Abstract
Background: Nm23‐H1 was the first metastasis suppressor discovered in most tumor models and reduction or loss of nm23‐H1 expression correlates with tumor progression and metastasis in non‐small‐cell lung cancer. Despite extensive studies, the regulatory mechanism of nm23‐H1 expression is far from elucidated. The transcriptional factor forkhead box (FOX)O3 has been reported to be involved in multiple regulatory signaling pathways in the biological behavior of tumors. Therefore, we aimed to study the relationship between FOXO3 activity and nm23‐H1 expression.
Methods: Real time reverse transcriptase‐polymerase chain reaction and Western blotting assays were employed to determine nm23‐H1 messenger ribonucleic acid and protein expression after being transformed by different FOXO3 plasmid in A549 cells. A dual luciferase reporter system and chromatin immunoprecipitation assay, were used to determine the promoter activity of the nm23‐H1 gene and to detect the binding of FOXO3 into the nm23‐H1 promoter, respectively.
Results: We found that activated FOXO3 decreased nm23‐H1 expression and dominant negative FOXO3 increased nm23‐H1 expression. Modulation of FOXO3 activity with FOXO3 pathway inhibitors altered nm23‐H1 promoter activity. Although there is a putative binding site of FOXO3 in the nm23‐H1 promoter, FOXO3 regulated nm23‐H1 expression in an indirect manner.
Conclusion: We demonstrated that the transcriptional factor FOXO3 decreased the expression levels of the tumor suppressor gene nm23‐H1 in the non‐small‐cell lung cancer A549 cell line and that the level of expression of nm23‐H1 was controlled by FOXO3 in an indirect manner. This finding provided an insight into the upstream regulation of nm23‐H1 and may provide promising targets for inhibition of the metastasis process.
Keywords: FoxO3, metastasis, nm23‐H1, non‐small‐cell lung cancer, metastasis suppressor gene
Introduction
Lung cancer is the leading cause of cancer‐related mortality worldwide.1 Approximately 85% of patients with lung cancer have non‐small‐cell lung cancer (NSCLC), three‐quarters of these with locally advanced or metastatic disease at the time of diagnosis.2 Metastasis is the most common cause for the high mortality of NSCLC; therefore, a strategy for treating, as well as preventing, metastatic diseases are necessary in order to increase the survival of NSCLC patients.2
Metastasis suppressor genes are defined by their capacity to inhibit metastasis without affecting the growth of the primary tumor.3 Nm23‐H1 was the first metastasis suppressor discovered in a mouse tumor model.4 During the past 30 years, the expression levels of nm23‐H1 have been widely studied in many human tumor samples, including lung cancer.5, 6, 7, 8 They function at various stages of the signaling cascade and regulate NSCLC metastasis. However, their mechanisms of action, for the most part, especially the upstream regulatory networks, remain unclear.9 Deciphering the molecular interactions of metastasis suppressors may provide promising targets for inhibition of the metastasis process.
Forkhead box O (FOXO) transcription factors are involved in multiple physiological and pathological processes, including apoptosis, aging, proliferation, metabolism, immunity, and tumorigenesis.10 FOXO3 is a member of the FOXO family of transcription factors.11 It is an important downstream effector in multiple signaling pathways, most notably those including protein kinase B (Akt), c‐Jun terminal kinase (JNK), and extracellular signal‐regulated kinase (ERK) pathways, which are notably involved in the pathology of tumors.12 Numerous studies have detected that Akt can phosphorylate FOXO and cause its cytoplasmic retention, leading to the inactivation of FOXO, whereas JNK can promote FOXO nuclear localization, leading to transcriptional activation of its target genes.13, 14, 15 However, the expression and regulatory mechanism of FOXO3 in the metastasis process of lung cancer has not yet been clearly demonstrated.16, 17, 18 In our present study, we detected that the transcriptional factor FOXO3 decreased the expression level of nm23‐H1 in the lung cancer A549 cell line. Further, we confirmed our result by inhibiting the signaling pathways upstream regulating FOXO3 factors and found that activated FOXO3 in nuclear downregulated nm23‐H1. Interestingly, analysis by the TRANSFAC database indicated that there may be a potential binding site of FOXO3 located in the promoter of the nm23‐H1 gene. However, we found that the FOXO3 regulated the metastasis suppressor gene nm23‐H1 in an indirect manner. We conclude that the metastasis suppressor gene nm23‐H1 is upstream negatively regulated by the transcriptional factor FOXO3 to modulate oncogenic properties in NSCLC. To the best of our knowledge our results represent the first report of such a conclusion.
