, affecting up to 47% of patients with diabetes1. The socioeconomic burden of diabetic complications is increasing rapidly in Asia2, and Japan is expected to remain one of the top 10 countries worldwide for diabetes prevalence in 20303. Up to 22% of patients with diabetes experience chronic diabetic neuropathic pain (DNP)4, 5. Symptoms of DNP include aching, burning, tingling and stabbing sensations6, and the condition is associated with decreased quality of life, sleep impairment, anxiety and depression7, 8, 9. Pharmacological treatments for DNP focus on pain relief, and include tricyclic antidepressants, anticonvulsants and serotonin noradrenaline reuptake inhibitors10, 11. However, the efficacy of some treatments can be compromised by adverse events, including cardiovascular complications, weight gain and hyperglycemia12, 13, 14.
Duloxetine, which selectively inhibits serotonin and noradrenaline reuptake from the central nervous system with relatively balanced affinity15, has shown promising efficacy and safety for the treatment of DNP16, 17, 18, 19, 20, 21, 22. Duloxetine provides relief from DNP16, 17, 19, 23, and has been associated with improvements in quality of life19 and cognitive function16. Both short‐ and long‐term duloxetine treatment have been reported as having generally favorable safety profiles17, 18, 21, 23, 24. However, duloxetine treatment has been associated with changes in bodyweight, fasting blood glucose and glycosylated hemoglobin (HbA1c) levels, particularly after longer treatment19, 25.
In Japan, duloxetine was approved for the treatment of DNP in February 2012, and was shown as being superior to placebo in a 12‐week, double‐blind, randomized, placebo‐controlled study of DNP in Japanese patients22. The primary efficacy outcome of 24‐h average pain score was significantly improved for patients treated with duloxetine (40 or 60 mg/day) compared with placebo, and no clinically important safety concerns were noted22. To examine long‐term duloxetine treatment in Japanese adults with DNP, we carried out a randomized, 52‐week, open‐label extension of that study. This is the first publication to present efficacy and safety (primary outcome) results from a long‐term study carried out in Asia with entirely Japanese patients.
Materials and Methods
Study Design
This was a randomized, 52‐week, open‐label study (ClinicalTrials.gov no. NCT00641719) carried out at 71 sites from March 2008 to March 2010, which recruited participants who completed a 12‐week, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, phase 3 study (NCT005‐52175)22. Both studies were approved by the ethical review board at each site, and carried out in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and all applicable laws and regulations. All participants provided written informed consent before any study‐related procedures.
Study Population
Participants who completed the double‐blind study and whose most recent HbA1c value was ≤9.4% were invited to enrol in the long‐term study. As described previously22, the double‐blind study included Japanese adults with sustained pain for at least 6 months as a result of polyneuropathy caused by diabetes. All participants met the Diabetic Neuropathy Study Group of Japan criteria for diabetic peripheral neuropathy26. Individuals were excluded if they had a neurological disorder unrelated to diabetic peripheral neuropathy or a psychiatric disease, or if they were taking drug treatments other than acetaminophen or insulin.
Treatment Protocol
The protocol for the double‐blind study has been described elsewhere22. Briefly, participants were randomized to receive duloxetine (Cymbalta®; Eli Lilly Japan K.K, Kobe, Japan, and Shionogi & Co. Ltd, Osaka, Japan) or placebo for 12 weeks. In the long‐term study described here, all participants, regardless of treatment during the double‐blind study, were re‐randomized (1:1) to receive duloxetine 40 mg/day or 60 mg/day. Participants were re‐randomized using a stochastic minimization method taking into account the following factors from the double‐blind study: (i) treatment group; (ii) improvement in Brief Pain Inventory (BPI) severity – average pain score of >30% or <30%; and (iii) investigational site.
The present long‐term study included tapering and treatment periods. Participants took duloxetine 20 mg/day during week 1, 40 mg/day during week 2, 40 or 60 mg/day (depending on treatment group) during weeks 3–50, 40 mg/day during week 51 and 20 mg/day during week 52.
