Abstract
Aims/Introduction
To assess glycated albumin (GA) as a potential glycemic index in managing gestational diabetes mellitus (GDM).
Materials and Methods
Eligible pregnant women were divided into the GDM group with abnormal result on a 75‐g oral glucose tolerance test (OGTT) and the control (normal) group. GA measurements, Pearson's correlation analysis, multiple logistic regression and receiver operating characteristic curve analysis were obtained at the follow‐up examination of participants in the two groups.
Results
A total of 2,118 women were assigned to the GDM group (n = 639) and control group (n = 1,479). The mean level of serum GA in GDM group was significantly greater than that in the control group at both 24–28 and 36–38 weeks of gestation (P < 0.05). The area under the receiver operating characteristic curve for GA defining good glycemic control in GDM was 0.874 (95% confidence interval 0.811–0.938). The cut‐off point for the GA levels derived from the receiver operating characteristic curve was 11.60%, which had sensitivity and specificity for detecting a poor glycemic status of 75.93% and 86.36%, respectively. The risk of birthweight ≥3,500 g and macrosomia increased significantly with GA levels ≥13.00% at 24–28 weeks and ≥12.00% at 36–38 weeks of gestation.
Conclusions
GA might be an appropriate and conveniently measured index that can detect poor glycemic control and predict birthweights in GDM women.
Keywords: Birthweight, Gestational diabetes, Glycated albumin
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Although GDM women have short disease durations, there are important, continuously graded relationships between higher maternal glucose and an increasing frequency of primary adverse outcomes that are independent of other risk factors1. Good glycemic control is key to reducing perinatal complications, such as macrosomia and neonatal hyperglycemia2, 3, 4. Therefore, markers that more accurately reflect variations in blood glucose levels and mean glycemic status for short‐term in GDM women are urgently required.
The current monitoring standards for diabetes involve a combination of a self‐monitored blood glucose (SMBG) procedure, continuous glucose monitoring, and glycated hemoglobin A1c (HbA1c) measurement. The values of the SMBG only reflect instantaneous blood glucose, which is susceptible to factors such as diet and emotion. Several studies have reported that neither SMBG testing nor the frequency of testing was associated with a glycemic benefit in type 2 diabetes patients regardless of treatment5, 6. Furthermore, the pain and inconvenience of collecting blood from a finger results in poor compliance with the SMBG. Continuous glucose monitoring, while reflecting the glycemic level in the preceding 3 days, is limited in application because of the complicated set‐up and high cost.
Although HbA1c provides a reliable assessment of chronic glycemic levels that are intimately related to the risk of diabetic complications, it could be a flawed indicator of blood glucose control in a short‐term period7, 8, and be not appropriate during pregnancy.
Increasing attention has been focused on the use of glycated albumin (GA) as a parameter of the short‐term glycemic status. GA is the product of non‐enzymatic glycosylation of plasma albumin. Because albumin has a relatively short half‐life (approximately 12–19 days) in the human body, GA measurement reflects the blood glucose levels of diabetic patients in the preceding 2–3 weeks9, 10. Previous studies have shown that this measurement has a higher sensitivity to glycemic fluctuations than HbAlc11, and provides useful information in evaluating blood glucose control in diabetic patients7, 8, 12. A study by Pan et al.13 showed that compared with HbA1c, GA is more closely correlated with fasting and postprandial glucose, regardless of insulin resistance and blood pressure, and might be a better monitoring index in women with GDM. Thus, GA is likely a more appropriate index for evaluating blood glucose in GDM women.
In the present study, we aimed to assess the clinical utility of GA in the management of GDM by monitoring the GA levels at 12–16, 24–28 and 36–38 weeks of gestation, and evaluating the association between glycemic control and birthweight with glycated albumin in Chinese women with GDM.
Materials and Methods
Study Population
The present study was carried out prospectively. Pregnant women were invited to participate in the study if they were between 12 weeks 0 days and 16 weeks 0 days of gestation, and presented for antenatal care and delivery at Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China, between December 2010 and February 2014. Women were excluded if they had pre‐existing diabetes and other endocrine diseases (e.g., hyperthyroidism, hypothyroidism and Cushing's syndrome), prior gestational diabetes, a history of stillbirth, in vitro fertilization, multiple pregnancy, asthma, or a history of chronic hypertension, cardiovascular disease, hematological disease, renal disease, cirrhosis or peritoneal dialysis, if they were taking corticosteroids, if there was a known fetal anomaly, or if an imminent or preterm delivery was likely because of maternal disease or fetal conditions. All participants provided written informed consent before being enrolled in the study. This research was ethically approved by the local research ethics committee in the Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai. Approval No: 2007‐45. Date of Review: 08/15/2007.