Materials and methods
Reagents, cells, and transfections
Anti‐nm23‐H1 antibody was obtained from Santa Cruz Biotechnology (Dallas, Texas, USA); anti‐Akt, anti‐JNK, and anti‐p21kip1 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA); and anti‐β‐actin from Sigma (Victoria, BC, Canada). The kinase inhibitor LY294002 and JNK inhibitor SP60012548 were purchased from Calbiochem (San Diego, CA, USA) and used at concentrations of 20 and 10 μM, respectively. Human A549 cells and A549‐99 cells were maintained in 1640 medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (Gibco BRL). For transfection, A549 cells and A549‐99 cells were grown in six‐well or 24‐well plates and transfected with polyjet purchased from SignaGen Laboratories (Carlsbad, CA, USA) according to the manufacturer's instructions.
Western blot analyses
A549 cells were seeded in six‐well plates (105 cells/well), and after various treatments they were lysed in pre‐warmed Laemmli buffer purchased from Bio‐Rad (Hercules, CA, USA). For each sample, the same amount of total protein was added to a well of 10% acrylamide gel and resolved by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis. A 1:500 dilution of anti‐nm23‐H1 and a 1:1000 dilution of all other primary antibodies were used. Blots were quantified by densitometry using Transparency Adapter (UTA‐2100XL) for PowerLook 2100XL obtained from UMAX (Mountain View, CA, USA).
Quantitative real time reverse transcriptase polymerase chain reaction
Total ribonucleic acid (RNA) was isolated at the indicated time using the RNA isolation reagent TRIzol (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed using the Reverse Transcriptase M‐MLV (RNase H−) kit (Takara Biotechnology, Dalian Co., Ltd, Liaoning, China). Each 25 μl of polymerase chain reaction (PCR) mixture was prepared using the SYBR Premix Ex Taq Kit (Takara), with amplification conditions according to the manufacturer's instructions. Each experiment was performed in triplicate by the 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA). The relative amount of messenger (m)RNA was calculated using the comparative CT method after normalization to glyceraldehyde 3‐phosphate dehydrogenase mRNA levels.
Dual luciferase reporter system
A549 cells were seeded in a 24‐well plate (2 × 104/well) 24 hours before transfection. For FOXO3 activity analysis, the cells were co‐transfected with 1 μg/well FOXO3‐TM or FOXO3‐δ‐DB plasmid and 1 μg/well nm23‐H1promoter‐luciferase reporter plasmid using polyjet transfection reagent. Luciferase activity was measured 48 hours after transfection using a Dual‐Glo luciferase assay system (Promega, Madison, WI, USA), and firefly luciferase activity was normalized to Renilla luciferase activity. The pcDNA3.1 vector served as a blank control. To confirm the relationship between FOXO3 and nm23‐H1, the cells were pretreated with different inhibitors for six hours after co‐transfection for 48 hours. The cells were washed, lysed, and assayed for luciferase activity. Normal or mutational nm23‐H1promoter‐ luciferase reporter plasmid was co‐transfected with 1 μg/well FOXO3‐TM or FOXO3‐δ‐DB plasmid in order to test whether FOXO3 could directly bind on the nm23‐H1 promoter. Experiments were performed in triplicate and repeated three times. Data are presented as means ± standard deviation.
