Abstract
The discovery that mutations in the succinate dehydrogenase (SDH) complex subunit (SDHA, B/C/D/AF2) genes predispose patients to the development of tumors has led to the identification of a large population of patients and relatives at risk for developing malignancies. The most frequent conditions associated with these mutations are the familial paraganglioma syndromes. Other tumors that are frequently associated with SDH mutations (SDHx) are gastrointestinal stromal tumors and renal cell carcinomas. A number of other rare associations have also been described. SDHx mutations are often clinically silent and metastatic, but they may also be aggressive in their presentation. The penetrance of these mutations is beginning to be understood, and the characteristics of the phenotype are being elucidated. However, the inability to accurately predict the appearance, nature, and location of tumors as well as their tendency to recur or metastasize pose challenges to those who counsel and manage patients with SDHx mutations. In this work, we present our approach for counseling these families in the context of the current uncertainties, while striving to maintain patient autonomy.
Keywords: counseling, paragangliomas, pheochromocytoma, subunits, succinate dehydrogenase
Introduction
An increasing number of families with mutations that lead to paraganglioma (PGL) syndromes are being identified since the description of the causative genetic defects, starting more than two decades ago (1). Our knowledge on the diagnosis and management of these disorders continues to improve, and more evidence regarding the natural history and the progression of these disorders is now available. However, the uncertainties about prognosis, morbidity, and mortality are still significant. Thus, helping patients cope with this diagnosis while keeping them active in the management of their condition continues to be a challenge. We present in this study our approach to a comprehensive counseling model, which includes patient education, psychosocial interventions and service delivery, for this population. Our discussion underscores the benefits of risk assessment, genetic testing, and educating patients on the importance of being actively involved in preventive and screening guidelines. In addition, we illustrate the importance of individualized preventive and screening guidelines for this group of disorders.
PGLs are tumors that originate from the chromaffin cells of the embryonic neural crest; these cells are distributed from the middle ear/skull to the pelvic floor. Based on their anatomical location and function, PGLs can be divided into two categories. The first category includes extradrenal tumors of the head and neck (HNPGLs) usually located at the carotid bifurcation, along the vagal nerve, in the jugular foramen, or in the middle ear space. The second category includes tumors located below the neck, which are most commonly found in the adrenal medulla (also known as pheochromocytoma; PCC), else-where in the abdomen, urinary bladder, and the upper mediastinum (2). The prevalence rates of these tumors are approximately 1:4500 and 1:1700, respectively, with an incidence of 3–8 cases/1 million per year in the general population (3).
PGLs/PCCs present in both hereditary and sporadic forms; there are ten genes that are involved in the pathogenesis of this condition. These include REarranged during transfection (RET [MIM 164761]) proto-oncogene, von Hippel-Lindau disease tumor suppressor gene (VHL [MIM 193300]), neurofibromatosis type 1 tumor suppressor gene (NF1 [MIM 162200]), genes encoding the succinate dehydrogenase (SDH) complex subunits (SDHB, SDHC, and SDHD genes), the gene encoding the enzyme responsible for the flavination of the SDHA subunit (SDHAF2 or SDH5 gene) (4–8), the tumor suppressor gene TMEM127 [MIM 613403] (9), and the Myc associated factor x gene (MAX [MIM 154950]), which are responsible mostly for sporadic PCCs (10). About 30%–35% of PCCs are due to mutations in these genes (11). Among the SDH genes, the first association with hereditary PGLs was identified for SDHD (5); this finding led to description of the other SDH subunit gene mutations. At this time, we know of four well defined PGL syndromes; PGL1 on 11q23.1 (12–15), PGL2 on 11q12.2 (13, 16, 17), PGL3 on 1q23.3 (8), and PGL4 on 1p36.1-p35 (4). The co-ocurrence of both PGLs and PCCs is well documented in these syndromes (18). SDHD, SDHAF2, SDHC, and SDHB are responsible for PGL1 (MIM 602690), PGL2 (MIM 601650), PGL3 (MIM 602413) and PGL4 (MIM 185470), respectively.
