Abstract
Aims/Introduction
The changes in metabolic parameters in type 2 diabetic patients who fast during Ramadan have not been studied in Singapore. This study aimed to examine the trends of glycated hemoglobin (HbA1c), systolic blood pressure, low‐density lipoprotein cholesterol, and triglycerides in diabetic patients with varying degrees of glycemic control and different types of therapeutic approaches during Ramadan.
Methods
The present retrospective study used a national electronic database to examine the metabolic parameter of Malay patients with type 2 diabetes. Eligible patients were stratified into three groups based on their mean HbA1c control before Ramadan: group 1 (HbA1c ≥10%), group 2 (HbA1c 7.1–9.9%) and group 3 (HbA1c ≤7%). Patients with a glomerular filtration rate <15 mL/min were excluded. The trends of metabolic parameters were traced before, during and after Ramadan.
Results
Of 13,565 patients examined, 5,172 patients (38.1%) were eligible for this study. Mean change of HbA1c varied from −1.4% to +0.2% during Ramadan, with the greatest reduction observed in group 1 (P < 0.001). A minimal systolic blood pressure reduction was observed in groups 2 and 3 (2 mmHg; P < 0.01). Low‐density lipoprotein cholesterol and triglycerides changes were insignificant. A small, 0.1%, reduction in mean HbA1c was observed in patients taking oral antidiabetic agents during Ramadan (P < 0.001).
Conclusions
Blood glucose was most affected during Ramadan, particularly in patients with mean baseline HbA1c ≥10%. The type of antidiabetic agent used did not seem to contribute to glycemic changes.
Keywords: Metabolic parameters, Ramadan, Type 2 diabetes
Introduction
Ramadan fasting is a religious observance carried out by Muslims all over the world. During Ramadan, Muslims abstain from eating, drinking and smoking during daylight hours1. Concerns have been raised over how the practice of fasting from dawn to sunset affects metabolic control in Muslim patients with diabetes2, 3.
Intermittent fasting has been widely observed to affect glucose metabolism changes in normal healthy adults. After several hours of fasting, blood glucose can decrease to a level as low as 3.3 mmol/L4. However, this decrease is not sustained because of the fall in insulin, and increase in glucagon and sympathetic activity that occur soon afterward4. The body's compensatory mechanism in maintaining normoglycemia then leads to an increase in gluconeogenesis, and a concomitant decrease in glycogen synthesis and glycolysis in the liver5. In patients with diabetes, however, the change in blood glucose levels during the fasting state is unpredictable. M'Guil et al.6 reported insignificant glycemic changes during Ramadan in patients with baseline HbA1c <7%, but another study reported HbA1c improvement of up to 0.8% in patients with baseline HbA1c of 8.3% after Ramadan (P < 0.001)7. Although the sample size in these studies was small, ranging from 67 to 120 patients, they nevertheless showed that different baseline glycemic controls might have differing effects on blood glucose levels during Ramadan.
Studies have shown that the prognosis for patients with diabetes without prior cardiovascular disease was worse than that for non‐diabetic patients with prior cardiovascular disease8, 9. Therefore, any changes in the lipid profile and blood pressure might provide a holistic view of the metabolic control of patients with diabetes during Ramadan. Khaled et al.10 found that although HbA1c improved by 0.8% during Ramadan, low‐density lipoprotein cholesterol (LDL‐C) and triglycerides (TG) worsened by 0.9 mmol/L and 0.5 mmol/L, respectively (P < 0.005). The findings of a prospective observational study involving 114 patients, however, reported insignificant changes in HbA1c, LDL‐C and TG during Ramadan11. Similarly, Ramadan fasting's effects on systolic blood pressure (SBP) have also varied in the limited number of studies examining the issue. In a study of the ambulatory monitoring of SBP, for example, SBP fluctuation was not significant12, whereas Dewanti et al.13 observed a significant change in SBP during Ramadan, a decline from a baseline of 125 mmHg to 117 mmHg (P < 0.05).