Cloning and DNA construction
Nm23‐H1 promoter reporter constructs were generated by PCR amplification using purified genomic DNA as the template and primers (Table 1). PCR products were inserted into the Bgl II/Hind III sites of pGL3 basic vector (Promega). Nm23‐H1 cDNA was generated by PCR amplification using purified total RNA as the template and primers (Table 1). PCR products were digested with BamHI/XbaI and inserted into the pcDNA3.1 (+) vector.
Table 1.
Primers used for polymerase chain reaction amplifications
| Nm23‐H1 promoter | Region | Primers |
|---|---|---|
| Promoter 969 | −969 to +7 | 5'‐aaaaagatcttagattggtcttttggtgtcgtc‐3' |
| 5'‐aaaaaagcttcacttgcacgcacggaacg‐3' | ||
| Promoter 747 | −747 to +7 | 5'‐aaaaagatctccatttttgtacctttcccccgtt‐3' |
| 5'‐aaaaaagcttcacttgcacgcacggaacg‐3' | ||
| Promoter 273 | −273 to +7 | 5'‐aaaaagatcttcaggcactctttggacttcacg‐3' |
| 5'‐aaaaaagcttacattgcacgcacggaacg‐3' | ||
| Promoter Mut | 5'‐gctagaaaaggtgaatacctacaaagcgggagcgaa‐3' | |
| 5'‐ttcgctcccgctttgtaggtattcaccttttctagc‐3' |
Point mutations in the nm23‐H1 promoter Mut were generated by site‐specific mutagenesis using the overlap PCR extension method. Nm23‐H1 promoter 969 was used as the template, and Mut PCR products were generated using primers (Table 1). The PCR products were digested with Bgl II/Hind III and subcloned into the pGL3‐luciferase vector.
Chromatin immunoprecipitation assay
A549 cells transfected separately with 2 μg FOXO3‐TM, FOXO3‐δ‐DB, and pcDNA3.1 plasmid were cross‐linked with 1% formaldehyde and quenched by glycine. After washing with phosphate buffered saline, cells were scraped and collected by centrifugation and cell pellets were lysed with lysis buffer. The resulting nuclear pellet was sonicated (Vibra cell, SONICS, Newtown, CT, USA) for four rounds of 15 pulses at 4% output power and 70% duty to obtain an average 1 kb sheared chromatin. The sonicated lysates were used for immunoprecipitation with biotinylated antibodies either against FOXO3 or normal goat immunoglobin G. The immune complexes were recovered with magnetic streptavidin beads. The immunoprecipitated and input DNA were extracted by incubation with chelating resin solution and boiled for 10 minutes to reverse the cross‐links. The DNA was purified with a DNA purification kit (Qiagen, Dusseldorf, Germany). PCR was performed with purified DNA and recommended reaction condition. The primers for human nm23‐H1 promoter were: forward: 5'‐aaaaagatcttagattggtcttttggtgtcgtc‐3'; and reverse: 5'‐aaaaaagcttcacttgcacgcacggaacg‐3'.
Statistical analysis
Data are expressed as the mean (± standard deviation), and statistical comparisons were performed by analysis of variance with Dunnett's correction for multiple comparisons, using SAS version 9 (SAS Institute Inc., Cary, NC, USA). A P value of <0.05 was considered statistically significant.