Mutations in the SDH subunit complex are inherited in an autosomal dominant manner with age-dependent and incomplete penetrance. However, mutations in the SDHD gene show a parent-of-origin effect (suggestive of maternal imprinting) (14, 19). PGL syndromes and other hereditary syndromes that involve predisposition to PGLs and PCCs are associated with high morbidity and significant complications, which lead to decreased lifespan and quality of life. Hence, early screening and therapeutic interventions are essential in improving disease management. However, the expression of the phenotype is variable and the penetrance of these mutations has not been clearly defined. In addition, other tumors [e.g., renal, neuroblastoma, and gastrointestinal stromal cell tumors (GIST)] have been associated with SDH mutations (20–23). These factors make it difficult to define the natural history and phenotypic characteristics of these mutations; therefore, the counseling of patients is filled with uncertainties. We describe here our approach to counseling 164 families with SDHB/C and D mutations who represent a clinically heterogeneous group. Our focus during each counseling session was centered on four main goals: to provide patients with new perspectives on their understanding of the disease, increase patient's control over their condition, provide accurate information, and improve management of the disease.
Materials and methods
Participants
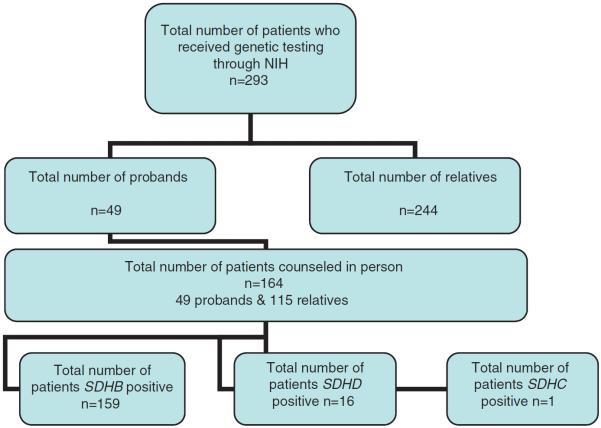
Both affected and unaffected family members were seen at the clinical center of the National Institutes of Health (NIH). Of the 293 patients tested at our facility, 246 had mutations in the SDHB gene (83%), 16 in SDHD (5.4%), and one in SDHC (0.3%). A total of 164 patients from this group were given one-on-one counseling (159 with SDHB, four with SDHD, and one with SDHC mutations; Figure 1). Individual medical and family histories were recorded during counseling, and all patients had been previously tested or evaluated to rule out VHL, MEN2, and NF1. All participants met with a genetic counselor and underwent pre- and post-test counseling for SDHB/C/D gene defects. The initial contact with a family was made through an affected family member (typically the proband) who presented with either PGL(s), PCC(s), or both. Upon confirmation of SDH subunit mutations on the proband, letters were sent to relatives (with proband's permission) for elective genetic counseling, testing and screening if positive.
Figure 1.
Flow chart of the patient population who received genetic testing and genetic counseling at NIH.
Genetic analysis
The sequencing of SDHB/C/D was performed by Mayo Clinic Laboratories. Genetic testing through NIH involved assessment for mutations or large deletions in the RET protooncogene, the VHL gene, and subunits B, C, and D of the SDH complex. Investigation for the more recently discovered SDHA, SDHAF2, and TMEM127 genes were not performed. Genotyping was performed in collaboration with several laboratories, including the Mayo Clinic in Rochester, Minnesota, USA, and the Department of Genetics of the European Georges Pompidou Hospital, Paris, France.
Genetic counseling procedures
The genetic testing of the different mutations associated with PGLs was done based on clinical presentation, medical and family history, and previous testing of relatives. Guidelines for testing patients with suspected PGL/PCC has been previously described in detail (24–26). The approach for managing and counseling PGL patients included several one-on-one in-person sessions with the patient (or family members). These meetings were divided into two categories, namely, pre-test and post-test.
Pre-Test
This was done to ensure that the person understood the implications of a positive test, and that he or she had enough balanced information to be able to formulate a truly informed consent. This session included an explanation of why the test was being offered, how the results might alter the individual's life, general information about the genes being tested, possible outcomes of tests, and brief discussion on management techniques to be discussed further if results are positive. During this initial session, we also addressed any misconceptions regarding the disease, prognosis, etiology, and management.
Post-Test
For positive patients, we discussed diagnosis, prognosis, assessment of the understanding of current treatment and/or management, explanation of recurrence risk, testing of relatives, future options (including prenatal diagnosis for younger patients), and coping with the results. All patients who tested positive were counseled extensively on the implications of the results, and were given screening and preventive guidelines according to their age group and mutation/disease status. This session lasted between 1 and 2 h depending on the patient's need for questions. In this part of the session, the focus was to allow patients to express emotions, doubts, and fears about the implications of the test results. The information part of the session was used to explain and discuss in detail the preventive and screening guidelines.