Furthermore, diabetes is a chronic, progressive condition; and diabetes‐related microvascular and macrovascular complications are inevitable without adequate glycemic control14. Hence, most patients with diabetes require life‐long medication as part of their disease management and efforts to maintain good glycemic control. Although different approaches to glycemic control have been investigated in relation to glycemic changes during Ramadan, with various outcomes reported, the association between these changes and the specific therapeutic approaches used have yet to be examined11, 15. Therefore, more insights on the trends in glycemic control in relation to the different therapeutic approaches could also provide better understanding on the changes in glycemia during Ramadan.
Singapore is a multiethnic country located in Southeast Asia, where 99% of its Malay population is Muslim16. However, the pharmaceutical management of this patient population with type 2 diabetes during Ramadan is not guideline‐specific, and the metabolic changes, if any, are yet to be examined. Therefore, the objectives of the present study were to describe and illustrate the trends of metabolic parameters, such as HbA1c, SBP, LDL‐C and TG, in our Malay patients with varying degrees of glycemic control and the patterns of glycemia in relation to different therapeutic approaches.
Methods
Database
Data were retrieved from the Chronic Disease Management System (CDMS) of the National Healthcare Group in Singapore17. The CDMS is an electronic, enterprise‐wide chronic disease database containing comprehensive information on patients' demographics, chronic care, diagnosis, medication, laboratory, and screening test records across the healthcare clusters of three hospitals and nine primary care clinics caring for up to 60% of patients in Singapore afflicted with chronic diseases. Data were extracted by the CDMS by a hierarchy of algorithms using the diagnostic codes in the International Classification of Diseases Ninth Revision18, pharmacy medication records and laboratory test records. This study was approved by the Singapore National Healthcare Group Domain Specific Review Board.
Inclusion and Exclusion Criteria, Data Handling and Stratification
Malay patients aged 21 years and older with type 2 diabetes were identified using the CDMS. Metabolic parameters defined as HbA1c, SBP, LDL‐C, TG and other pertinent information of patients were extracted from the CDMS. Those with at least one value of HbA1c, SBP, LDL‐C, TG or glomerular filtration rate (GFR) before, during and after Ramadan 2010 were included, whereas those with missing data or GFR <15 mL/min were excluded. Patients with GFR <15 mL/min were excluded in order to eliminate inaccurate HbA1c values produced as a result of immature hemoglobin generated by patients with severely reduced kidney function19, 20. Eligible patients were further stratified into three groups based on their HbA1c values before Ramadan (group 1: HbA1c ≥10%, group 2: HbA1c 7.1–9.9% and group 3: HbA1c ≤7%). The metabolic parameters of each group, which were mainly measured by venous blood, were traced from March 2010 to March 2011 for a total of 13 months with Ramadan in 2010 falling on 11 August 2010 to 9 September 2010. The pre‐, during and post‐Ramadan periods were defined as March to August 2010, September to November 2010, and December 2010 to March 2011, respectively21.
Statistical Analysis
HbA1c changes from pre‐Ramadan to Ramadan and Ramadan to post‐Ramadan were analyzed using a general linear model, followed by pairwise comparisons with Bonferroni adjustment22. Split file analysis among the different glycemic control groups for SBP, LDL‐C, and TG from pre‐Ramadan to Ramadan and Ramadan to post‐Ramadan were analyzed using a linear mixed model, followed by pairwise comparisons with Bonferroni adjustment22. All analyses were adjusted for age and sex, whereas the P‐value was multiplied by the number of repeated analyses. Computations were carried out using SPSS for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two‐tailed and carried out at a 5% level of significance. All data are presented herein as actual numbers, percentages or means ± standard deviations.
