Abstract
Background
The brain is a frequent site of metastases from non‐small cell lung cancer (NSCLC). The purpose of this study was to detect the expression of CUG‐binding protein 1 (CUGBP1) messenger ribonucleic acid (mRNA) and Ki‐67 in metastasized brain tissue from NSCLC and determine the relationship between CUGBP1 and brain metastases.
Methods
The expression of CUGBP1 mRNA and Ki‐67 in metastasized brain tissue from NSCLC was investigated by semiquantitative polymerase chain reaction and immunohistochemistry, respectively. The expression of CUGBP1 and Ki‐67 in metastasized brain tissue from NSCLC was related to clinical characteristics, as assessed using the chi‐square test. The prognostic significance was assessed by univariate and multivariate analyses using the Cox hazard model.
Results
The expression of CUGBP1 mRNA and Ki‐67 was overexpressed in metastasized brain tissue from NSCLC and was correlated with differentiation. In addition, by both univariate and multivariate survival analyses, CUGBP1 expression, Ki‐67 expression, and age were noted to be independent indicators of a shorter postsurgical survival.
Conclusion
The expression of CUGBP1 is an important factor in the development of brain metastases from NSCLC.
Keywords: Brain metastases, CUGBP1, Ki‐67, non‐small cell lung cancer
Introduction
Lung cancer, particularly non‐small cell type, is the most frequent type of cancer worldwide.1 Non‐small cell lung cancer (NSCLC) is the leading cause of cancer‐related deaths with brain metastasis being one of the direst complications.2 Brain metastasis is an important prognostic factor of NSCLC.3 More accurate assessment of brain metastasis is an important part in the management of lung cancer, as an early diagnosis would contribute to a better survival rate. Many investigations have been conducted to diagnose lung cancer and brain metastasis through the identification of molecular targets.4, 5, 6, 7 However, to date, a significant correlation between CUG‐binding protein 1 (CUGBP1) expression and brain metastases in lung cancer has not been reported.
CUG‐binding protein 1 is a member of the CEFE (CUGBP and embryonic lethal abnormal vision‐like factor) family of human ribonucleic acid (RNA)‐binding proteins.8 RNA CUG repeats are expanded in the 3′‐untranslated region (UTR) of the gene encoding the myotonic dystrophy protein kinase (DMPK) and cause myotonic dystrophy type 1 disease (DM1).9, 10 CUGBP1 is involved in the control of splicing, deadenylation, messenger (m)RNA decay, and translation.10, 11, 12 In addition to its role in embryonic and cardiac development, skeletal muscle and adipose tissue differentiation, and germ cell formation, CUGBP1 plays an important role in genesis and deterioration of certain tumors. The overexpression of CUGBP1 has been reported in DM1 myoblasts, the heart, esophageal epithelial cells, skeletal muscle tissues, NSCLC, and some DM1 mouse models.13 Although the overexpression of CUGBP1 has been evaluated in NSCLC, the correlation between this expression and brain metastasis remains unclear. The Ki‐67 antigen has been developed to investigate the cell cycle, as well as cell proliferation. In lung cancer, Ki‐67 has been reported to be a marker for evaluating cell proliferative activity and cancer metastasis.4, 14 We used this marker in our study to investigate the expression of CUGBP1 and Ki‐67, and we assessed whether there was an association between CUGBP1 expression and brain metastasis and NSCLC prognosis.
Materials and methods
Patients
In total, 68 NSCLC patients with metachronous brain metastasis who underwent brain metastasis tumor resection in the Department of Neurosurgery at the Affiliated Hospital of Qingdao University from January 2009 to April 2014 were enrolled in our investigation. Written and informed consent was obtained from all patients and the ethical committee of our hospital approved the investigation. Brain metastasis tissue was obtained via surgery and immediately stored at −80°C until processing. The clinical and pathological data of the 68 patients were recorded according to the 7th edition of the tumor node metastasis (TNM) classification and staging system for lung cancer, published by the International Association for the Study of Lung Cancer (IASLC), the International Union Against Cancer (UICC), and the American Joint Committee on Cancer (AJCC), enacted on 1 January 2010.15 All patients underwent radiotherapy after surgery in the Department of Oncology at the Affiliated Hospital of Qingdao University. The clinico‐pathological data analyzed included gender, age, smoking, histology, T‐stage, and differentiation. The characteristics of the patients are listed in Table 1.