At the first prenatal visit, the patient's GA levels were measured to establish a hospital record in the clinic at 12–16 weeks of gestation. At 24–28 weeks of gestation, a 50‐g glucose challenge test (GCT) or a 75‐g oral glucose‐tolerance test (OGTT) was carried out, and the GA levels were determined. At 36–38 weeks of gestation, the GA levels were measured. Other relevant data were also collected, recorded, organized and analyzed.
The following diagnostic criteria were used: (i) pregestational diabetes mellitus was defined as fasting plasma glucose (FPG) ≥7.0 mmol/L, HbA1c ≥6.5% or random plasma glucose ≥11.1 mmol/L at the first perinatal visit, and women meeting the aforementioned criteria were excluded from the study; and (ii) participants with FPG <7.0 mmol/L underwent a GCT at 24–28 weeks of gestation. Women with capillary‐blood glucose levels ≥7.2 mmol/L after a 1‐h 50‐g glucose challenge underwent a 75‐g OGTT within a week, after an 8–12‐h overnight fast. Using the 2010 International Association of Diabetes and Pregnancy Study Groups criteria14, GDM was diagnosed when one or more of the following conditions equalled or exceeded the established thresholds on a 75‐g OGTT: FPG 5.1 mmol/L; 1‐h plasma glucose 10.0 mmol/L; and 2‐h plasma glucose 8.5 mmol/L. If all OGTT values were less than the aforementioned thresholds, the participant was defined as normal.
The participants were divided into two groups (the normal group as the control group and the GDM group), according to the OGTT results. GDM women were divided into the following three categories based on the results of the OGTT: (i) the GDM1 group consisted of participants who equalled or exceeded one of the three thresholds; (ii) the GDM2 group consisted of participant who equalled or exceeded two thresholds; and (iii) the GDM3 group consisted of participant who met all three conditions.
GDM women received ongoing care by the attending obstetrical team with a physician's support and the following interventions were given. Individualized dietary advice would be provided by the qualified dietitian based on the pre‐pregnancy weight, activity level, dietary intake and weight gain during pregnancy. The participant were given instructions on how to monitor blood glucose with the portable, memory‐based reflectance meter, and the participant were also requested to test the blood glucose four times daily (fasting, and 2‐h postprandial measurements) until the glucose levels fell into the recommended range. For women with abnormal plasma glucose levels after 2 weeks of the aforementioned interventions, insulin would be prescribed.
According to the clinical practice recommendations proposed by the American Diabetes Association15, the targets for glycemic control of gestational hyperglycemia are as follows: FPG ≤5.3 mmol/L, and 2‐h postprandial blood glucose ≤6.7 mmol/L. Good glycemic control was defined as equal to and more than 60% of daily SMBG meeting the targets, without hunger or ketosis. Poor glycemic control was defined as less than 60% of daily SMBG meeting the targets.
Pre‐pregnancy weight, weight gain during pregnancy and height were recorded, and the body mass index (BMI) was calculated as the weight (kg) divided by the height (m) squared. Macrosomia was defined as a birthweight over 4,000 g.
GA and Plasma Glucose Measurements
GA was measured using a liquid enzymatic method with a Lucica® GA‐L enzymatic kit assay on serum samples (Asahi Kasei Pharma Corp., Tokyo, Japan), and GA analysis was carried out using an automated biochemical instrument (Glamour2000; Molecular Devices, Sunnyvale, CA, USA) with intergroup and intragroup coefficient variations of <3 and 5.1%, respectively, according to the precision test. GA was hydrolyzed to amino acids by an albumin‐specific proteinase and was then oxidized by ketoamine oxidase to produce hydrogen peroxide, which was measured quantitatively. The GA value was calculated as the percentage of GA relative to the total albumin, and it was measured using the bromocresol purple method on the same serum sample16. The GA assay was unaffected by the physiological concentrations of ascorbic acid, bilirubin or glucose up to 55 mmol/L. Plasma glucose levels were measured promptly using a glucose oxidase method (Shanghai Kehua Bioengineering, Shanghai, China) on a Glamour 2000 autoanalyzer.