Results
A total of 49 probands were seen initially for clinical evaluation, counseling, and testing. Letters were sent to 278 unaffected relatives notifying them of the risk and offering mutation analysis; 248 unaffected family members elected to be tested (80 were tested and counseled on other centers) and only four declined testing. A total of 164 patients were found to have mutations, and out of this group, 21 were found to have tumors (PCC/PGL) by imaging studies (12.8%). The four relatives who declined testing gave the following reasons: they had no offspring and did not want to know for themselves, they had other chronic health conditions and did not want to deal with more health information now, or they did not want to participate unless it helped their relative's current health. All patients expressed an improvement over worries and concerns after post-testing counseling, stating that the preventive and screening guidelines were helpful and provided a frame for management of the disorder.
Family history
Out of the total 49 probands counseled at the NIH, 33 family histories were obtained; the rest of the histories were incomplete or provided inaccurate information. All 33 family histories were from probands of SDHB mutation-positive families. In our sample of families from SDHB probands, the following incidences were reported for associated cancers (Table 1): four families reported relatives with colon cancer (12%), seven families reported pancreatic cancer (21%), one family reported thyroid cancer (3%), 12 families reported breast cancer (36%), four families reported neuroblastoma (12%), three families reported uterine cancer (9%), and four families reported ovarian cancers (12%). In this study, we did not control for known risk factors or tested for other mutations associated with hereditary cancer syndromes (ongoing studies are addressing these issues).
Table 1.
Family history of 33 probands with SDHB mutations and the number of reported cancer cases.
| Type of cancer | Colon cancer | Breast cancer | Thyroid cancer | Neuroblastoma | Pancreatic cancer | Uterine cancer | Ovarian cancer |
|---|---|---|---|---|---|---|---|
| Number of cases | 4 | 12 | 1 | 4 | 7 | 3 | 4 |
| Percentage of total families | 12% | 36% | 3% | 12% | 21% | 9% | 12% |
Observations and recommendations
In this patient population, most of the emotional burden was focused on the uncertainty of the appearance of tumors (for those who were asymptomatic/no tumors) and risk of malignancy (for those with tumors).
Based on our experience with counseling this group of patients, we can derive several observations and recommendations, which are listed below.
-
–
The critical component of the genetic counseling process in this group was determination and communication of risk (including risk of malignancy).
-
–
The information collected from each patient should include a thorough personal medical history not targeted to PGL-related symptoms. Seemingly unrelated findings proved to be valuable information for risk assessment as well as the indentificaton of additional risk factors.
-
–
Family history should comprise data from at least four generations with targeted questions, in order to elicit the necessary information for risk assessment and identifications of individuals at risk. Pedigree should be updated as additional information becomes available.
-
–Several misconceptions were identified at the initial pre-test sessions. The most common ones include the following:
-
–the belief that if a mutation is idenfied in a person, his/her prognosis will be exactly the same as their affected relative;
-
–perception of risk is higher than actual risk for both penetrance and risk of malignancy; and
-
–The belief that the mutation is more penetrant in younger generations.
-
–
-
–
Education regarding the genetics of PGL syndromes, and discussions on preventing and screening options proved to be most beneficial. All patients, except one (see below) reported reduction of anxiety, increased sense of control, improved accuracy of perceived risk, and increased knowledge about the condition.
-
–
A small subset of patients (2/164) was more vulnerable to testing distress and demonstrated excessive anxiety upon receiving test results. They required additional counseling sessions aimed at identifying their adapting techniques, and coping strategies.
-
–
Patients who presented with tumors and were found to have a deleterious mutation in one of the SDH genes were mostly concerned with risk of malignancy. This concern was more evident in the SDHB-positive group due to the increased risk of malignancy.
-
–
Unaffected family members who were found to have a mutation were mostly concerned with risk of appearance of tumors and passing the mutation on to their offspring.
-
–
Frustration about uncertainty of recurrence/malignancy was reduced by emphasizing the importance of following preventive and screening guidelines (e.g., imaging, blood & urine tests).