Table 1.
Correlation between CUGBP1 expression and clinicopathological characteristics
| Clinicopathological characteristics | Cases (N) | CUGBP1 mRNA | P value | Ki‐67 | P value | P value | ||
|---|---|---|---|---|---|---|---|---|
| Negative (N) | Positive (N) | Negative (N) | Positive (N) | |||||
| All patients | 68 | 22 | 46 | 19 | 49 | |||
| Gender | 0.536 | 0.284 | ||||||
| Male | 46 | 16 | 30 | 11 | 35 | |||
| Female | 22 | 6 | 16 | 8 | 14 | |||
| Age | 0.577 | 0.232 | ||||||
| <60 | 28 | 8 | 20 | 10 | 18 | |||
| ≥60 | 40 | 14 | 26 | 9 | 31 | |||
| Smoking | 0.946 | 0.681 | ||||||
| Non‐smoker | 9 | 3 | 6 | 2 | 7 | |||
| Smoker | 59 | 19 | 40 | 17 | 42 | |||
| Histology | 0.257 | 0.903 | ||||||
| Squamous cell carcinoma | 12 | 3 | 9 | 3 | 9 | |||
| Adenocarcinoma | 40 | 16 | 24 | 12 | 28 | |||
| Other | 16 | 3 | 13 | 4 | 12 | |||
| T‐stage | 0.678 | 0.062 | ||||||
| T1, T2 | 44 | 15 | 29 | 9 | 35 | |||
| T3 | 24 | 7 | 17 | 10 | 14 | |||
| Differentiation | 0.024 | 0.007 | ||||||
| Well/moderate | 36 | 16 | 20 | 15 | 21 | |||
| Poor | 32 | 6 | 26 | 4 | 28 | |||
CUGBP1, CUG‐binding protein 1; mRNA, messenger ribonucleic acid.
Semi‐quantitative reverse transcriptase‐polymerase chain reaction of CUG‐binding protein 1 (CUGBP1) messenger ribonucleic acid
Each sample, including cancer and normal tissues from the same patient, was frozen in liquid nitrogen immediately after surgical resection before the extraction of RNA. Trizol was used to isolate the total RNA of the metastasized brain tissue according to the manufacturer's protocol. cDNA was prepared from each total RNA sample using 10 ng RNA for reverse transcription. The polymerase chain reaction (PCR) conditions were as follows: 95°C for five minutes; 40 cycles of 94°C for 15 seconds, 57°C for 20 seconds, and 72°C for 60 seconds; and a final extension at 72°C for 10 minutes. The sequences of CUGBP1 and glyceraldehyde 3‐phosphate dehydrogenase primers were as follows: forward: 5′‐GTCAGTGGTGGACCTGACCT‐3′ and 5′‐TGACTTCAACAGCGACACCCA‐3′, reverse: 5′‐AGGGGTCTACATGGCAACTG‐3′ and 5′‐CACCCTGTTGCTGTAGCCAAA‐3′. Samples were separated using 2% agarose gel electrophoresis. CUGBP1 mRNA samples were quantified using a FluoroImager scanner (Molecular Dynamics, Sunnyvale, CA, USA) and analyzed with ImageQuant software (Molecular Dynamics). We selected 0.6 as the cut‐off value for CUGBP1 mRNA expression. If the value of CUGBP1 mRNA was above the cut‐off value, the patient was considered positive for CUGBP1 mRNA, otherwise, the patient was considered negative. Each assay was performed at least three times to verify the results.