Statistical Analysis
All analyses were carried out using spss version 17.0 (SPSS Inc., Chicago, IL, USA). Enumeration data that were normally distributed were compared with the χ2‐test and exact probability test. Measurement data for the normal distributions are presented as the mean ± standard deviation. The means of two groups were compared by t‐test, and the means among groups were compared by anova. To examine the correlation between the GA levels and factors throughout pregnancy, Pearson's correlation was used to assess simple correlation, and multiple logistic regression was used to assess multivariable analysis. Non‐parametric statistics were used to assess data that were not normally distributed. A receiver operating characteristic (ROC) analysis curve was used to identify the optimal cut‐off point (the maximum of the combined sensitivity and specificity) of the GA levels used to diagnose GDM and good glycemic control, and P < 0.05 was determined to be statistically significant.
Results
Demographic Data Analysis of Normal Pregnant and GDM Women
Figure 1 shows the screening, enrolment and follow up of the study participants.
Figure 1.
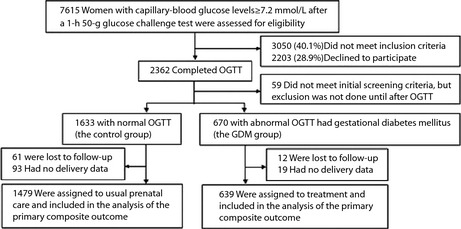
Screening, enrolment, assignment and follow up of the study participants. OGTT, oral glucose tolerance test.
The study population consisted of 2,118 pregnant women, with 1,479 in the normal (control) group and 639 in the GDM group. Statistically significant differences between the normal group and the GDM group (P < 0.05) were observed in family history of diabetes mellitus (P < 0.01), pre‐pregnancy weight over 90 kg (P = 0.01) and macrosomia delivery history (P < 0.01), whereas there were no significant between‐group differences for primiparas, as well as having a history of recurrent spontaneous abortion and stillbirth (P = 0.14, 0.25, 0.37, respectively). Regarding the general clinical data from the two groups, the differences between the pre‐pregnancy BMI were statistically significant (P < 0.01), whereas the differences between age and height were not (P = 0.09, 0.14, respectively).
Comparison of the GA Values in the Two Groups, and Analysis of the Correlation Between GA and Other Metabolic Parameters
Comparison of the GA Values in the Two Groups
Although no significant between‐group differences were observed for the GA levels taken at 12–16 weeks of gestation, the levels were significantly greater in the GDM group than in the normal group at 24–28 weeks of gestation and 36–38 weeks of gestation (P < 0.05). The GA levels gradually decreased as pregnancy progressed in both groups, as shown in Table 1.
Table 1.
Clinical characteristics of the study participants
| Variables | Normal group n = 1479 | GDM group n = 639 |
|---|---|---|
| Age (years) | 30.36 ± 3.98 | 30.77 ± 4.29 |
| Pre‐pregnancy BMI | 20.68 ± 2.79 | 22.23 ± 3.49* |
| Macrosomia history (n) | 12 | 19* |
| 12–16 weeks | ||
| Weight gain (kg) | 6.84 ± 3.20 | 6.88 ± 3.40 |
| FPG (mmol/L) | 4.66 ± 0.39 | 4.92 ± 0.62* |
| GA (%) | 12.53 ± 1.38 | 12.49 ± 1.52 |
| 24–28 weeks | ||
| Weight gain (kg) | 9.14 ± 3.30 | 9.04 ± 3.70 |
| OGTT (mmol/L) | ||
| FPG | 4.40 ± 0.31 | 4.88 ± 0.74* |
| 1 h‐PG | 7.91 ± 1.18 | 10.03 ± 1.42* |
| 2 h‐PG | 6.24 ± 1.2 | 8.54 ± 1.74* |
| 3 h‐PG | 4.96 ± 1.31 | 6.36 ± 1.70* |
| GA (%) | 11.53 ± 1.16 | 11.72 ± 1.49* |
| 36–38 weeks | ||
| Weight gain (kg) | 14.92 ± 4.23 | 13.78 ± 4.70* |
| FPG | 4.47 ± 0.35 | 4.78 ± 2.29* |
| GA (%) | 10.23 ± 1.17 | 10.69 ± 1.43* |
| Antepartum BMI | 26.43 ± 3.04 | 27.54 ± 3.67* |
| Birth weight (g) | 3357.98 ± 394.39 | 3426 ± 498.99* |
| Family history of diabetes | 16 | 27* |
BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); FPG, fasting plasma glucose; GA, glycated albumin; GDM, gestational diabetes mellitus; PG, post‐load plasma glucose. Values are given as mean ± standard deviation or n. *P < 0.05 compared with the normal group.