Results
Forkhead box O (FOXO)3 inhibits the expression of metastasis suppressor gene nm23‐H1 in vitro
It has been reported that the expression of nm23‐H1 is reduced or lost in most tumors. During analysis using the TRANSFAC database, we found that there is a potential binding site of FOXO3 located in the nm23‐H1 promoter. Therefore, we transfected A549 cells with 2 μg FOXO3‐WT (wild type), FOXO3‐TM (constitutively activated), and FOXO3‐δ‐DB (dominant negative) plasmid to examine nm23‐H1expression levels. Western blot analysis showed that the expression of nm23‐H1 decreased in the cells transfected with FOXO3‐WT, and FOXO3‐TM plasmids, but increased with FOXO3‐δ‐DB plasmid transfection (Fig 1a). Furthermore, we transfected A549 cells with nm23‐H1 promoter reporter constructs, and the transcriptional activity of nm23‐H1 in cells was detected by dual luciferase reporter system. Results showed that FOXO3‐δ‐DB could induce luciferase activity of the nm23‐H1 promoter, while there was a significant decrease using FOXO3‐TM (Fig 1b,c). The mRNA were extracted from A549 cells, which were transfected with FOXO3‐TM or FOXO3‐δ‐DB plasmid. Real‐time PCR indicated that nm23‐H1 expression decreased after transfecting with FOXO3‐TM, but increased with FOXO3‐δ‐DB plasmid transfection (Fig 1d). Our study shows that the FOXO3 protein can inhibit nm23‐H1 expression.
Figure 1.
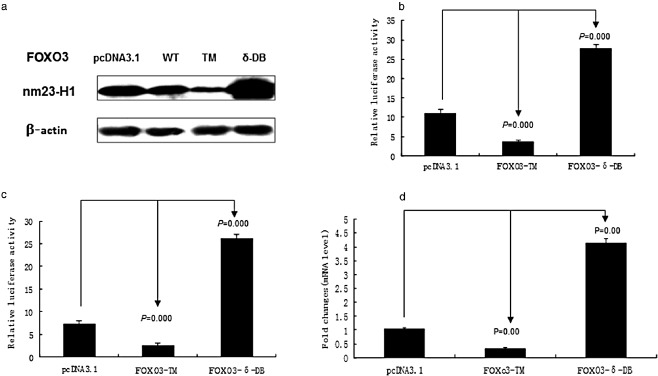
Forkhead box O (FOXO3) inhibits the expression of metastasis suppressor gene nm23‐H1 in in vitro experiments. (a) Western blot shows that the expression of nm23‐H1 decreased in the cells which were transfected with FOXO3‐WT, FOXO3‐TM plasmid, but increased with FOXO3‐δ‐DB. (b) and (c) Results show that in both A549 and A549‐99 cells, FOXO3‐TM decreased luciferase activity, while FOXO3‐δ‐DB significantly induced luciferase activity. (d) Results indicate that nm23‐H1 expression decreased after transfection with FOXO3‐TM, which increased with FOXO3‐δ‐DB.
The FOXO3 pathway inhibitors regulate the expression of nm23‐H1
It has been demonstrated that Akt is a negative regulator, while JNK is a positive regulator to FOXO3. Therefore, we treated A549 cells with Akt inhibitor LY294002 or with JNK inhibitor SP60012548 to examine nm23‐H1 expression. Western blot showed that the expression of nm23‐H1 was decreased in A549 cells after treatment with LY294002 (20 μmol), but increased with SP60012548 (10 μmol) (Fig 2a,b). The transcriptional activity of nm23‐H1 was examined after treatment with LY294002 and SP60012548 by dual luciferase reporter system. The luciferase activity significantly decreased in the LY294002 treatment, and increased in the SP60012548 addition (Fig 2c). These results indicate that FOXO3 inhibits the expression of nm23‐H1 through both the Akt and JNK pathways.
Figure 2.
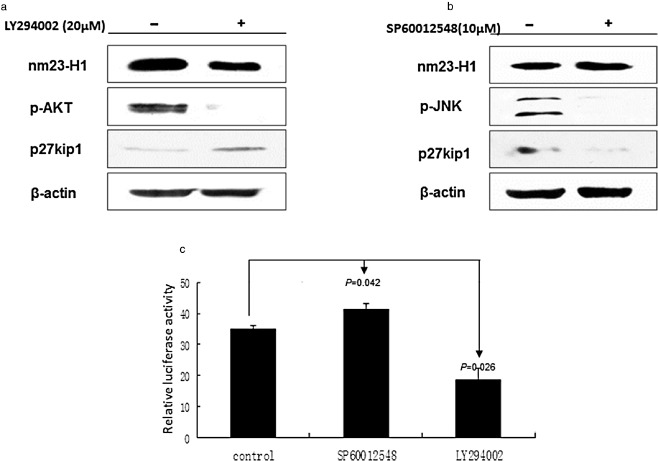
The Forkhead box O (FOXO3) pathway inhibitors regulate the expression of nm23‐H1. (a) and (b) Western blot shows that the expression of nm23‐H1 decreased in A549 cells after treatment with LY294002, but increased with SP60012548. (c) Luciferase activity decreased significantly in the LY294002 group, but increased in the SP60012548 group.