Study visits occurred biweekly during the first 4 weeks and every 4 weeks thereafter. Study visits also occurred at weeks 50 and 51 for the 60 mg/day and 40 mg/day treatment groups, respectively, before the final study visit at week 52 (or at discontinuation). Baseline was defined as the measurement obtained immediately before the start of duloxetine or placebo treatment in the double‐blind study. The study end‐point was the last available observation. The maximum duration of treatment with duloxetine was 52 weeks for participants who had received a placebo during the double‐blind study and 65 weeks for participants who had received duloxetine during both studies.
Efficacy Measures
The efficacy outcomes in this long‐term study were pain and quality of life. Pain was measured by the participant‐rated BPI severity (from 0 [no pain] to 10 [pain as bad as you can imagine]) and interference (from 0 [does not interfere] to 10 [completely interferes]) scales22, 27. The BPI severity subscores included average pain, worst pain, least pain and current pain. The BPI interference scale measured patient functionalities, including general activity, mood, walking, work, relations with others, sleep, enjoyment of life and the mean of these seven items. Quality of life was also evaluated by the Patient's Global Impression of Improvement (PGI‐I) scale (from 1 [very much better] to 7 [very much worse])28.
Safety/Tolerability Measures
The primary outcome of the long‐term study was the safety/tolerability of duloxetine treatment. This was determined by the frequencies of adverse events (AEs), adverse drug reactions (ADRs) and serious adverse events (SAEs), the rate of discontinuation as a result of AEs, and the severity of AEs (mild, moderate or severe). Events that occurred after the start of the long‐term study or that continued from the double‐blind study were counted as AEs. All AEs were coded and summarized using the Medical Dictionary for Regulatory Activities, Version 11.1. Adverse drug reactions were AEs judged by the investigator to be definitely related, probably related or possibly related to the study drug. The change from the start of the double‐blind study to the end of the long‐term study in metabolic measures (fasting plasma glucose, HbA1c, lipid profile), vital signs (heart rate, systolic blood pressure, diastolic blood pressure), bodyweight and electrocardiogram (defined as normal, borderline or abnormal by the investigator) were also determined. HbA1c was measured according to recommendations from the Japanese Diabetes Society (JDS) and converted to National Glycohemoglobin Standardization Program (NGSP) equivalent values (NGSP %HbA1c = [1.02 × JDS %HbA1c] + 0.25%)29.
Statistical Analysis
Participants were included in the efficacy analysis if they had at least one assessment after the start of the long‐term study, and in the safety analysis if they had taken at least one dose of duloxetine. Efficacy results were also stratified by treatment group in the previous double‐blind study. Values for mean and standard deviation (SD) were calculated for efficacy measures, laboratory measures, and vital signs. Summary statistics were calculated for the change from the start of the double‐blind study (vital signs, metabolic measures, bodyweight) or from the start of the long‐term study (efficacy measures) to the last observation during treatment. Changes in efficacy and laboratory measures were analyzed by Wilcoxon's signed‐rank test. Statistical significance was set at P < 0.05. Analyses were carried out using SAS® Version 9.1.3 (SAS Institute Inc, Cary, NC, USA).
Results
Participant Disposition and Characteristics
In the double‐blind study, 448 potential participants were screened, 339 were randomized and 293 completed the study (Figure 1)22. A total of 258 participants enrolled in the long‐term study, and were re‐randomized to receive duloxetine 40 mg/day (129 participants) or 60 mg/day (129 participants; Figure 1). A total of 99 (77%) and 91 (71%) participants in the 40 mg/day and 60 mg/day groups, respectively, completed the long‐term study. The characteristics of participants in each group in the long‐term study were broadly similar (Table 1).
Figure 1.