Analyses of the Correlations Between the GA Levels and Other Parameters in the GDM Group
The GA values in different GDM groups were compared, and the results were summarized in Figure 2a,b. Univariate correlation analysis showed a negative correlation between the pre‐pregnancy BMI and GA levels (r = –0.255, P = 0.011), and a positive correlation between the GA levels and FPG (r = 0.135, P = 0.011) at 12–16 weeks of gestation. There was a positive correlation between the GA values and the FPG, 1‐h plasma glucose, 2‐h plasma glucose, severity of GDM, and neonatal birthweight (r = 0.297, r = 0.107, r = 0.208, r = 0.119, r = 0.223, respectively, P < 0.05) at 24–28 weeks of gestation. There was a positive correlation between the GA values at 36–38 weeks of gestation and FPG, poor glycemic control, and birth weight (r = 0.297, P < 0.001; r = 0.361, P < 0.001; and r = 0.426, P < 0.001, respectively).
Figure 2.
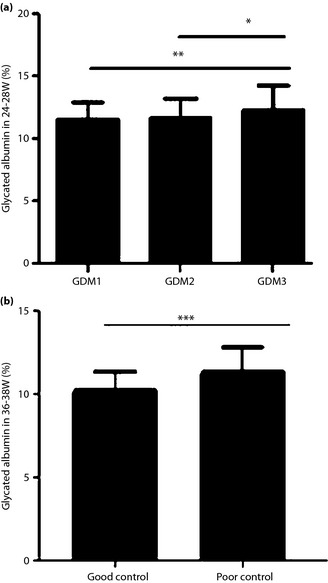
A comparison of the glycated albumin values in different gestational diabetes mellitus (GDM) groups. (a) The different severity of GDM at 24–28 weeks. (b) The status of the glycemic control in GDM patients at 36–38 weeks: good and poor glycemic control. A P‐value of <0.05 is considered to be statistically significant. *P < 0.05; **P < 0.01; ***P < 0.001. GDM1, participants with one abnormal oral glucose‐tolerance test value; GDM2, participants with two abnormal oral glucose‐tolerance test value values; GDM3, participants with three abnormal oral glucose‐tolerance test values. W, weeks of gestation.
Using the GA levels at different weeks of gestation as dependent variables and age, parity, pre‐pregnancy BMI, weight gain during pregnancy, abnormal results of the OGTT, glycemic control status, and birth weight as independent variables, multiple linear regression analysis was carried out. These data suggest that pre‐pregnancy BMI (β = –0.363, P < 0.001) and abnormal results in an OGTT (β = 0.265, P = 0.019) were the main factors influencing the GA levels at 24–28 weeks of gestation, and poor glycemic control was the main influential factor for the GA levels at 36–38 weeks of gestation (β = 1.062, P < 0.001), as shown in Table 2.
Table 2.
Multiple linear regression analysis of glycated albumin value at different weeks of gestation in women with gestational diabetes mellitus
| GA value | Pre‐pregnancy BMI | Weight gain during pregnancy | Abnormal results of the OGTT | Poor glycemic control | Birthweight | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| 12–16 weeks | –0.106 | 0.004 | –0.011 | 0.717 | 0.271 | 0.216 | 0.414 | 0.118 | –0.027 | 0.934 |
| 24–28 weeks | –0.363 | 0.000 | –0.013 | 0.381 | 0.265 | 0.019 | –0.081 | 0.618 | 0.002 | 0.915 |
| 36–38 weeks | –0.033 | 0.143 | –0.031 | 0.068 | 0.170 | 0.218 | 1.062 | 0.000 | 0.773 | 0.062 |
β, Non‐standardized coefficients; BMI, body mass index; OGTT, oral glucose tolerance test; GA, glycated albumin. P‐value <0.05 is considered to be statistically significant.