Results
Among the 13,565 patients identified from CDMS, 8,393 patients were excluded because 61.4% had an incomplete clinical laboratory test record and 0.5% had a GFR <15 mL/min. The total sample thus comprised of 5,172 patients (38.1%) with 397 in group 1 (HbA1c ≥10%), 2,877 in group 2 (HbA1c 7.1–9.9%) and 1,898 in group 3 (HbA1c ≤7%). The mean age across the three groups were 60.1 ± 10.7 years, and comprised 2,006 (38.8%) men and 3,166 (61.2%) women. Patients with a higher mean baseline HbA1c value were younger, whereas the body mass index values were similar across the three groups. From group 1 to 3, the creatinine level was increasing, whereas the GFR level was decreasing in trend. The use of oral antidiabetic agents dominated in all three groups (Table 1).
Table 1.
Demographics of study population
| Characteristics No. patients | Baseline HbA1c control | |||
|---|---|---|---|---|
| Total 5,172 | Group 1 397 | Group 2 2,877 | Group 3 1,898 | |
| Oral antidiabetic agents† | 3,707 | 249 | 2,181 | 1,277 |
| Insulin‐containing regimen† | 736 | 130 | 538 | 68 |
| No antidiabetic agent† | 729 | 18 | 158 | 553 |
| Male/female† | 2,006/3,166 | 164/233 | 1,110/1,767 | 732/1,166 |
| Age (years) | 60.1 ± 10.7 | 55.2 ± 9.8 | 59.0 ± 10.2 | 62.7 ± 10.9 |
| BMI (kg/m2) | 29.5 ± 5.3 | 29.4 ± 5.2 | 29.8 ± 5.3 | 29.0 ± 5.2 |
| Creatinine (μmol/L) | 84.9 ± 33.9 | 78.2 ± 36.9 | 84.0 ± 33.4 | 88.0 ± 33.6 |
| GFR (mL/min) | 77.8 ± 28.6 | 90.5 ± 34.4 | 79.0 ± 28.5 | 72.9 ± 26.0 |
Age, body mass index (BMI), creatinine and glomerular filtration rate (GFR) are represented as means ± standard deviations. Group 1: glycated hemoglobin (HbA1c) ≥10%; group 2: HbA1c 7.1–9.9%; group 3: HbA1c ≤7%. †Number of patients.
Overall, metformin was the most commonly prescribed oral antidiabetic agent (n = 4,029; mean daily dose 1,726 ± 773 mg), followed by glipizide (n = 2,277; mean daily dose 17.7 ± 10.5 mg). Other oral antidiabetic agents included acarbose (n = 393; mean daily dose 197 ± 85 mg), gliclazide (n = 192; mean daily dose 194 ± 109 mg) and rosiglitazone (n = 18; mean daily dose 5.6 ± 2.1 mg). Of the insulin products, premixed insulin, which contains 70% neutral protamine hagedorn and 30% regular insulin (n = 402; mean daily dose 56.5 ± 28.5 units), was used most frequently, followed by neutral protamine hagedorn (n = 231; mean daily dose 18.2 ± 16.7 units). Other insulin products used included glargine (n = 54; mean daily dose 11.8 ± 6.1 units), detemir (n = 51; mean daily dose 17.4 ± 12.5 units) and regular insulin (n = 47; mean daily dose 22.9 ± 17.4 units).
Glycemic Control
The mean HbA1c values of groups 1 and 2 decreased by 1.4% and 0.1%, respectively, between the pre‐Ramadan and Ramadan periods (P < 0.001; Figure 1a). However, these reductions were not sustained in the post‐Ramadan period. From Ramadan to post‐Ramadan periods, there were an insignificant increase in group 1 (0.1%, P > 0.05) and a significant increase in group 2 (0.2%, P < 0.001). The mean HbA1c values for group 3 increased steadily by 0.2% (P < 0.001) from pre‐Ramadan to Ramadan and by 0.2% from Ramadan to post‐Ramadan (P < 0.001).
Figure 1.