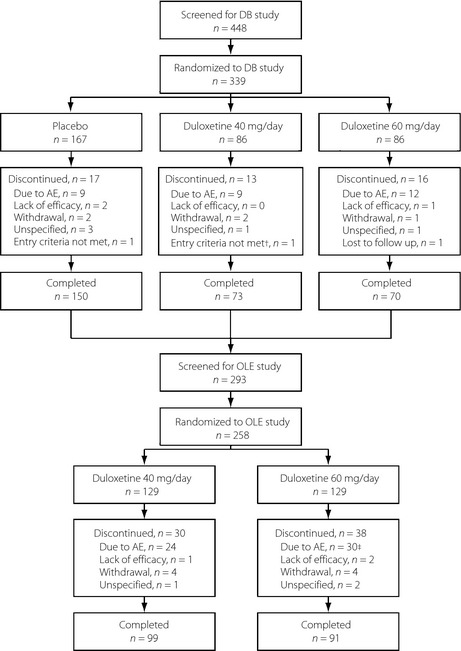
Participant flow diagram for the double‐blind (DB) and open‐label extension (OLE) studies. †One participant did not receive any study drug and was not assessed at any study visits after the start of the double‐blind study; this participant was excluded from the analysis. ‡One participant discontinued during the taper phase, not the treatment phase. AE, adverse event.
Table 1.
Participant demographics and characteristics at baseline
| Characteristic | Duloxetine 40 mg/day (n = 129) | Duloxetine 60 mg/day (n = 129) | Combined Duloxetine (n = 258) |
|---|---|---|---|
| Mean age (years) | 60.2 ± 10.5 | 60.0 ± 9.6 | 60.1 ± 10.0 |
| Male, n (%) | 89 (69.0) | 107 (82.9) | 196 (76.0) |
| Mean weight (kg) | 63.9 ± 12.1 | 65.0 ± 11.6 | 64.5 ± 11.8 |
| Duration of diabetes, n (%) | |||
| <5 years | 23 (17.8) | 25 (19.4) | 48 (18.6) |
| 5–10 years | 27 (20.9) | 23 (17.8) | 50 (19.4) |
| ≥10 years | 76 (58.9) | 79 (61.2) | 155 (60.1) |
| Unknown | 3 (2.3) | 2 (1.6) | 5 (1.9) |
| Type of diabetes, n (%) | |||
| Type 1 | 8 (6.2) | 4 (3.1) | 12 (4.7) |
| Type 2 | 121 (93.8) | 125 (96.9) | 246 (95.3) |
| Mean duration of DPN (years) | 3.9 ± 3.2 | 4.3 ± 3.9 | 4.1 ± 3.6 |
| Mean HbA1c (%)a | 7.02 ± 0.93 | 7.17 ± 0.87 | 7.09 ± 0.90 |
| Mean plasma glucose (mg/dL) | 145.5 ± 57.8 | 148.7 ± 61.3 | 147.1 ± 59.5 |
Glycosylated hemoglobin (HbA1c) was measured according to recommendations from the Japanese Diabetes Society and converted to National Glycohemoglobin Standardization Program equivalent values (%HbA1c = [1.02 × Japanese Diabetes Society %HbA1c] + 0.25%)29. DPN, diabetic peripheral neuropathy; HbA1c, glycosylated hemoglobin; SD, standard deviation.
Efficacy Outcome Measures
Pain
The severity of pain, as rated by participants, and the interference caused by that pain, improved during this long‐term study. The BPI severity – average pain score and all other pain subscores improved significantly (P < 0.0001) from the start of the long‐term study to the end‐point (Figure 2; Table S1). The BPI severity – average pain score decreased from the first week of the study (potentially because some participants had received a placebo in the previous double‐blind study) and continued to decline throughout the study period (Figure 3). Changes in the other BPI severity and interference subscores followed a similar time‐course (data not shown). There was no difference between the 40 mg/day and 60 mg/day groups for any BPI measurement of pain severity subscores (Table S1). However, there were greater improvements in most, but not all, measures of BPI severity in participants who had received a placebo in the previous double‐blind study compared with participants who had received duloxetine (Table S1).
Figure 2.
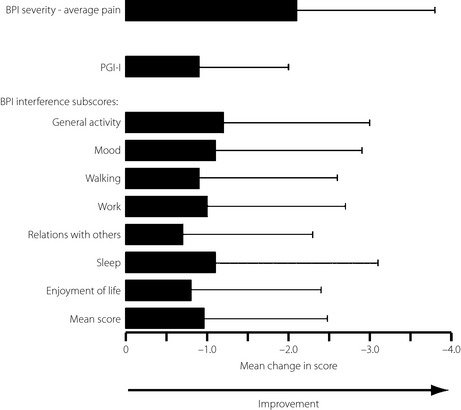
Mean (±standard deviation) change from the start of the long‐term study in Brief Pain Inventory (BPI) severity – average pain score, Patient's Global Impression of Improvement (PGI‐I) score and BPI interference subscores at the end of the long‐term study. Results from the combined duloxetine group (40 mg/day and 60 mg/day, n = 257) are presented. All scores decreased significantly from the start of the long‐term study (P < 0.0001 for all scores; Wilcoxon's signed‐rank test).