Clinical Use of GA in Evaluating Glycemic Control in GDM
For a variety of reasons, some GDM patients and their healthcare providers do not achieve the desired treatment goals. Using the cut‐off point derived from the ROC analysis of GA for glycemic control, the numbers of GDM women with good and poor glycemic control were 444 and 195, respectively. The area under the ROC curve for GA defining good glycemic control in GDM was 0.874 (95% confidence interval 0.811–0.938, standard error 0.031, P < 0.001). At GA ≥11.60%, the cut‐off point for poor glycemic control, the sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio were 75.93%, 86.36%, 82%, 81.43%, 5.57, and 0.28, respectively. These results are shown in Figure 3.
Figure 3.
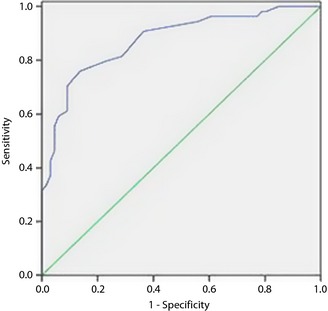
Receiver operating characteristic curves for glycated albumin defining good glycemic control in gestational diabetes mellitus women. The area under the curve was 0.874 (95% confidence interval 0.811–0.938) for glycated albumin.
Correlation Between Birthweight and the GA Levels at 24–28 Weeks and 36–38 Weeks of Gestation
Association Between the GA Levels and Birthweight
According to the aforementioned analysis, there was a positive correlation between the GA levels (during 24–28 weeks and 36–38 weeks of gestation) and birthweight. The neonatal birthweights were divided into the following four groups: (i) <3,000 g; (ii) 3,000–3,499 g; (iii) 3,500–3,999 g; and (iv) ≥4,000 g. The neonatal birthweight increased along with elevated GA levels at 24–28 weeks and 36–38 weeks of gestation (Table 3).
Table 3.
Different birthweight and corresponding glycated albumin levels at 24–28 weeks and 36–38 weeks
| Variables | <3,000 g (n = 87) | 3,000–3,499 g (n = 249) | 3,500–3,999 g (n = 205) | ≥4,000 g (n = 98) |
|---|---|---|---|---|
| 24–28 weeks | 11.530 ± 1.089 | 11.830 ± 1.050 | 12.250 ± 1.153* ①② | 13.760 ± 2.126* ①②③ |
| 36–38 weeks | 10.687 ± 1.226 | 11.356 ± 0.765* ① | 11.476 ± 0.928* ① | 12.840 ± 1.382* ①②③ |
*P < 0.01 compared with different birthweight groups, ① with <3,000 g; ② with 3,000–3,499 g; ③ with 3,500–3,999 g.
Association of the GA Levels with Birthweight ≥3,500 g and Macrosomia at 24–28 Weeks of Gestation
To further explore the association between the GA levels at 24–28 weeks of gestation with birthweight, we divided the neonates into two groups based on their birthweights, birthweight ≥3,500 g and birthweight <3,500 g; then, we classified the GA levels into the following six grades: 0 (<11.0%), 1 (11.0–11.9%), 2 (12.0–12.9%), 3 (13.0–13.9%), 4 (14.0–14.9%) and 5 (≥15%). With the minimum GA level (0: <11.0%) of the pregnant women as the exposure level, we calculated the odds ratio (OR) of the GA levels in each grade (Table 4). We found that the ORs for GA grades ≥13.0% (grades 3, 4 and 5) were 2.614, 4.182 and 4.530 (P < 0.05), respectively, which showed that the risk of a birthweight ≥3,500 g was elevated markedly in GDM women with GA levels ≥13.0% at 24–28 weeks of gestation (Table 4).
Table 4.
Association of glycated albumin levels during 24–28 weeks with the birthweight
| Birthweight | Exposure classification (GA)† | Total (n) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| ≥3,500 g | 55 | 78 | 60 | 40 | 16 | 13 | 262 |
| <3,500 g | 115 | 130 | 86 | 32 | 8 | 6 | 377 |
| OR | – | 1.255 | 1.459 | 2.614 | 4.182 | 4.530 | – |
| 95% CI | – | 0.819–1.992 | 0.921–2.312 | 1.485–4.599 | 1.688–10.363 | 1.635–12.555 | – |
| P | – | 0.297 | 0.107 | 0.001 | 0.001 | 0.002 | – |
CI, confidence interval; OR, odds ratio. †Exposure classification (glycated albumin [GA]): 0 (<11.0%), 1 (11.0–11.9%), 2 (12.0–12.9%), 3 (13.0–13.9%), 4 (14.0–14.9%), and 5 (≥15%).