FOXO3 negatively regulated nm23‐H1 expression in an indirect manner
Our experiments revealed that FOXO3 inhibits the expression of nm23‐H1, and the effect may occur at the promoter level. We constructed the plasmids with different lengths of nm23‐H1 promoter (Fig 3a), and transfected them into A549 cells. The activity of the different lengths of nm23‐H1 promoter was detected by dual luciferase reporter system. The luciferase activity was decreased in all of the fragments after transfection with FOXO3‐TM, but increased with FOXO3‐δ‐DB (Fig 3b). After bio‐information analysis of the nm23‐H1 promoter, the predicted binding site of FOXO3 may lie in ‐309bp to ‐304bp; therefore the putative binding site was mutated from TAAACA into TAGGTA (Fig 3c). We used the dual luciferase report system to detect the wild type and mutant promoter activity. The nm23‐H1 activity decreased after being transfected with FOXO3‐TM and increased with FOXO3‐δ‐DB (Fig 3d). This indicated that a mutation of the putative binding site of FOXO3 on the nm23‐H1 promoter could not interrupt the effect of FOXO3 on the nm23‐H1 promoter. Co‐immunoprecipitation was further used to verify whether FOXO3 protein could be bound on the nm23‐H1 promoter, but the result indicated that FOXO3 negatively regulated nm23‐H1 expression in an indirect manner (Fig 3e).
Figure 3.
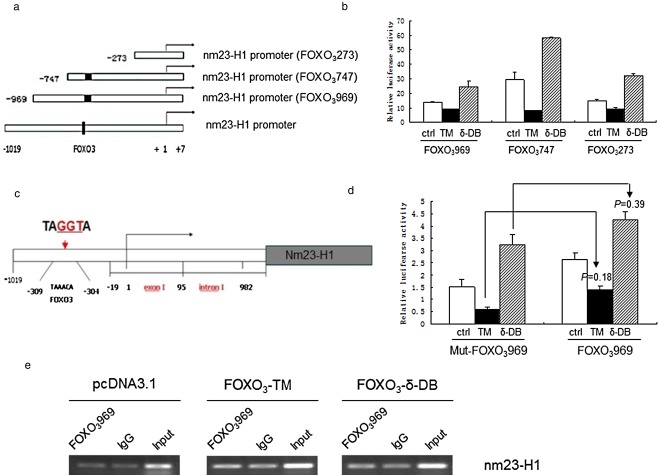
Study of the relationship between Forkhead box O (FOXO3) and different fragments of the nm23‐H1 promoter. (a) Different length of nm23‐H1 promoter. (b) Luciferase activity decreased in all of the fragments after transfection with FOXO3‐TM, but increased with FOXO3‐δ‐DB. (c) The nm23‐H1 promoter with mutant predicted binding site of FOXO3. (d) With mutant and un‐mutant nm23‐H1 promoter, luciferase activity decreased after transfection with FOXO3‐TM, but increased with FOXO3‐δ‐DB. (e) Compared with the negative control (IgG) and positive control (Input), the co‐immunoprecipitation result is negative.