Figure 3.
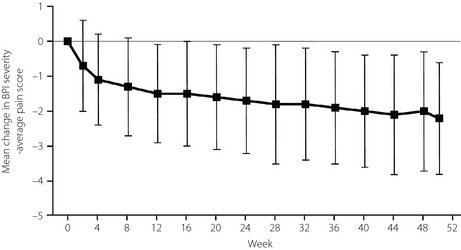
Mean (±standard deviation) change in Brief Pain Inventory (BPI) severity – average pain scores during the long‐term study (50/51 weeks). Results from the combined duloxetine group (40 mg/day and 60 mg/day, n = 191–258) are presented.
Quality of Life
Patient functionality was assessed by BPI interference scores. All BPI interference subscores decreased significantly from the start of the long‐term study, showing improvements in general activity, mood, walking, work, relations with others, sleep and enjoyment of life (Figure 2), and continued to decline throughout the study period. All changes in BPI interference scores were similar regardless of treatment group in the double‐blind study (Table S1). The quality of life of participants was mainly measured by PGI‐I (Figure 2). The PGI‐I scores significantly (P < 0.0001) decreased from the start of the long‐term study and continued to decline slowly throughout the study. There was no difference between the 40 mg/day and 60 mg/day groups (Table S1). However, there were greater improvements in PGI‐I in participants who had received a placebo in the previous double‐blind study compared with participants who had received duloxetine (Table S1). In addition, the proportion of participants reporting improvements in quality of life increased during the long‐term study (Figure 4).
Figure 4.
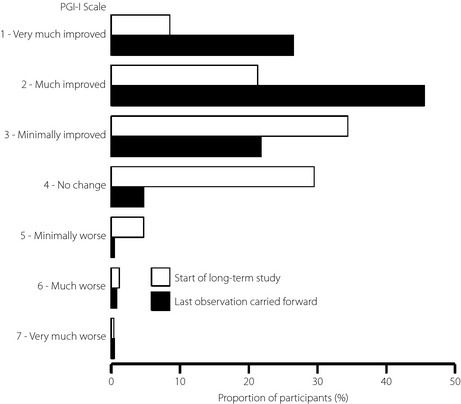
Proportion of participants at each Patient's Global Impression of Improvement (PGI‐I) scale level at the start (n = 258) and at the end of the long‐term study (last observation carried forward; n = 257). Results from the combined duloxetine group (40 mg/day and 60 mg/day) are presented.
Safety and Tolerability
Incidence of Adverse Events
Most participants (248/258; 96.1%) in the present long‐term study experienced an AE. As in the double‐blind study22, there was no significant difference in the incidence of AEs between the 40 mg/day (126/129; 97.7%) and 60 mg/day (122 of 129; 94.6%) groups. Overall, 54 (20.9%) of 258 participants in the long‐term study discontinued because of an AE (Figure 1); one of these participants (60 mg/day group) discontinued during the taper phase (i.e., after finishing the full dose), not during the treatment phase.
In the present long‐term study, there were 12 SAEs in 11 participants in the 40 mg/day group and 34 SAEs in 22 participants in the 60 mg/day group. One participant in the 40 mg/day group died as a result of myocardial ischemia, which was considered unrelated to treatment. There were 11 ADRs in the long‐term study: cerebral infarction, lymphoma and colon cancer in the 40 mg/day group, and generalized edema, cardiac failure, clavicle fracture, thoracic vertebral fracture, spinal compression fracture, lung injury, pneumothorax and gastric cancer in the 60 mg/day group.