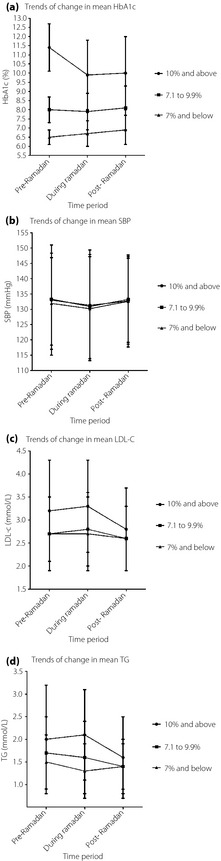
Trends of changes in metabolic parameters over the three time periods in patients with various baseline glycated hemoglobin (HbA1c) values. LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglycerides.
All three groups showed a significant proportional change in HbA1c from pre‐Ramadan to Ramadan (P < 0.001; Figure 1a). Group 1 experienced the largest change, followed by group 3 (−0.1 ± 0.2% vs 0.03 ± 0.1%) and group 2 (−0.01 ± 0.1%), which had the smallest. The proportional change in HbA1c from Ramadan to post‐Ramadan was significant only in group 3 (0.03 ± 0.1%; P = 0.045). Group 2 (0.03 ± 0.1%) and group 1 (0.02 ± 0.1%) showed no significant change (P > 0.05).
Blood Pressure
From pre‐Ramadan to Ramadan, the mean SBP decreased marginally by 2 mmHg in all three groups, with significant changes observed in groups 2 and 3 (P < 0.01) (Figure 1b). However, these changes were not sustained beyond Ramadan. From Ramadan to post‐Ramadan, the mean SBP increased significantly by 2 mmHg in groups 2 and 3 (P < 0.001).
Lipids
Changes in the mean LDL‐C were insignificant from pre‐Ramadan to Ramadan. The mean LDL‐C increased by 0.1 mmol/L in groups 1 and 2 during Ramadan (P > 0.05), but remained unchanged in group 3 (Figure 1c). From Ramadan to post‐Ramadan, however, it decreased in all three groups with significant change seen in groups 1 and 2 (group 1: 0.5 mmol/L, P < 0.05; group 2: 0.2 mmol/L, P < 0.05; group 3: 0.1 mmol/L, P > 0.05). The mean TG increased by 0.1 mmol/L in group 1 (P > 0.05), and decreased by 0.1 mmol/L in groups 2 (P > 0.05) and 3 (P > 0.05) during Ramadan (Figure 1d). From Ramadan to post‐Ramadan, it decreased in groups 1 (0.4 mmol/L; P > 0.05) and 2 (0.2 mmol/L; P < 0.05), and increased by 0.03 mmol/L in group 3 (P > 0.05). Changes in TG were significant only in group 2, between Ramadan to post‐Ramadan periods.
Types of Therapeutic Approaches
The mean HbA1c of those patients on OAD (n = 3,707) decreased by 0.1% (P < 0.001) during Ramadan and increased by 0.2% post‐Ramadan (P > 0.05) (Figure 2). However, those patients who were not taking an antidiabetic agent (n = 729) or who were on an insulin‐containing regimen (n = 736) did not show any changes in the mean HbA1c during Ramadan (P > 0.05). The post‐Ramadan period saw the mean HbA1c increases of 0.2% and 0.3% among patients who had no antidiabetic agent and were on an insulin‐containing regimen, respectively (P > 0.05).
Figure 2.
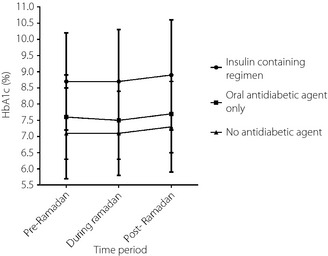
Change in mean glycated hemoglobin (HbA1c) trends in relation to different therapeutic approaches.
Discussion
This was a large study that examined changes in metabolic parameters in type 2 diabetic patients who observe Ramadan fasting in Singapore. It is also the first in the literature to show that the varying degrees of change in these parameters during Ramadan appeared to be related to patients' glycemic control before Ramadan. In the present study, the largest change observed during Ramadan was in HbA1c, whereas the other metabolic parameters did not appear to change significantly.