Discussion
Increasing experimental evidence indicates that a decreased expression of metastasis suppressor gene nm23‐H1 is involved in the progression of a variety of human malignancies, including NSCLC.8, 9 The mechanism underlying its ability to suppress metastasis has been investigated in a number of studies. However, the mechanism of how the nm23‐H1 gene has been downregulated in cancer cells is far from elucidated. Our study provided evidence showing that transcriptional factor FOXO3 is a negative regulator of nm23‐H1 in lung cancer cell lines.
Members of the FOXO family contain four homologous mammalian proteins: FOXO1, FOXO3, FOXO4, and FOXO6.11 Alteration of these factors is often linked to tumorigenesis in the form of breast, prostate, glioblastoma, rhabdomyosarcoma, and leukemic cancers.12 However, their mechanism of function in lung cancer has not been investigated at great length. It has been reported that the FOXO3 gene is frequently deleted in early‐stage lung adenocarcinoma, which indicates that FOXO3 is a suppressor of lung adenocarcinoma carcinogenesis.16, 17, 18 In addition, FOXO3 is a relevant mediator of the cytotoxic effects of cisplatin in lung cancer cells. The phosphorylation (at Thr32, Akt phosphorylation site) of FOXO3 is significantly inhibited by cisplatin and nuclear accumulation of FOXO3 is detected, which, in turn, increases the expression of FOXO3‐dependent apoptosis.19 But whether the expression of FOXO3 is related to tumor metastasis of lung cancer is unclear. In our study, we reported that FOXO3 negatively regulated a known metastasis suppressor gene nm23‐H1, which suggested that FOXO3 might be a metastasis promoter or regulator in lung cancer. However, the mechanism of how they functionally linked with each other still needs to be investigated.
Subcellular localization of FOXO factors plays an important role in the regulation of their activities and functions.20 These activities are tightly controlled by multiple signaling cascades, including phosphatidylinositol 3‐kinase (PI3K)/Akt, Ras/Erk, and stress/JNK. Generally speaking, the activation of survival/growth ERK and Akt signaling causes the retention of FOXO factors in the cytoplasm or degradation, and the stress/JNK pathway has been shown to phosphorylate 14‐3‐3, releasing FOXO to enter the nucleus, inducing apoptosis.13, 15 However, the upstream regulated network of FOXO‐nm23‐H1 transduction is complicated. Studies have demonstrated that ultraviolet irradiation activates JNK, which, in turn, inactivates ERK and Akt, leading to FOXO3 translocation into the nucleus and Bim expression.21 In addition, the stress stimuli override the sequestration of FOXO by growth factors in mammalian cells during stress events, and FOXO remain in the nucleus despite the presence of survival and growth factors.22 Moreover, the transcriptional function of FOXO factors is regulated by the transforming growth factor‐β/Smad, PI3K/Akt, and FOXG1 pathways together in the glioma cell to regulate the expression of p21Cip1 and cell proliferation.23 Expressed as part of a neural progenitor proliferation program, FOXG1 binds to the FOXO‐Smad complex in the nucleus, inhibiting the transcriptional activity of p21Cip1, and leading to tumor survival.24 In our study, we demonstrated that inhibiting the Akt signaling pathways promotes the activation of FOXO3 in the nucleus and downregulates nm23‐H1 expression. The degradation of FOXO3 in plasma regulated by JNK inhibitor SP600025 upregulated the expression of nm23‐H1. In addition, we also detected that Akt signaling pathway activator, nicotine, could also downregulate nm23‐H1 expression. Thus, diverse signals and a wide range of other upstream factors together control the regulation network of FOXO3‐nm23‐H1 signaling transduction. These signals and factors need to be identified in the future.