Ki‐67 immunohistochemistry staining
Tumor sections (4‐mm‐thick) were obtained from 68 formalin‐fixed, paraffin‐embedded archival brain metastasis tumor tissues. The slides were dewaxed in xylene and gradually rehydrated with alcohol. The slides were heated in a pressure cooker for 50 minutes in 10 mM citrate buffer (pH 6.0), treated with 0.3% H2O2 for five minutes, and finally incubated with MIB‐1 monoclonal antibody (DAKO Corp., Carpinteria, CA, USA) for 10 minutes. After incubation with a secondary biotinylated antibody for 10 minutes and treatment with a streptavidin peroxidase reagent (DAKO Corp.), the slides were rinsed and then stained with diaminobenzidine chromogen solution (ResGen Invitrogen Corp., Carlsbad, CA, USA). After a light counterstaining with hematoxylin and dehydration, coverslips were mounted. To evaluate the percentage of cancer cells with Ki‐67 nuclear immunoreactivity, at least 1000 tumor cells per slide were counted. The median value of this series (41% of positive cells) was used as the cut‐off value to distinguish tumors with a low index of cell proliferation (0–40%) from those with a high index of cell proliferation (41–100%). If the extent of staining was above the cut‐off value, the patient was considered positive for Ki‐67, otherwise the patient was considered negative.
Statistical analysis
The association between the expression of CUGBP1 and clinicopathological characteristics were analyzed using the chi‐square test. Cox univariate analysis was performed to compare time to progression (TTP) survival between patients, using the log‐rank test. The value of the independent prognostic factors was assessed in multivariate analysis using the Cox hazard model. All statistical analyses were performed with the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at P < 0.05 in all tests.
Results
Patient characteristics
In total, 68 patients were enrolled in this study (Table 1). The age of the patients (46 men and 22 women) ranged from 39–72 years, with a mean age of 58 years. Smoking history was reported in 59 out of 68 patients (86.8%). The postsurgical pathological stage was determined using the current TNM classification. Histologically, 40 patients had adenocarcinoma, 12 had squamous cell carcinoma, and 16 had other cell carcinomas. Intraoperative therapy was not performed on any patient.
Relationship between the expression of CUGBP1 and, Ki‐67 and brain metastasis in non‐small cell lung cancer
Using immunohistochemistry and PCR analyses, we observed 67.6% CUGBP 1 mRNA expression (x2 = 5.892, P = 0.015) and 72.1% Ki‐67 expression (x2 = 10.903, P = 0.001) had positive significance (Table 1). CUGBP1 and Ki‐67 expression was associated with the differentiation and brain metastasis (Tables 1, 2). The expression of CUGBP1 and Ki‐67 is shown in Figure 1. The relationship between CUGBP1 and Ki‐67 is shown in Table 3.
Table 2.
The expression of CUGBP1, Ki‐67 in NSCLC with brain metastasis
| Items | Cases | CUGBP1 mRNA | X2 | P | Ki‐67 | X2 | P | ||
|---|---|---|---|---|---|---|---|---|---|
| Negative N (%) | Positive N (%) | Negative N (%) | Positive N (%) | ||||||
| Cancer Group | 68 | 22 (32.4) | 46 (67.6) | 5.892 | 0.015 | 19 (27.9) | 49 (72.1) | 10.903 | 0.001 |
| Normal group | 68 | 36 (52.9) | 32 (47.1) | 38 (55.9) | 30 (44.1) | ||||
CUGBP1, CUG‐binding protein 1; mRNA, messenger ribonucleic acid; NSCLC, non‐small cell lung cancer.
Figure 1.
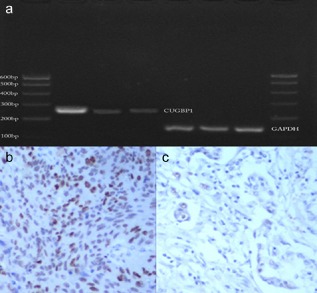
(a) Expression of CUG‐binding protein 1 (CUGBP1) and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) messenger ribonucleic acid mRNA detected by reverse‐transcription polymerase chain reaction. (b,c) Representative immunohistochemical staining for Ki‐67. (b) Positive expression of Ki‐67. (c) Negative expression of Ki‐67.
Table 3.
Correlation between CUGBP1 mRNA and Ki‐67 expression
| CUGBP1 mRNA expression | Ki‐67 | X2 | P | Consistency test | ||
|---|---|---|---|---|---|---|
| Negative | Positive | Kappa | 95% CI | |||
| Negative (N) | 11 | 11 | 7.86 | 0.005 | 0.338 | 1.532–14.723 |
| Positive (N) | 8 | 38 | ||||
CI, confidence interval; CUGBP1, CUG‐binding protein 1; mRNA, messenger ribonucleic acid.