-
–
Surprisingly, knowledge of carrier status of SDH mutations did not deter young couples/patients from having a desire to conceive in the future. Therefore, prenatal counseling was an important part of our study; we had one couple who conceived successfully through in-vitro fertilization with prenatal-genetic diagnosis.
-
–
In total, there were four couples with one partner identified as a carrier of an SDH mutation (one with metastatic PGL/PCC). All four couples expressed the desire to conceive and requested prenatal counseling. They all reported gaining benefits from this session.
-
–
One out of 164 patients decided not to implement any preventive guidelines and screening measures in her two positive offspring due to concerns that it will disrupt their life, bring anxiety, and remind them of the disease. Additional counseling sessions were scheduled with this family, along with referrals to local specialists.
Discussion
This paper describes the NIH approach for genetic counseling of those who are part of the PGL/PCC patient population who undergo SDH testing. Based on our observations of 164 mutation positive patients, we found that providing patients with structured preventive and screening guidelines (see supplemental information) according to their mutation/disease status decreased anxiety and gave them an increased sense of empowerement.
The information that we gathered from 33 family histories yielded high rate of reported cancer cases (Table 1). The presence of thyroid, pancreatic, breast, and renal cancer in families with SDH mutations has been noted before (23, 27–31). In our study, the incidence of these tumors in the patient population is higher than in other studies. One explanation for this may be that our patients are ascertained through affected relatives, indicating that our sample may reflect a more homogeneous population. Future studies are on the way to further caharacterize the family history in a more extensive number of patients with SDH mutations, and patients with other mutations that predispose to PGLs and PCCs.
The counseling sessions were aimed at exploring the impact of the diagnosis on both affected and unaffected family members, assisting families and individuals as they adjusted to the diagnosis, and collecting pertient medical information to set the basis for future studies.
The information is complex; thus, we focused our approach in making it personally relevant to the patient, while addressing their emotional concerns and reactions as needed. During this part, it was imperative to engage patients in the process of cognitive assimilation of genetic information so that they can understand and organize the information in terms of their respective values, beliefs, and lifestyles. There are no established genetic counseling guidelines for SDH mutation carrier. Our counseling approach was based on the information gathered from review of the studies on this patient population and our own studies. This part proved to be the most challenging due to the following factors: lack of explicit guidelines in the literature, lack of knowledge of physicians taking care of these families, and the need for different screening and preventing guidelines according to the stage of disease, mutation, and other factors (see above; counseling guidelines). These measures are important because of the uncertainty associated with them and the lack of means by which to accurately predict the appearance and location of tumors and their tendency to recur. In addition to informed decision-making regarding genetic testing, we were primarily concerned with decreasing anxiety, increasing the patient's sense of control, and improving accuracy of risk perception.
If left untreated, PGLs can result in significant clinical morbidity and mortality. Thus, early treatment and the identification of individuals who have higher risks for PGLs are thus imperative. The counseling approach highlighted here is aimed at improving adherence to screening recommendations, which then decreases morbidity and mortality. The clinical manifestations of PGLs are broad, and the majority of symptoms can mimic minor ailments (e.g., headaches, palpitations). Therefore, once a mutation has been identified, individuals should be monitored closely with a lower threshold for further evaluation of symptoms by a physician. Many studies are currently being conducted to characterize further the genotype-phenotype correlations, with the hope that more specific guidelines can be generated for this patient population.
Practice implications
Genetic counselors can effectively counsel these patients by providing specific preventive and screening guidelines according to the type of mutation, tumor, stage, and/or patient's clinical presentation (see supplemental information). In addition, it is important to address the uncertainty of the appearance of tumors (for patients who are asymptomatic/no tumors) and risk of malignancy (for patients who have tumors). In our patient population, anxiety towards this uncertainty was decreased by providing specific screening and preventive plans that address their particular situation. We offer further guidelines for genetic counselors and points to consider when working with patients who have SDH mutations. The information is complex, and counseling should be personally relevant to the patient, while addressing their emotional concerns and reactions as needed.
Study limitations and future recommendations
For genetic counselors who work with these patients, there are unique challenges that remain, and guidelines will change as we move forward with research. Our study was limited by the the small sample size, the ascertainment of patients through affected probands, and the lack of a quantitative study design based on these qualitative data. These limitations will be addressed in future studies with larger sample size. Future research should also be aimed at further characterizing the genotype-phenotype correlations with the hope that more specific guidelines can be generated for this patient population. This is the first publication of counseling patients with SDH mutations, and as such, we have provided valuable insights and recommendations when dealing with this patient population.