The correlation of FOXO3 and nm23‐H1 levels was observed in this study; however, the specific mechanisms of how the nm23‐H1 level was regulated by FOXO3 are not clear. The TRANSFAC database provided us a potential binding site of FOXO3 located in the region from −747 to −273 in the promoter of the nm23‐H1 gene. However, our results negate the hypothesis that FOXO3 binds to the promoter of nm23‐H1 to regulate its transcription. Recent studies have demonstrated that interacting proteins with nm23‐H1 may act as modulators of the metastasis suppressor activity. nm23‐H1 can be phosphorylated by casein kinase I and Aurora‐A/STK15, which alter the binding affinity of nm23‐H1 with interacting proteins and play a final effecter of signal transduction.25, 26 In addition, proteins act as bi‐directional binding partners with nm23‐H1, such as PRUNE, serine‐threonine kinase receptor‐associated protein (STRAP), ribosomal protein small subunit 3 (rpS3), and signal transducer and activator of transcription 3, involved in the regulation of metastasis and tumorigenesis. 27 , 28 , 29 , 30 The interaction of these factors and FOXO3 in the regulation of nm23‐H1 in lung cancer needs investigation.
Conclusion
In summary, this study established the important role of transcriptional factor FOXO3 in the regulation of metastasis suppressor gene nm23‐H1. This finding provided an insight into the upstream regulation of nm23‐H1; however, the function and regulation mechanism of FOXO3‐nm23‐H1 signaling transduction still needs to be identified in future studies of NSCLC.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by the National High Technology Research and Development Program of China (No. 2012AA02A502), the National Natural Science Foundation of China (No. 30973364), the National Natural Science Foundation of China (No. 81272359), the Key Project of Priorities of Development of Research Fund for the Doctoral Program of Higher Education of China (20131202130001), the Key Project of Sichuan Natural Science Foundation (No. 0 6SG005‐002‐2), the Application Foundation and Front Technology Research Program of Tianjin (No. 13JCQNJC12500), and the High School Science and Technology Fund Planning Project of Tianjin (No. 20130112).
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. (Published erratum appears in CA Cancer J Clin 2014; 64: 364) CA Cancer J Clin 2014; 64: 9–29.24399786 [Google Scholar]
- 2. D'Addario G, Früh M, Reck M et al Metastatic non‐small‐cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2012; 21 (Suppl. 5): 116–119. [DOI] [PubMed] [Google Scholar]
- 3. Steeg PS, Bevilacqua G, Kopper L et al Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988; 80: 200–204. [DOI] [PubMed] [Google Scholar]
- 4. Boissan M, Lacombe ML. [NM23, an example of a metastasis suppressor gene.] Bull Cancer 2012; 99: 431–440. (In French.) [DOI] [PubMed] [Google Scholar]
- 5. Parhar RS, Shi Y, Zou M, Farid NR, Ernest P, al‐Sedairy ST. Effects of cytokine‐mediated modulation of nm23 expression on the invasion and metastatic behavior of B16F10 melanoma cells. Int J Cancer 1995; 60: 204–210. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki E, Ota T, Tsukuda K et al nm23‐H1 reduces in vitro cell migration and the liver metastatic potential of colon cancer cells by regulating myosin light chain phosphorylation. Int J Cancer 2004; 108: 207–211. [DOI] [PubMed] [Google Scholar]
- 7. Hartsough MT, Morrison DK, Salerno M et al Nm23‐H1 metastasis suppressor phosphorylation of kinase suppressor of Ras via a histidine protein kinase pathway. J Biol Chem 2002; 277: 32389–32399. [DOI] [PubMed] [Google Scholar]
- 8. Goncharuk VN, del‐Rosario A, Kren L et al Co‐downregulation of PTEN, KAI‐1, and nm23‐H1 tumor/metastasis suppressor proteins in non‐small cell lung cancer. Ann Diagn Pathol 2004; 8: 6–16. [DOI] [PubMed] [Google Scholar]