In the present study, we observed a significant correlation between the expression of CUGBP1 and Ki‐67 and brain metastasis in NSCLC (Table 2). We observed a significant correlation between the levels of CUGBP1 and the Ki‐67 expression (x2 = 7.86, P = 0.005) (Table 3). No significant correlation was found between CUGBP1 mRNA expression and the histologic types of the tumors or the gender of the patients. The median TTP of all patients was five months (range: 1–13 months). Results of the log‐rank test were marginally significant (x2 = 8.417, P = 0.004): individuals without an elevated CUGBP1 had a TTP of 7.868 months, while those with an elevated CUGBP1 had a TTP of 5.076 months, as shown in Figure 2. In univariate analysis, our data indicated that survival rates were closely related to CUGBP1 (P = 0.001) and Ki‐67 expression (P = 0.004) (Table 4). Cox regression multivariate analysis of all of these factors influencing TTP revealed that CUGBP1 expression in NSCLC patients with brain metastasis was an independent prognostic factor (hazard ratio [HR] =2.411, 95% confidence interval [CI] 1.331–4.370), independent of Ki‐67 expression (HR = 2.376, 95% CI 1.240–4.553) (Table 5).
Figure 2.
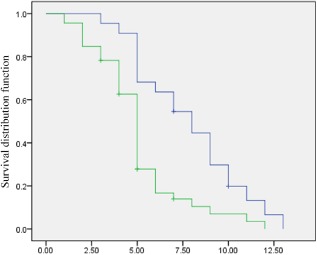
Cox model postsurgical survival according to CUG‐binding protein 1 (CUGBP1) expression. The blue line represents patients with a low CUGBP1 expression (negative); the green line represents patients with a high CUGBP1 expression (positive).
Table 4.
Cox univariate analysis of initial variables
| Variables | Number of patients N (%) | X2 | P value |
|---|---|---|---|
| Gender | 0.593 | 0.441 | |
| Male | 46 (67.6) | ||
| Female | 22 (32.4) | ||
| Age | 1.512 | 0.216 | |
| <60 | 28 (41.2) | ||
| ≥60 | 40 (58.8) | ||
| Smoking | 0.056 | 0.813 | |
| Non‐smoker | 9 (13.2) | ||
| Smoker | 59 (86.8) | ||
| Histology | 2.237 | 0.312 | |
| Squamous cell carcinoma | 12 (17.6) | ||
| Adenocarcinoma | 40 (58.8) | ||
| Other | 16 (23.6) | ||
| T‐stage | 1.474 | 0.225 | |
| T1, T2 | 44 (64.7) | ||
| T3 | 24 (32.3) | ||
| Differentiation | 1.392 | 0.238 | |
| Well/moderate | 36 (52.9) | ||
| Poor | 32 (47.1) | ||
| CUGBP1 | 10.834 | 0.001 | |
| Negative | 22 (32.4) | ||
| Positive | 46 (67.6) | ||
| Ki‐67 | |||
| Negative | 19 (27.9) | 8.134 | 0.004 |
| Positive | 49 (72.1) |
CUGBP1, CUG‐binding protein 1.
Table 5.
Cox multivariate analysis of prognostic factors of NSCLC with brain metastasis
| Characteristics | X2 | P value | HR | 95% CI |
|---|---|---|---|---|
| CUGBP1 | 8.417 | 0.004 | 2.411 | 1.331–4.370 |
| Ki‐67 | 6.804 | 0.009 | 2.376 | 1.240–4.553 |
CI, confidence interval; CUGBP1, CUG‐binding protein 1; HR, hazard ratio; NSCLC, non‐small cell lung cancer.