Similarly, the ORs for GA grades ≥14% (grades 4 and 5) were 15.56 and 40.00, respectively, both of which were risk factors of macrosomia (P < 0.05), showing that the risk of macrosomia was increased in GDM women with GA levels ≥14% at 24–28 weeks of gestation.
Association Between the GA Levels at 36–38 Weeks of Gestation and Macrosomia
Using the same method, the GA levels were also distributed into five grades, 0 (<11.0%), 1 (11.0–11.9%), 2 (12.0–12.9%), 3 (13.0–13.9%) and 4 (≥14.0%), to analyze the association between the GA levels at 36–38 weeks of gestation and macrosomia. With the lowest GA level of the pregnant women as the exposure level, the OR for the GA at each grade was calculated. We found that the ORs for the GA grades ≥12.0% (grades 2, 3 and 4) were 10.941, 41.333 and 62.000 (P = 0.033, 0.003 and 0.009, respectively); thus, the risk of the incidence of macrosomia increased in GDM women with GA levels ≥12.0%.
Discussion
The present retrospective analysis of prospectively collected data has shown that the GA levels were significantly higher after 24 weeks of gestation in the GDM women compared with controls. Furthermore, we found that abnormal OGTT results were the main factors influencing the GA levels in the second trimester, and two or three of the abnormal OGTT results had a high, positive correlation with the GA levels. We also observed that elevated GA levels had a positive association with the incidence of babies with birthweights ≥3,500 g, and macrosomia in GDM women with poor glycemic control. Additionally, we found that the GA levels decreased as pregnancy progressed with or without GDM, although the decrease from 24–28 weeks to 36–38 weeks of gestation was relatively small, whereas pre‐pregnancy BMI was an important, influential factor in the GA levels in both groups. These results show that the GA levels could directly reflect the severity of glucose tolerance impairment for GDM women, and could be a useful marker for monitoring short‐term glycemic status. However, these suggest that BMI and gestational age should be considered as the complicating factors when we assessed the validity of GA in controlling for GDM.
There was little information on the GA value for glycemic control in pregnant women with GDM. The present study further identified the value of a GA ≥11.60% level, which was derived from the ROC curve, as the cut‐off point for identifying poor glycemic control in GDM women, and provided the optimal sensitivity (75.93%) and specificity (86.36%). Meanwhile, the present study also found that in GDM women, the risks of birthweight ≥3,500 g and macrosomia increased significantly with GA levels ≥13.0 and ≥14.0%, respectively, during 24–28 weeks of gestation. Furthermore, the incidence of macrosomia in GDM women with GA levels ≥12.0% was increased at 36–38 weeks of gestation. It could also have an impact on screening and detecting birthweight ≥3,500 g and macrosomia.
The present study had some limitations. This was a retrospective study. In addition, the GA levels were determined at just 12–16 weeks, 24–28 weeks and 36–38 weeks of gestation. Our observations will need to be confirmed in a large‐scale prospective multicenter study in different gestational periods. However, various conditions, such as chronic hypertension, thyroid disorders, and hepatic and renal disease, could affect the turnover of serum albumin, leading to anomalous measurements of GA levels. Individuals with these disorders were excluded from the present study, and further work will be required to determine whether these conditions affect GA levels. Caution is therefore advised in interpreting GA measurements in patients with such disorders.
Hiramatsu et al.17 showed similar changes of GA levels, which gradually decreased as pregnancy progressed toward the third trimester, in healthy pregnant women. One of the reasons why GA decreases from early to late pregnancy is considered to be the decrease in plasma glucose levels. Yi et al.18 also found that the GA reduction continued as pregnancy progressed in both normal and GDM pregnant women.
Research worldwide has documented that BMI negatively influences GA levels. One study showed that GA levels decreased with increasing BMI in 2,563 subjects with normal glucose tolerance19. These findings were also confirmed in type 2 diabetes patients7 and obese children without diabetes20. The underlying mechanism of the decreased GA levels and BMI elevations might be that obese individuals have shorter‐lived albumin and are in a state of chronic inflammation21.
Recent several studies have identified that, in the intensive treatment of diabetes, changes in serum GA levels are closely associated with HbA1c, and fasting and postprandial blood glucose22. The result of a survey of endocrinologists provides an assessment of the degree of utility and acceptance of GA as a new monthly management tool for diabetes23.