- 9. Kim HD, Youn B, Kim TS, Kim SH, Shin HS, Kim J. Regulators affecting the metastasis suppressor activity of Nm23‐H1. Mol Cell Biochem 2009; 329: 167–173. [DOI] [PubMed] [Google Scholar]
- 10. Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal 2011; 14: 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005; 24: 7410–7425. [DOI] [PubMed] [Google Scholar]
- 12. Yang JY, Hung MC. Deciphering the role of forkhead transcription factors in cancer therapy. Curr Drug Targets 2011; 12: 1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen MD, Luo X, Biteau B, Syverson K, Jasper H. 14‐3‐3 Epsilon antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell 2008; 7: 688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang JY, Zong CS, Xia W et al ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2‐mediated degradation. Nat Cell Biol 2008; 10: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt‐mediated survival signals by phosphorylating 14‐3‐3. J Cell Biol 2005; 170: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikse OR, Blake DC Jr, Jones NR et al FOXO3 encodes a carcinogen‐activated transcription factor frequently deleted in early‐stage lung adenocarcinoma. Cancer Res 2010; 70: 6205–6215. [DOI] [PubMed] [Google Scholar]
- 17. Blake DC Jr, Mikse OR, Freeman WM, Herzog CR. FOXO3a elicits a pro‐apoptotic transcription program and cellular response to human lung carcinogen nicotine‐derived nitrosaminoketone (NNK). Lung Cancer 2010; 67: 37–47. [DOI] [PubMed] [Google Scholar]
- 18. Herzog CR, Blake DC Jr, Mikse OR, Grigoryeva LS, Gunderman EL. FoxO3a gene is a target of deletion in mouse lung adenocarcinoma. Oncol Rep 2009; 22: 837–843. [DOI] [PubMed] [Google Scholar]
- 19. Liu H, Yin J, Wang C, Gu Y, Deng M, He Z. FOXO3a mediates the cytotoxic effects of cisplatin in lung cancer cells. Anticancer Drugs 2014; 25: 898–907. [DOI] [PubMed] [Google Scholar]
- 20. Zanella F, Rosado A, García B, Carnero A, Link W. Chemical genetic analysis of FOXO nuclear‐cytoplasmic shuttling by using image‐based cell screening. Chembiochem 2008; 9: 2229–2237. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Chen WR, Xing D. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol 2012; 227: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 22. Brunet A, Datta SR, Greenberg ME. Transcription‐dependent and ‐independent control of neuronal survival by the PI3K‐Akt signaling pathway. Curr Opin Neurobiol 2001; 11: 297–305. [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Chen WR, Xing D. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol 2012; 227: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 24. Seoane J, Le HV, Shen L, Anderson SA, Massagué J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 2004; 117: 211–223. [DOI] [PubMed] [Google Scholar]
- 25. Garzia L, D'Angelo A, Amoresano A et al Phosphorylation of nm23‐H1 by CKI induces its complex formation with h‐prune and promotes cell motility. Oncogene 2008; 27: 1853–1864. [DOI] [PubMed] [Google Scholar]
- 26. Du J, Hannon GJ. The centrosomal kinase Aurora‐A/STK15 interacts with a putative tumor suppressor NM23‐H1. Nucleic Acids Res 2002; 30: 5465–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garzia L, Roma C, Tata N, Pagnozzi D, Pucci P, Zollo M. H‐prune‐nm23‐H1 protein complex and correlation to pathways in cancer metastasis. J Bioenerg Biomembr 2006; 38: 205–213. [DOI] [PubMed] [Google Scholar]
- 28. Jung H, Seong HA, Ha H. NM23‐H1 tumor suppressor and its interacting partner STRAP activate p53 function. J Biol Chem 2007; 282: 35293–35307. [DOI] [PubMed] [Google Scholar]
- 29. Wan F, Anderson DE, Barnitz RA et al Ribosomal protein S3: A KH domain subunit in NFkappaB complexes that mediates selective gene regulation. Cell 2007; 131: 927–939. [DOI] [PubMed] [Google Scholar]
- 30. Gong L, Wu Z, Guo L et al Metastasis suppressor Nm23‐H1 inhibits STAT3 signaling via a negative feedback mechanism. Biochem Biophys Res Commun 2013; 434: 541–546. [DOI] [PubMed] [Google Scholar]