Discussion
As NSCLC is the leading cause of cancer death, determining the molecular markers associated with progression and prognosis is of vital importance.2, 3, 16 To the best of our knowledge, this is the first study in which the relationship between CUGBP1 expression and clinicopathological features, with special attention given to the prognostic significance of NSCLC with brain metastases, has been investigated. This study provides strong evidence that the overexpression of CUGBP1 is an independent indicator of poor prognosis, which provides us with new insights into the detection, treatment, and prognosis of NSCLC patients with brain metastases. On the basis of our statistical data, we suggest that CUGBP1 should be routinely detected by screening NSCLC patients with brain metastases in future clinical practice.
CUGBP1 is a human RNA‐binding protein implicated in DM1, a neuromuscular disease associated with a CUG triplet expansion in the 3′‐UTR of the DMPK gene. Various functions have been reported for CUGBP1, such as protein translation regulation, RNA stability, and splicing. In addition, CUGBP1 plays an important role in tumor genesis.17 Arnal‐Estape et al. reported the significance of CUGBP1 in the genesis and the deterioration of certain tumors.18 Rattenbacher et al. reported that CUGBP1 and its binding target transcripts define a posttranscriptional regulatory network that functions to control cellular growth and homeostasis and suggested that disruptions in this network correlate with the development of cancer.19 Wang et al. reported that silencing of CUGBP1 by RNA interference could be developed as a promising therapeutic approach for gastric cancer.20 In addition, Jiao et al. found that CUGBP1 is overexpressed in NSCLC, and may be used as a biomarker in conjunction with other methods for clinical diagnosis.21 The underlying biological mechanisms that might explain the relationship between CUGBP1 expression and brain metastasis in NSCLC are not known, but our data suggest that CUGBP1 plays an important role in the genesis and deterioration of tumors.
Ki‐67 has also been previously defined as an important biomarker for metastasis in NSCLC.22 Ki‐67 is a nuclear antigen in proliferating human cells. It is expressed in mid G1, S, and G2; it reaches its peak in the M phase, and is very rapidly degraded at the end of the M phase.22, 23 Ki‐67 expression is an important biomarker for proliferation and metastasis and has been reported in all types of cancer, including lung, breast, and prostate cancers and colorectal liver metastasis. Li et al. and Verhoven et al. reported that Ki‐67 is an important prognostic factor for metastasis.14, 24 Bubb et al. reported that the expression of Ki‐67 has a positive correlation with brain metastasis in NSCLC.25 Furthermore, Yamayoshi et al. reported that the overexpression of Ki‐67 is significantly associated with metastasis in lung cancer.26 In our study, the expressions of CUGBP1 (P = 0.015) and Ki‐67 (P = 0.001) were similar to previous studies. There is a significant correlation between the level of CUGBP1 mRNA and Ki‐67 antigen expression (x2 = 7.86, P = 0.005). Although a relationship between the expression of CUGBP1 and brain metastasis has not previously been reported in the literature, our data indicate that the level of expression of CUGBP1 is an important factor related to brain metastasis. Using Cox univariate survival analysis, the TTP of NSCLC patients with brain metastasis is associated with the variables Ki‐67 (x2 = 8.134, P = 0.004) and CUGBP1 (x2 = 10.834, P = 0.001). Cox multivariate survival analysis revealed that overexpression of CUGBP1 predicted a poor survival (HR = 2.411), independent of other powerful predictors, such as Ki‐67 (HR = 2.376), similar to conclusions found in previous studies.27 Therefore, CUGBP1 is an excellent tumor marker for detecting brain metastasis in patients.
Although the extent to which administered therapy determines survival for a brain metastasis patient with elevated CUGBP1 expression is unclear, our data indicate that CUGBP1 expression is a strong and consistent determinant of superior survival, regardless of other independent predictors.28 No direct evidence from previous studies or formal clinical trials exists to guide treatment decisions for the individual patient with brain metastasis on the basis of CUGBP1 expression; however, our study provides a direction for future clinical research.29 A combination of age, and Ki‐67 and CUGBP1 expression may be used to classify patients as having a low, intermediate, or high risk of death, which represents a new direction for treatment of NSCLC patients with brain metastasis because inhibition of the molecular markers of expression can be applied in future clinical trials.