Common Adverse Events
In the previous double‐blind study, several AEs occurred in at least 5% of duloxetine‐treated participants, and were at least twice as common in these participants than in placebo‐treated participants. These AEs included the symptoms somnolence, nausea, dizziness, malaise and vomiting, and the laboratory measures increased aspartate aminotransferase and increased white blood cell count22. The incidence of these AEs during the long‐term study (Table 2) was generally similar to the incidence during the double‐blind study22. Other common AEs during the long‐term study, experienced by >10% of participants, were nasopharyngitis (67 participants; 26.0%), increased HbA1c (60; 23.3%), constipation (34; 13.2%), increased blood glucose (26; 10.1%) and hypoglycemia (26; 10.1%). Only one participant experienced nasopharyngitis that was possibly related to the study drug. The incidence of these AEs during the double‐blind study was similar in both the duloxetine and placebo groups22.
Table 2.
Incidence of adverse events during the long‐term study
| Preferred terma | Duloxetine 40 mg/day (n = 129) | Duloxetine 60 mg/day (n = 129) | Combined Duloxetine (n = 258) |
|---|---|---|---|
| Symptoms | |||
| Somnolence |
17 (17) 13.2% |
18 (18) 14.0% |
35 (35) 13.6% |
| Nausea |
14 (16) 10.9% |
13 (15) 10.1% |
27 (31) 10.5% |
| Dizziness |
10 (12) 7.8% |
8 (11) 6.2% |
18 (23) 7.0% |
| Malaise |
6 (6) 4.7% |
5 (5) 3.9% |
11 (11) 4.3% |
| Vomiting |
9 (11) 7.0% |
10 (12) 7.8% |
19 (23) 7.4% |
| Laboratory measures | |||
| AST increased |
13 (13) 10.1% |
12 (14) 9.3% |
25 (27) 9.7% |
| WBC count increased |
13 (16) 10.1% |
8 (8) 6.2% |
21 (24) 8.1% |
According to the Medical Dictionary for Regulatory Activities, Version 11.1. AST, aspartate aminotransferase; WBC, white blood cell.
Data are presented as number of participants, (number of events), and percentage incidence. The table presents events that were experienced by at least 5% of participants in any group during the double‐blind study and were at least twice as common in the combined duloxetine group compared with the placebo group22.
Most AEs were mild (824/1,914; 43.1%) or moderate (1,055/1,914; 55.1%) in severity and occurred during the first week of treatment (data not shown). The proportion of moderate AEs was somewhat higher in the long‐term study than in the double‐blind study (30.3%)22.
Vital Signs and Metabolic Measures
Vital signs and metabolic measures were assessed from the start of the double‐blind study (baseline) to the end of the long‐term study. There were no clinically significant changes in vital signs, electrocardiogram or bodyweight. There were small, but statistically significant, increases in heart rate (mean change +2.6 b.p.m.; n = 242; P = 0.0009) and bodyweight (mean change, +1.4 kg; n = 241; P < 0.0001). There was a significant increase in HbA1c (mean [25th percentile, 75th percentile]) from baseline to end‐point (change +0.45% [−0.10, 0.92]; P < 0.0001; n = 240). There were also significant increases (mean [25th percentile, 75th percentile]) in plasma glucose (+18.4 [−19.5, 59.0] mg/dL; P < 0.0001), total cholesterol (+6.4 [−11.0, 29.0] mg/dL; P = 0.0004), and low‐density lipoprotein cholesterol (+2.1 [−11.0, 19.0] mg/dL; P = 0.0439; n = 240 for all measures). There were no significant changes in high‐density lipoprotein cholesterol or triglycerides.
Discussion
This is the first publication of a long‐term (52‐week), randomized, open‐label study carried out in Asia entirely in Japanese patients that evaluated the efficacy and safety of duloxetine in adults with DNP. Long‐term duloxetine treatment was associated with sustained improvements in pain severity, consistent with findings from other studies. For example, 6 months of duloxetine treatment in Caucasian participants with DNP was associated with improvements in BPI severity and the Clinical Global Impression of Severity scores30. Although that study was shorter than the present long‐term study, the improvement in pain was similar. Together, these studies suggest that duloxetine continues to be analgesic over the long term in patients with DNP. Notably, the improvement in pain during our long‐term study was observed regardless of the treatment participants had received during the double‐blind study.