Muslim patients with uncontrolled HbA1c >7% at baseline were observed to experience a decrease in HbA1c during Ramadan, with the greatest effect seen in those with HbA1c ≥10%. The implication is that the acute dietary and lifestyle changes occurring during Ramadan could contribute to a decrease in average blood glucose, which is reflected as improved HbA1c. Studies have shown that the shortened interval of food consumption and reduced number of meals per day result in the meals consumed by fasting Muslims during Ramadan to be hypocaloric23, 24. Furthermore, many Muslim patients experience an increase in physical activity during Ramadan as a result of increased participation in communal prayers and other religious activities in mosques25. Patients with higher HbA1c are also associated with consumption of higher caloric food26. Thus, decrease in energy intake and increase in energy expenditure are likely explanations for the improvement in glycemic control seen among patients with higher baseline HbA1c during Ramadan. The improvement in HbA1c, however, appears to be of short duration, with a trend toward increased HbA1c as patients return to their regular dietary and lifestyle habits after Ramadan.
In patients with optimally controlled HbA1c ≤7%, no further improvement in HbA1c was seen during Ramadan. In fact, a gradual increase in mean HbA1c over the 13‐month study period was observed. The increased HbA1c trend in these patients appeared to be consistent with the progressive decline in β‐cell function over time that has been associated with the normal disease progression of type 2 diabetes regardless of the use of glucose‐lowering therapies27. Our observation of an increased HbA1c trend in Muslim patients with HbA1c ≤7% is also similar to the observation of Al‐Shafei28 of HbA1c deterioration during Ramadan in patients with good baseline control.
In the present study, no clinically significant effects on SBP in any of the glycemic control groups were observed during Ramadan. The marginal reduction in mean SBP was not sustained after the fasting period. Other studies reporting similar trends in SBP change have attributed the SBP decrease during Ramadan to weight loss13, 29, and the subsequent post‐Ramadan increase to the resumption of usual behavioral patterns30.
We also found the changes in LDL‐C and TG during Ramadan to be clinically insignificant across the three groups regardless of baseline glycemic control. A prospective study of 120 patients who fasted during Ramadan also reported insignificant changes in LDL‐C and TG levels before and after Ramadan6. The slight fluctuations in LDL‐C and TG in the present patient population might be related to the quantity and quality of food consumed during the Ramadan period31.
Furthermore, the findings of the present study show that the different types of therapeutic approaches did not contribute to changes in glycemia during Ramadan. Although patients who were on an OAD showed a statistically significant improvement in glycemic control during Ramadan, the reduction in HbA1c was minimal, and did not reach clinical significance. A study of 65 Muslim patients also reported that patients who were on OAD saw an insignificant reduction in HbA1c of between 0.1% and 0.3%11. Similarly, Sari et al.31 reported that patients on OAD experienced an insignificant improvement in HbA1c (0.2% or less) during Ramadan.
Despite these important observations, the study had several limitations. First, it was a retrospective study, and thus patient behavior that could have contributed to changes in metabolic parameters during Ramadan could not be assessed or accounted for. However, our sample was large and highly representative of Singapore's Muslim population16. Second, we were unable to capture incidents of acute complications as a result of Ramadan fasting. Although the changes in glycemic trends and the minimal changes shown in the other metabolic parameters suggested that such acute complications were unlikely, they should still be looked out for. Third, although the use of fructosamine might reflect shorter‐term changes in glycemia, it is less commonly used in the clinical setting. HbA1c as a surrogate marker of glucose control is commonly used, and it is well defined and accepted in the clinical setting32. Fourth, the present findings reflected the metabolic changes in diabetic patients who observe Ramadan fasting in Singapore. This might not be generalized to other Muslim patients who fast, as the practice of Ramadan fasting might differ. Fifth, a large number of patients were excluded from this study because of missing data during the month of Ramadan. It was unavoidable, as many patients chose not to visit healthcare institutions during the holy month. Finally, we were unable to analyze changes in body mass index or fasting blood glucose values, if any, because of missing data. Self‐monitoring of blood glucose was rarely carried out among the Singaporean patients with diabetes33, hence HbA1c levels were utilized, as they were more readily available. Future research should focus on prospective findings that account for patient behavior, patient‐reported symptoms and changes in management, if any, that can be determined through a comprehensive questionnaire administered before, during and after Ramadan.