However, GA, as a new index of plasma glucose, lacks a widely recognized reference interval. The reference range of GA in the Japanese population from the Japan Diabetes Society in 2006 is 12.3–16.9% (n = 699, mean ± 2 SD)24. The GA range for the Chinese population, as recommended by the Shanghai Diabetes Institute in 2009, is 11–17%25, and for healthy Americans, as proposed by Kohzuma et al.26, the range is 11.9–15.8%.
Fewer studies have evaluated the normal range of GA levels during pregnancy. Hiramatsu et al.17 studied 574 normal Japanese pregnant women and proposed that the reference interval for GA in normal pregnant women was 11.5–15.7%. In our previous study, 1,046 healthy pregnant women were selected, and the reference intervals of the GA levels were determined to be 9.20–14.60% throughout pregnancy, 10.20–15.18% at 12–16 weeks, 9.70–13.98% at 24–28 weeks and 8.70–13.20% at 36–38 weeks of gestation, respectively27.
Few studies have assessed the validity of GA in GDM management. The primary utility of the GA cut‐off level of 11.60% is to detect approximately 80% of subjects with poor glycemic control, to positively affect GDM management largely and to permit the early identification of subjects who are at imminent risk of disease development, and who can then be referred for further evaluation and appropriate management. In addition, GA use could be more sensitive to short‐term glycemic variations than HbA1c28, and also relegate SMBG testing. Thereby, increasing compliance and improving GDM women empowerment, which might result in significant healthcare cost savings.
In clinical practice, birthweight ≥3,500 g predicts an increased risk of a difficult vaginal delivery, and macrosomia is the strongest risk factor for maternal/fetal birth injuries1, 29, 30, 31 and increases the risk of obesity32, and cardiovascular diseases in the offspring. No single measure was clearly superior in predicting macrosomia. When the GA measures were analyzed as continuous variables, GA levels ≥12.0% at 36–38 weeks of gestation were highly predictive of macrosomia.
In summary, GDM women had greater GA levels than normal pregnant women. There was a strongly positive correlation between the GA levels and blood glucose, and the severity of GDM. GA can be used to assess glycemic control in GDM women during the third trimester of pregnancy. We recommend a GA level ≥11.60% as the cut‐off point for poor glycemic control in GDM. The regular monitoring of GA of these women (once/3–4 weeks) helps to reduce the frequency of SMBG, thereby to lower healthcare costs, and increase patient compliance. In addition, in GDM women, the risk of macrosomia significantly increases when the GA levels are ≥12.0% in the third trimester. In conclusion, the results reported in the present study provide strong support for the use of GA measurements, as a complement to finger stick glucose, for assessing short‐term glycemic control and predicting large birthweight in the Chinese GDM women.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Natural Science Foundation of Shanghai (12ZR1422200) from the Science and Technology Commission of Shanghai Municipality, China, and by the Science and Technology Fund Project of the Shanghai Jiao Tong University School of Medicine (11XJ21059).
J Diabetes Investig 2016; 7: 48–55
References
- 1. HAPO Study Cooperative Research Group , Metzger BE, Lowe LP, Dyer AR, et al Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 2. Yang X, Hsu‐Hage B, Zhang H, et al Women with impaired glucose tolerance during pregnancy have significantly poor pregnancy outcomes. Diabetes Care 2002; 25: 1619–1624. [DOI] [PubMed] [Google Scholar]
- 3. Landon MB, Spong CY, Thom E, et al Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med 1994; 45: 245–260. [DOI] [PubMed] [Google Scholar]
- 5. Schütt M, Kern W, Krause U, et al Is the frequency of self‐monitoring of blood glucose related to long‐term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes 2006; 114: 384–388. [DOI] [PubMed] [Google Scholar]
- 6. Davis WA, Bruce DG, Davis TM. Does self‐monitoring of blood glucose improve outcome in type‐2 diabetes? The Fremantle Diabetes Study. Diabetologia 2007; 50: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koga M, Matsumoto S, Saito H, et al Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J 2006; 53: 387–391. [DOI] [PubMed] [Google Scholar]
- 8. Tahara Y. Analysis of the method for conversion between levels of HbA1c and glycated albumin by linear regression analysis using a measurement error model. Diabetes Res Clin Pract 2009; 84: 224–229. [DOI] [PubMed] [Google Scholar]