Patients with DNP typically have impaired quality of life, which might be related to the extent and severity of pain8. Participants in the present study reported improved quality of life after long‐term duloxetine treatment, consistent with a 12‐month study in Caucasian patients (in which quality of life was assessed using the 36‐Item Short Form Health Survey and European Quality of Life Instrument‐5D)23. Participants in our study also reported significant improvements in BPI interference, an indirect measure of quality of life. Although the improvements in interference were smaller than those in a 6‐month study30, the present study used lower doses of duloxetine than the shorter study (120 mg/day). We believe the improvements in pain severity and interference experienced by the present study participants contributed to a sustained improvement in quality of life.
Our findings show that duloxetine has an acceptable safety and tolerability profile in Japanese adults with DNP, consistent with findings from long‐term studies in Caucasians23, 24. The incidence of AEs was relatively high in both the previous double‐blind study22 and the current long‐term study, again consistent with previous studies18, 21, 23, 24, 25. However, the rate of discontinuation as a result of an AE in our long‐term study (20.9%) was greater than that among duloxetine‐treated participants in both the 12‐week, double‐blind study (12.2%)22 and in other 52‐week studies (5.6%23 and 9.3%24). This result suggests that Japanese patients might be more inclined to discontinue as a result of AEs during long‐term duloxetine treatment. However, post‐hoc analyses of data from the double‐blind study show that the most common AEs experienced by duloxetine‐treated participants were not persistent. The majority (77.6%) of somnolence, nasopharyngitis and nausea occurrences resolved during the 12‐week study, with mean (±SD) durations of 22.6 ± 27.1 days, 14.7 ± 12.4 days and 10.8 ± 13.2 days, respectively.
As noted, there were no significant or remarkable differences between dose groups in the efficacy or safety of duloxetine in the present long‐term study. Given the similar efficacy between the dose groups, the recommended optimal dose of duloxetine for the treatment of DNP in Japanese patients is considered to be the lower dose of 40 mg/day, at least for 1 year of the dosing period. However, if efficacy is insufficient, the dose might be increased to 60 mg/day with minimal additional risk of adverse events.
In the present study, long‐term duloxetine treatment was associated with changes in metabolic measures, particularly HbA1c and blood glucose, which were greater than those reported for other 52‐week studies of duloxetine23, 24. However, the observed increase in HbA1c (0.45%) was below the level at which a clinically meaningful change is likely (0.5%)31. The increase in HbA1c in the long‐term study contrasts with that of the 12‐week, double‐blind study (placebo 0.10%; duloxetine 0.06%)22, suggesting that weekly visits during the double‐blind study might have provided a better opportunity to control participants’ diabetes than the monthly visits during the long‐term study. In addition, increased HbA1c is generally associated with long‐term, rather than short‐term, duloxetine treatment25, and could result from progression of diabetes rather than from duloxetine treatment itself. Similarly, the bodyweight changes in our long‐term study were consistent with changes seen in other long‐term duloxetine studies25, 32, and, like HbA1c, could be related to diabetes progression rather than duloxetine.
Management of elderly patients with DNP can be complicated by comorbidities and concomitant medications, and the safety and tolerability of some treatments are of particular concern in this population33. To examine the efficacy and safety of duloxetine in the elderly population, we carried out subgroup analyses of data from the double‐blind study. Among elderly participants (aged ≥65 years), the improvement in 24‐h average pain score was significantly greater (P < 0.001) in those treated with duloxetine (n = 62) than in those treated with placebo (n = 53). Furthermore, the incidence of AEs among elderly participants was similar to that of the overall study population, and no SAEs occurred in this subgroup. Together with the results of other studies33, these results suggest that duloxetine might be an effective and well‐tolerated option for the treatment of elderly patients with DNP, including those from Asia.
The present study is strengthened by the duration of treatment, the involvement of multiple study centers and the exclusive enrolment of Japanese participants with DNP, which allowed evaluation of long‐term duloxetine treatment for the first time in an Asian population. In addition, participants with psychiatric diseases requiring pharmacotherapy were excluded, thereby controlling for the potential confounding effects of treatment with antidepressants. However, the open‐label design and lack of placebo or routine care control group are limitations of the long‐term study. Also, because participants were re‐randomized in the long‐term study, the participants’ total exposure to duloxetine varied.