Several limitations of our study must be acknowledged. First, as a prospective study, the evaluation of clinical response and TTP is often imprecise. Second, the number of NSCLC patients with brain metastases in our study was small, thereby reducing the statistical power of our results. A larger study is necessary to validate our data reporting the influence of CUGBP1 levels on brain metastasis in NSCLC.
Conclusion
In summary, CUGBP1 may play a major role in the development of brain metastasis in NSCLC. Our study may have an impact on future developments for the prevention, diagnosis, and therapy of NSCLC patients with brain metastasis.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This study was supported by the Shandong Natural Science Foundation (2009HW024); the Shandong Excellent Young Scientist Research Award Fund Project (2006BSB14114; BS2010YY013); and Shandong Tackle Key Problems in Science and Technology (2010GSF10245).
References
- 1. Grinberg‐Rashi H, Ofek E, Perelman M et al The expression of three genes in primary non‐small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res 2009; 15: 1755–1761. [DOI] [PubMed] [Google Scholar]
- 2. Zhao N, Wilkerson MD, Shah U et al Alterations of LKB1 and KRAS and risk of brain metastasis: Comprehensive characterization by mutation analysis, copy number, and gene expression in non‐small‐cell lung carcinoma. Lung Cancer 2014; 86: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iuchi T, Shingyoji M, Itakura M et al Frequency of brain metastases in non‐small‐cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2014. doi: 10.1007/s10147‐014‐0760‐9 [DOI] [PubMed] [Google Scholar]
- 4. Nguyen VN, Mirejovsky P, Mirejovsky T, Melinová L, Mandys V. Expression of cyclin D1, Ki‐67 and PCNA in non‐small cell lung cancer: Prognostic significance and comparison with p53 and bcl‐2. Acta Histochem 2000; 102: 323–338. [DOI] [PubMed] [Google Scholar]
- 5. Fernández‐Figueras MT, Puig L, Musulen E et al Prognostic significance of p27Kip1, p45Skp2 and Ki67 expression profiles in Merkel cell carcinoma, extracutaneous small cell carcinoma, and cutaneous squamous cell carcinoma. Histopathology 2005; 46: 614–621. [DOI] [PubMed] [Google Scholar]
- 6. He J, Zhou F, Shao K et al Overexpression of Pin1 in non‐small cell lung cancer (NSCLC) and its correlation with lymph node metastases. Lung Cancer 2007; 56: 51–58. [DOI] [PubMed] [Google Scholar]
- 7. Jin YL, Wang ZQ, Qu H et al Annexin A7 gene is an important factor in the lymphatic metastasis of tumors. Biomed Pharmacother 2013; 67: 251–259. [DOI] [PubMed] [Google Scholar]
- 8. Marquis J, Paillard L, Audic Y et al CUG‐BP1/CELF1 requires UGU‐rich sequences for high‐affinity binding. Biochem J 2006; 400: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watanabe T, Takagi A, Sasagawa N, Ishiura S, Nakase H. Altered expression of CUG binding protein 1 mRNA in myotonic dystrophy 1: Possible RNA‐RNA interaction. Neurosci Res 2004; 49: 47–54. [DOI] [PubMed] [Google Scholar]
- 10. Ward AJ, Rimer M, Killian JM, Dowling JJ, Cooper TA. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum Mol Genet 2010; 19: 3614–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng Y, Miskimins WK. CUG‐binding protein represses translation of p27Kip1 mRNA through its internal ribosomal entry site. RNA Biol 2011; 8: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu TX, Rao JN, Zou T et al Competitive binding of CUGBP1 and HuR to occludin mRNA controls its translation and modulates epithelial barrier function. Mol Biol Cell 2013; 24: 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Timchenko NA, Patel R, Iakova P, Cai ZJ, Quan L, Timchenko LT. Overexpression of CUG triplet repeat‐binding protein, CUGBP1, in mice inhibits myogenesis. J Biol Chem 2004; 279: 13129–13139. [DOI] [PubMed] [Google Scholar]