In conclusion, among the known metabolic parameters, the greatest change was observed in glycemia during Ramadan. Patients with a mean baseline HbA1c value of 10% or more experienced the greatest HbA1c reduction. However, none of the changes we observed during Ramadan were sustained after Ramadan. Furthermore, the different therapeutic approaches did not seem to contribute to the metabolic parameter changes seen during Ramadan.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
Parts of this study were published in abstract form in Diabetes June 2012; 61 (supplement) 2571‐PO and July 2013; 62 (supplement) 1592P.
J Diabetes Investig 2016; 7: 70–75
References
- 1. Al‐Arouj M, Assaad‐Khalil S, Buse J, et al Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care 2010; 33: 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salti I, Bénard E, Detournay B, et al A population‐based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care 2004; 27: 2306–2311. [DOI] [PubMed] [Google Scholar]
- 3. Jaleel MA, Raza SA, Fathima FN, et al Ramadan and diabetes: as‐saum (the fasting). Indian J Endocrinol Metab 2011; 15: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azizi F. Medical aspects of Islamic fasting. Med J Islam Repub Iran 1996; 10: 241–246. [Google Scholar]
- 5. Vasan SK, Karol R, Mahendri NV, et al A prospective assessment of dietary patterns in Muslim subjects with type 2 diabetes who undertake fasting during Ramadan. Indian J Endocrinol Metab 2012; 16: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. M'Guil M, Ragala MA, El Guessabi L, et al Is Ramadan fasting safe in type 2 diabetic patients in view of the lack of significant effect of fasting on clinical and biochemical parameters, blood pressure, and glycemic control? Clin Exp Hypertens 2008; 30: 339–357. [DOI] [PubMed] [Google Scholar]
- 7. Maislos M, Abou‐Rabiah Y, Zuili I, et al Improved diabetes control after prolonged fasting — the Ramadan model. Pract Diabetes Int 2001; 18: 149–151. [Google Scholar]
- 8. Juutilainen A, Lehto S, Ronnemaa T, et al Type 2 diabetes as a “coronary heart disease equivalent”: an 18‐year prospective population‐based study in Finnish subjects. Diabetes Care 2005; 28: 2901–2907. [DOI] [PubMed] [Google Scholar]
- 9. Haffner SM, Lehto S, Ronnemaa T, et al Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234. [DOI] [PubMed] [Google Scholar]
- 10. Khaled BM, Bendahmane M, Belbraouet S. Ramadan fasting induces modifications of certain serum components in obese women with type 2 diabetes. Saudi Med J 2006; 27: 23–26. [PubMed] [Google Scholar]
- 11. Cesur M, Corapcioglu D, Gursoy A, et al A comparison of glycemic effects of glimepiride, repaglinide, and insulin glargine in type 2 diabetes mellitus during Ramadan fasting. Diabetes Res Clin Pract 2007; 75: 141–147. [DOI] [PubMed] [Google Scholar]
- 12. Perk G, Ghanem J, Aamar S, et al The effect of the fast of Ramadan on ambulatory blood pressure in treated hypertensives. J Hum Hypertens 2001; 15: 723–725. [DOI] [PubMed] [Google Scholar]
- 13. Dewanti L, Watanabe C, Sulistiawati, et al Unexpected changes in blood pressure and hematological parameters among fasting and nonfasting workers during Ramadan in Indonesia. Eur J Clin Nutr 2006; 60: 877–881. [DOI] [PubMed] [Google Scholar]
- 14. Stratton IM, Adler AI, Neil HAW, et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hui E, Bravis V, Salih S, et al Comparison of humalog mix 50 with human insulin mix 30 in type 2 diabetes patients during Ramadan. Int J Clin Pract 2010; 64: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 16. Singapore department of statistics . Census of population 2010. Available from: http://www.singstat.gov.sg [accessed on December 13, 2013]
- 17. Toh MP, Leong HS, Lim BK. Development of a diabetes registry to improve quality of care in the national healthcare group in Singapore. Ann Acad Med Singapore 2009; 38: 546–551. [PubMed] [Google Scholar]
- 18. International classification of diseases, ninth revision (ICD‐9): Centers for Disease Control and Prevention. [updated September 1, 2009]. Available from: http://www.cdc.gov/nchs/icd/icd9.htm. [accessed on September 15, 2014].