Supplementary Material
Table 2.
Penetrance by age.
Table 3.
Clinical manifestations.
| Phenotype | SDHB | SDHC | SDHD | References |
|---|---|---|---|---|
| Multiple primary tumors | Not commom | Not common | Common | Raygada et al. (1); Pasini and Stratakis (25) |
| Single primary tumors | Common | Common | Not common | Raygada et al. (1); Pasini and Stratakis (25) |
| Extra adrenal tumors | Common (50%–67%) | Less common | Less common (18%–21%) | |
| HNPGL | Less common (27%–31%) | Common | Common (71%–89%) | Niemann et al. (8) |
| Risk for malignancy | High (34%–37%) | Low | Low (8%) | Bardella et al. (32); Benn et al. (33); |
Note: Many studies are still analyzing data and associated risks may change as more cases are reported.
Acknowledgments
This work was funded by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Conflict of interest statement: The authors declared no conflict of interest.
References
- 1.Raygada M, Pasini B, Stratakis CA. Hereditary paragangliomas. Adv Otorhinolaryngol. 2011;70:99–106. doi: 10.1159/000322484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winship T, Kloop CT, Jenkins WH. Glomus jugularis tumors. Cancer. 1948;1:441. doi: 10.1002/1097-0142(194809)1:3<441::aid-cncr2820010310>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Beard CM, Sheps SG, Kurland LT, Kurland LT, Carney JA, et al. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983;58:802–4. [PubMed] [Google Scholar]
- 4.Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–51. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 6.Burnichon N, Briere JJ, Libé R, Vescovo L, Rivière J, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–20. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;28:1139–42. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–70. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 9.Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42:229–33. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, Landa I, Leandro-GarcÃa LJ, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43:663–7. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- 11.Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–66. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- 12.Baysal BE, Farr JE, Rubinstein WS, Galus RA, Johnson KA, et al. Fine mapping of an imprinted gene for familial nonchromaffin paragangliomas, on chromosome 11q23. Am J Hum Genet. 1997;60:121–32. [PMC free article] [PubMed] [Google Scholar]
- 13.Mariman EC, van Beersum SE, Cremers CW, van Baars FM, Ropers HH. Analysis of a second family with hereditary non-chromaffin paragangliomas locates the underlying gene at the proximal region of chromosome 11q. Hum Genet. 1993;91:357–61. doi: 10.1007/BF00217356. [DOI] [PubMed] [Google Scholar]
- 14.Heutink P, van Schothorst EM, van der Mey AG, Bardoel A, Breedveld G, et al. Further localization of the gene for hereditary paragangliomas and evidence for linkage in unrelated families. Eur J Hum Genet. 1994;2:148–58. doi: 10.1159/000472358. [DOI] [PubMed] [Google Scholar]
- 15.Milunsky J, DeStefano AL, Huang XL, Baldwin CT, Michels VV, et al. Familial paragangliomas: linkage to chromosome 11q23 and clinical implications. Am J Med Genet. 1997;72:66–70. doi: 10.1002/(sici)1096-8628(19971003)72:1<66::aid-ajmg14>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Mariman EC, van Beersum SE, Cremers CW, Struycken PM, Ropers HH. Fine mapping of a putatively imprinted gene for familial non-chromaffin paragangliomas to chromosome 11q13.1: evidence for genetic heterogeneity. Hum Genet. 1995;95:56–62. doi: 10.1007/BF00225075. [DOI] [PubMed] [Google Scholar]
- 17.Niemann S, Becker-Follmann J, Nürnberg G, Rüschendorf F, Sieweke N, et al. Assignment of PGL3 to chromosome 1 (q21–q23) in a family with autosomal dominant non-chromaffin paraganglioma. Am J Med Genet. 2001;98:32–6. [PubMed] [Google Scholar]
- 18.Sato T, Saito H, Yoshinaga K, Shibota Y, Sasano N. Concurrence of carotid body tumor and pheochromocytoma. Cancer. 1974;34:1787–95. doi: 10.1002/1097-0142(197411)34:5<1787::aid-cncr2820340529>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.van der Mey AG, Maaswinkel-Mooy PD, Cornelisse CJ, Schmidt PH, van de Kamp JJ. Genomic imprinting in hereditary glomus tumours: evidence for new genetic theory. Lancet. 1989;2:1291–4. doi: 10.1016/s0140-6736(89)91908-9. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong R, Greenhalgh KL, Rattenberry E, Judd B, Shukla R, et al. Succinate dehydrogenase subunit B (SDHB) gene deletion associated with a composite paraganglioma/neuroblastoma. J Med Genet. 2009;46:215–6. doi: 10.1136/jmg.2008.060749. [DOI] [PubMed] [Google Scholar]