In conclusion, the present findings suggest that duloxetine is an effective long‐term therapy with an acceptable safety and tolerability profile in Japanese patients with DNP. Given that all treatments for DNP are symptomatic only11, duloxetine could be a suitable long‐term treatment option, because it was associated with improvements in both pain severity and the quality of life of patients.
Disclosure
HY has received research funding, consultancy fees, and speaker fees from Shionogi & Co. Ltd and Eli Lilly Japan K.K. NH has received consultancy fees and speaker fees from Shionogi & Co. Ltd and Eli Lilly Japan K.K. MK has received research funding, consultancy fees, and speaker fees from Shionogi & Co. Ltd and Eli Lilly Japan K.K. AK has received research funding, consultancy fees, and speaker fees from Shionogi & Co. Ltd and Eli Lilly Japan K.K. RK has received research funding, consultancy fees, and speaker fees from Shionogi & Co. Ltd and Eli Lilly Japan K.K. TY and YB are employees of Shionogi & Co. Ltd. LA and KN are employees of Eli Lilly Japan K.K.
Supporting information
Table S1 ¦ Change in efficacy measures from the start of the long‐term study to the end of treatment (overall and stratified by treatment group during the double‐blind study).
Acknowledgments
The authors are deeply grateful to the 84 primary investigators, subinvestigators and staff at the 73 study sites, and all the patients who participated in this study. This study was sponsored by Eli Lilly Japan K.K, the licensee, with Shionogi & Co. Ltd, of Cymbalta® in Japan. In compliance with the Uniform Requirements for Manuscripts, established by the International Committee of Medical Journal Editors, the sponsor of this study did not impose any impediment, directly or indirectly, on the publication of the study's results. Medical writing assistance was provided by Rebecca Lew, PhD, CMPP, and Janelle Keys, PhD, of ProScribe – part of the Envision Pharma Group, funded by Eli Lilly Japan K.K. ProScribe's services complied with international guidelines for Good Publication Practice (GPP2). Eli Lilly Japan K.K. and Shionogi & Co. Ltd were involved in the study design, data collection, data analysis, and preparation of the manuscript.
J Diabetes Investig 2016; 7: 100–108
References
- 1. Barrett AM, Lucero MA, Le T, et al Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med 2007; 8(Suppl 2): S50–S62. [DOI] [PubMed] [Google Scholar]
- 2. Chan JC, Chan SP, Deerochanawong C, et al Diabetic dyslipidaemia in Asian populations in the Western Pacific Region: what we know and don't know. Diabetes Res Clin Pract 2011; 94: 1–13. [DOI] [PubMed] [Google Scholar]
- 3. Wild S, Roglic G, Green A, et al Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 4. Hanaoka K, Ogawa S, Hotta N, et al Current status of neuropathic pain treatment in Japan and scope in future – proposal from the consensus conference of specialists. Pain Clinic 2009; 30: 1395–1408 (Japanese). [Google Scholar]
- 5. Sadosky A, McDermott AM, Brandenburg NA, et al A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract 2008; 8: 45–56. [DOI] [PubMed] [Google Scholar]
- 6. Boulton AJ, Vinik AI, Arezzo JC, et al Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962. [DOI] [PubMed] [Google Scholar]
- 7. Gore M, Brandenburg NA, Dukes E, et al Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005; 30: 374–385. [DOI] [PubMed] [Google Scholar]
- 8. Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health‐related quality of life: review and implications. Neurology 2007; 68: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 9. Zelman DC, Brandenburg NA, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain 2006; 22: 681–685. [DOI] [PubMed] [Google Scholar]
- 10. Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig 2011; 2: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tesfaye S, Vileikyte L, Rayman G, et al Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev 2011; 27: 629–638. [DOI] [PubMed] [Google Scholar]
- 12. Lustman PJ, Griffith LS, Clouse RE, et al Effects of nortriptyline on depression and glycemic control in diabetes: results of a double‐blind, placebo‐controlled trial. Psychosom Med 1997; 59: 241–250. [DOI] [PubMed] [Google Scholar]