- 19. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus 2011; 4: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morgan L, Marenah CB, Jeffcoate WJ, et al Glycated proteins as indices of glycaemic control in diabetic patients with chronic renal failure. Diabet Med 1996; 13: 514–519. [DOI] [PubMed] [Google Scholar]
- 21. Siaw MYL, Chew DEK, Dalan R, et al Evaluating the effect of Ramadan fasting on Muslim patients with diabetes in relation to use of medication and lifestyle patterns: a prospective study. Int J Endocrinol 2014; 2014: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gamst G, Meyers LS, Guarino AJ. Analysis of Variance Designs: A Conceptual and Computational Approach With SPSS and SAS. Cambridge University Press, New York, USA, 2008. [Google Scholar]
- 23. Larijani B, Zahedi F, Sanjari M, et al The effect of Ramadan fasting on fasting serum glucose in healthy adults. Med J Malaysia 2003; 58: 678–680. [PubMed] [Google Scholar]
- 24. Mafauzy M, Mohammed WB, Anum MY, et al A study of the fasting diabetic patients during the month of Ramadan. Med J Malaysia 1990; 45: 14–17. [PubMed] [Google Scholar]
- 25. Sadiya A, Ahmed S, Siddieg HH, et al Effect of Ramadan fasting on metabolic markers, body composition, and dietary intake in Emiratis of Ajman (UAE) with metabolic syndrome. Diabetes Metab Syndr Obes 2011; 4: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu J, Eilat‐Adar S, Loria CM, et al Macronutrient intake and glycemic control in a population‐based sample of American Indians with diabetes: the strong heart study. Am J Clin Nutr 2007; 86: 480–487. [DOI] [PubMed] [Google Scholar]
- 27. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009; 32: S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al‐Shafei AI. Ramadan fasting ameliorates arterial pulse pressure and lipid profile, and alleviates oxidative stress in hypertensive patients. Br J Med Health Sci 2013; 1: 1–13. [DOI] [PubMed] [Google Scholar]
- 29. Shariatpanahi ZV, Shariatpanahi MV, Shahbazi S, et al Effect of Ramadan fasting on some indices of insulin resistance and components of the metabolic syndrome in healthy male adults. Br J Nutr 2008; 100: 147–151. [DOI] [PubMed] [Google Scholar]
- 30. Shehab A, Abdulle A, El Issa A, et al Favorable changes in lipid profile: the effects of fasting after Ramadan. PLoS ONE 2012; 7: e47615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sari R, Balci MK, Akbas SH, et al The effects of diet, sulfonylurea, and repaglinide therapy on clinical and metabolic parameters in type 2 diabetic patients during Ramadan. Endocr Res 2004; 30: 169–177. [DOI] [PubMed] [Google Scholar]
- 32. Saudek CD, Brick JC. The clinical use of hemoglobin A1c. J Diabetes Sci Technol 2009; 3: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JY, Wong S. Development and implementation of signs‐ and symptoms‐based insulin adjustment algorithm. Am J Health Syst Pharm 2010; 67: 1503–1506. [DOI] [PubMed] [Google Scholar]