- 14. Verhoven B, Yan Y, Ritter M et al Ki‐67 is an independent predictor of metastasis and cause‐specific mortality for prostate cancer patients treated on Radiation Therapy Oncology Group (RTOG) 94‐08. Int J Radiat Oncol Biol Phys 2013; 86: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rami‐Porta R, Bolejack V, Goldstraw P. The new tumor, node, and metastasis staging system. Semin Respir Crit Care Med 2011; 32: 44–51. [DOI] [PubMed] [Google Scholar]
- 16. Wang F, Ning F, Liu C et al Comparison of Gefitinib versus VMP in the combination with radiotherapy for multiple brain metastases from non‐small cell lung cancer. Cell Biochem Biophys 2015; 71: 1261–1265. [DOI] [PubMed] [Google Scholar]
- 17. Cui YH, Xiao L, Rao JN et al miR‐503 represses CUG‐binding protein 1 translation by recruiting CUGBP1 mRNA to processing bodies. Mol Biol Cell 2012; 23: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arnal‐Estapé A, Tarragona M, Morales M et al HER2 silences tumor suppression in breast cancer cells by switching expression of C/EBPss isoforms. Cancer Res 2010; 70: 9927–9936. [DOI] [PubMed] [Google Scholar]
- 19. Rattenbacher B, Beisang D, Wiesner DL et al Analysis of CUGBP1 targets identifies GU‐repeat sequences that mediate rapid mRNA decay. Mol Cell Biol 2010; 30: 3970–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Wang H, Ji F, Zhao S, Fang X. Lentivirus‐mediated knockdown of CUGBP1 suppresses gastric cancer cell proliferation in vitro. Appl Biochem Biotechnol 2014; 173: 1529–1536. [DOI] [PubMed] [Google Scholar]
- 21. Jiao W, Zhao J, Wang M et al CUG‐binding protein 1 (CUGBP1) expression and prognosis of non‐small cell lung cancer. Clin Transl Oncol 2013; 15: 789–795. [DOI] [PubMed] [Google Scholar]
- 22. Pugsley JM, Schmidt RA, Vesselle H. The Ki‐67 index and survival in non‐small cell lung cancer: A review and relevance to positron emission tomography. Cancer J 2002; 8: 222–233. [DOI] [PubMed] [Google Scholar]
- 23. Dijkman HB, Weening JJ, Smeets B et al Proliferating cells in HIV and pamidronate‐associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 2006; 70: 338–344. [DOI] [PubMed] [Google Scholar]
- 24. Li R, Heydon K, Hammond ME et al Ki‐67 staining index predicts distant metastasis and survival in locally advanced prostate cancer treated with radiotherapy: An analysis of patients in radiation therapy oncology group protocol 86‐10. Clin Cancer Res 2004; 10: 4118–4124. [DOI] [PubMed] [Google Scholar]
- 25. Bubb RS, Komaki R, Hachiya T et al Association of Ki‐67, p53, and bcl‐2 expression of the primary non‐small‐cell lung cancer lesion with brain metastatic lesion. Int J Radiat Oncol Biol Phys 2002; 53: 1216–1224. [DOI] [PubMed] [Google Scholar]
- 26. Yamayoshi T, Nagayasu T, Matsumoto K, Abo T, Hishikawa Y, Koji T. Expression of keratinocyte growth factor/fibroblast growth factor‐7 and its receptor in human lung cancer: Correlation with tumour proliferative activity and patient prognosis. J Pathol 2004; 204: 110–118. [DOI] [PubMed] [Google Scholar]
- 27. Thorsteinsson H, Jonsson S, Alfredsson H, Isaksson HJ, Gudbjartsson T. [Results of pneumonectomy for non‐small cell lung cancer in Iceland]. Laeknabladid 2009; 95: 823–829. (In Icelandic.) [PubMed] [Google Scholar]
- 28. Dundar E, Oner U, Peker BC, Metintas M, Isiksoy S, Ak G. The significance and relationship between mast cells and tumour angiogenesis in non‐small cell lung carcinoma. J Int Med Res 2008; 36: 88–95. [DOI] [PubMed] [Google Scholar]
- 29. Hoody DW, Beckett CF, Zielenski C, Moore GD. Quality of drug information database research for clinical decision support. Int J Clin Pharmacol 2011; 33: 599–602. [DOI] [PubMed] [Google Scholar]