- 13. Ray WA, Meredith S, Thapa PB, et al Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther 2004; 75: 234–241. [DOI] [PubMed] [Google Scholar]
- 14. Zimmermann U, Kraus T, Himmerich H, et al Epidemiology, implications and mechanisms underlying drug‐induced weight gain in psychiatric patients. J Psychiatr Res 2003; 37: 193–220. [DOI] [PubMed] [Google Scholar]
- 15. Bymaster FP, Dreshfield‐Ahmad LJ, Threlkeld PG, et al Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology 2001; 25: 871–880. [DOI] [PubMed] [Google Scholar]
- 16. Boyle J, Eriksson ME, Gribble L, et al Randomized, placebo‐controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care 2012; 35: 2451–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstein DJ, Lu Y, Detke MJ, et al Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005; 116: 109–118. [DOI] [PubMed] [Google Scholar]
- 18. Raskin J, Pritchett YL, Wang F, et al A double‐blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005; 6: 346–356. [DOI] [PubMed] [Google Scholar]
- 19. Skljarevski V, Frakes EP, Sagman D, et al Review of efficacy and safety of duloxetine 40 to 60 mg once daily in patients with diabetic peripheral neuropathic pain. Pain Res Treat 2012; 2012: 898347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanenberg RJ, Irving GA, Risser RC, et al Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open‐label, randomized, noninferiority comparison. Mayo Clin Proc 2011; 86: 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wernicke JF, Pritchett YL, D'Souza DN, et al A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006; 67: 1411–1420. [DOI] [PubMed] [Google Scholar]
- 22. Yasuda H, Hotta N, Nakao K, et al Superiority of duloxetine to placebo in improving diabetic neuropathic pain: results of a randomized controlled trial in Japan. J Diabetes Investig 2011; 2: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wernicke JF, Wang F, Pritchett YL, et al An open‐label 52‐week clinical extension comparing duloxetine with routine care in patients with diabetic peripheral neuropathic pain. Pain Med 2007; 8: 503–513. [DOI] [PubMed] [Google Scholar]
- 24. Raskin J, Smith TR, Wong K, et al Duloxetine versus routine care in the long‐term management of diabetic peripheral neuropathic pain. J Palliat Med 2006; 9: 29–40. [DOI] [PubMed] [Google Scholar]
- 25. Hall JA, Wang F, Oakes TM, et al Safety and tolerability of duloxetine in the acute management of diabetic peripheral neuropathic pain: analysis of pooled data from three placebo‐controlled clinical trials. Expert Opin Drug Saf 2010; 9: 525–537. [DOI] [PubMed] [Google Scholar]
- 26. Diabetic Neuropathy Study Group . Abbreviated diagnostic criteria for distal symmetric polyneuropathy. Peripher Nerv 2003; 14: 225 (Japanese). [Google Scholar]
- 27. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore 1994; 23: 129–138. [PubMed] [Google Scholar]
- 28. Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication, National Institute of Mental Health, Rockville, 1976; 129–138. [Google Scholar]
- 29. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raskin J, Wang F, Pritchett YL, et al Duloxetine for patients with diabetic peripheral neuropathic pain: a 6‐month open‐label safety study. Pain Med 2006; 7: 373–385. [DOI] [PubMed] [Google Scholar]
- 31. National Institute for Health and Clinical Excellence (NICE) . Short Clinical Guideline 87. Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0033486/. Effective May 2009. Accessed 13 March 2013. [PubMed]
- 32. Hardy T, Sachson R, Shen S, et al Does treatment with duloxetine for neuropathic pain impact glycemic control? Diabetes Care 2007; 30: 21–26. [DOI] [PubMed] [Google Scholar]
- 33. Wasan AD, Ossanna MJ, Raskin J, et al Safety and efficacy of duloxetine in the treatment of diabetic peripheral neuropathic pain in older patients. Curr Drug Saf 2009; 4: 22–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Change in efficacy measures from the start of the long‐term study to the end of treatment (overall and stratified by treatment group during the double‐blind study).