- 9. Cohen MP, Clements RS. Measuring glycated proteins: clinical and methodological aspects. Diabetes Technol Ther 1999; 1: 57–70. [DOI] [PubMed] [Google Scholar]
- 10. Kouzuma T, Uemastu Y, Usami T, et al Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta 2004; 346: 135–143. [DOI] [PubMed] [Google Scholar]
- 11. Abe F, Miyamoto N, Tahara Y, et al Serum glycated albumin concentrations during pregnancy. Ann Clin Biochem 1993; 30: 198–200. [DOI] [PubMed] [Google Scholar]
- 12. Yoshiuchi K, Matsuhisa M, Katakami N, et al Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008; 55: 503–507. [DOI] [PubMed] [Google Scholar]
- 13. Pan J, Zhang F, Zhang L, et al Influence of insulin sensitivity and secretion on glycated albumin and hemoglobin A1c in pregnant women with gestational diabetes mellitus. Int J Gynaecol Obstet 2013; 121: 252–256. [DOI] [PubMed] [Google Scholar]
- 14. International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, et al International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycaemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association . Standards of medical care in diabetes–2011. Diabetes Care 2011; 34(Suppl 1): S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paroni R, Ceriotti F, Galanello R, et al Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem 2007; 40: 1398–1405. [DOI] [PubMed] [Google Scholar]
- 17. Hiramatsu Y, Shimizu I, Omori Y, et al Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J 2012; 59: 145–151. [DOI] [PubMed] [Google Scholar]
- 18. Yi XM, Li QF, Guo HQ. Assessment of glycosylated hemoglobin and glycosylated serum protein as a screening and diagnosing tests in Gestational Diabetes Mellitus. Int J Lab Med 2010; 3: 670–672. [Google Scholar]
- 19. Wang FF, Ma XJ, Hao YP, et al Serum glycated albumin is inversely influenced by fat mass and visceral adipose tissue in Chinese with normal glucose tolerance. PLoS ONE 2012; 7: e51098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishimura R, Kanda A, Sano H, et al Glycated albumin is low in obese, non‐diabetic children. Diabetes Res Clin Pract 2006; 71: 334–338. [DOI] [PubMed] [Google Scholar]
- 21. Piva SJ, Tatsch E, De Carvalho JA, et al Assessment of inflammatory and oxidative biomarkers in obesity and their associations with body mass index. Inflammation 2013; 36: 226–231. [DOI] [PubMed] [Google Scholar]
- 22. Lee EY, Lee BW, Kim D, et al Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol 2011; 48: 167–172. [DOI] [PubMed] [Google Scholar]
- 23. Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol 2008; 2: 1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tominaga M, Makino H, Yoshino G, et al Report of the committee on standardization of laboratory testing related to diabetes mellitus of the Japan diabetes society: determination of reference intervals of hemoglobin A1c (IFCC) and glycoalbumin in the Japanese population. J Jpn Diabetes Soc 2006; 49: 825–833. [Google Scholar]
- 25. Zhou J, Li H, Yang WY, et al A multi‐center clinical study of the reference value of serum glycated albumin. Zhonghua Nei Ke Za Zhi 2009; 48: 469–472. [PubMed] [Google Scholar]
- 26. Kohzuma T, Yamamoto T, Uematsu Y, et al Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol 2011; 5: 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang FH, Li HP. Influential factors, change trends, and reference values of serum glycated albumin in pregnancy. J Shanghai Jiao Tong Univ 2014; 34: 347–351. [Google Scholar]
- 28. Takahashi S, Uchino H, Shimizu T, et al Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c)in type 2 diabetic patients: usefulness of GA for evaluation of short‐term changes in glycemic control. Endocr J 2007; 54: 139–144. [DOI] [PubMed] [Google Scholar]
- 29. Weiss PA, Haeusler M, Tamussino K, et al Can glucose tolerance test predict fetal hyperinsulinism. BJOG 2000; 107: 1480–1485. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Decker A, Platt RW, et al How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol 2008; 198: 517.e1–e6. [DOI] [PubMed] [Google Scholar]
- 31. Esakoff TF, Cheng YW, Sparks TN, et al The association between birth weight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol 2009; 200: 672.e1–e4. [DOI] [PubMed] [Google Scholar]
- 32. Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4–7 years of age. Diabetes Care 1999; 22: 1284–1291. [DOI] [PubMed] [Google Scholar]