- 21.Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2010;108:314–8. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst. 2008;100:1260–2. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 23.Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peçzkowska M, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–9. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnichon N, Rohmer V, Amar L, Herman P, Leboulleux S, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab. 2009;94:2817–27. doi: 10.1210/jc.2008-2504. [DOI] [PubMed] [Google Scholar]
- 25.Pasini B, Stratakis CA. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med. 2009;266:19–42. doi: 10.1111/j.1365-2796.2009.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karasek D, Frysak Z, Pacak K. Genetic testing for pheochromocytoma. Curr Hypertens Resp. 2010;12:456–64. doi: 10.1007/s11906-010-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baysal BE. Clinical and molecular progress in hereditary paraganglioma. J Med Genet. 2008;45:689–94. doi: 10.1136/jmg.2008.058560. [DOI] [PubMed] [Google Scholar]
- 28.Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, et al. European-American Paraganglioma Study Group 2004 Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. J Am Med Assoc. 2004;292:943–51. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 29.Ni Y, He X, Chen J, Moline J, Mester J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowdenand Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum Mol Genet. 2012;21:300–10. doi: 10.1093/hmg/ddr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Y, Zbuk KM, Sadler T, Patocs A, Lobo G, et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83:261–8. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schimke RN, Collins DL, Stolle CA. Paraganglioma, neuroblastoma, and a SDHB mutation: resolution of a 30-year-old mystery. Am J Med Genet. 2010;152A:1531–5. doi: 10.1002/ajmg.a.33384. [DOI] [PubMed] [Google Scholar]
- 32.Bardella C, Pollard PJ, Tomlinson I. SDH mutations in Cancer. Biochim Biophys Acta. 2011;1807:1432–43. doi: 10.1016/j.bbabio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91:827–36. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- 34.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, et al. Plasma methoxytyramine: A novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2011;48:1739–49. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–20. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prodanov T, Havekes B, Nathanson KL, Adams KT, Pacak K. Malignant paraganglioma associated with succinate dehydrogenase subunit B in an 8-year-old child: the age of first screening? Pediatr Nephrol. 2009;24:1239–42. doi: 10.1007/s00467-008-1111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Algeciras-Schimnich A, Preissner CM, Young WF, Jr, Singh RJ, Grebe SK. Plasma chromogranin A or urine fractionated metanephrines follow-up testing improves the diagnostic accuracy of plasma fractionated metanephrines for pheochromocytoma. J Clin Endocrinol Metab. 2008;93:91–5. doi: 10.1210/jc.2007-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoegerle S, Ghanem N, Altehoefer C, Schipper J, Brink I, et al. 18F-DOPA positron emission tomography for the detection of glomus tumours. Eur J Nucl Med Mol Imaging. 2003;30:689–94. doi: 10.1007/s00259-003-1115-3. [DOI] [PubMed] [Google Scholar]
- 39.King KS, Chen CC, Alexopoulos DK, Whatley MA, Reynolds JC, et al. Functional imaging of SDHx-related head and neck paragangliomas: comparison of 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine, 18F-fluoro-2-deoxy-D-glucose PET, 123I-metaiodobenzylguanidine scintigraphy, and 111In-pentetreotide scintigraphy. J Clin Endocrinol Metab. 2011;96:2779–85. doi: 10.1210/jc.2011-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King KS, Whatley MA, Alexopoulos DK, Reynolds JC, Chen CC, et al. The use of functional imaging in a patient with head and neck paragangliomas. J Clin Endocrinol Metab. 2010;95:481–2. doi: 10.1210/jc.2009-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–67. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solanki KK, Bomanji J, Moyes J, Mather SJ, Trainer PJ, et al. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG) Nucl Med Commun. 1992;13:513–21. doi: 10.1097/00006231-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 43.King KS, Prodanov T, Kantorovich V, Fojo T, Hewitt JK, et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29:4137–42. doi: 10.1200/JCO.2011.34.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.