Abstract
Middle ear surgery is strongly influenced by anatomical and functional characteristics of the middle ear. The complex anatomy means a challenge for the otosurgeon who moves between preservation or improvement of highly important functions (hearing, balance, facial motion) and eradication of diseases. Of these, perforations of the tympanic membrane, chronic otitis media, tympanosclerosis and cholesteatoma are encountered most often in clinical practice. Modern techniques for reconstruction of the ossicular chain aim for best possible hearing improvement using delicate alloplastic titanium prostheses, but a number of prosthesis‐unrelated factors work against this intent. Surgery is always individualized to the case and there is no one‐fits‐all strategy. Above all, both middle ear diseases and surgery can be associated with a number of complications; the most important ones being hearing deterioration or deafness, dizziness, facial palsy and life‐threatening intracranial complications. To minimize risks, a solid knowledge of and respect for neurootologic structures is essential for an otosurgeon who must train him‐ or herself intensively on temporal bones before performing surgery on a patient.
Keywords: Surgical anatomy, middle ear, facial nerve, tympanic membrane, otitis media
Introduction
The ear is an accurate, powerful and reliable instrument of perception. From the evolutionary standpoint, the ear represents the most important warning sensor of the body, a function which it combines with its fundamental role in social communication. In addition to the acoustic organ, it also houses the equilibrium organ and thus forms the basis of two human sensory systems. Next to the sense of sight, the sense of hearing is our most important biological source of information. The importance of the hearing sense for the social life of man was aptly described by Immanuel Kant: ‘Blindness separates people from things. Deafness separates people from people’.
The middle ear is in many circumstances a remarkable organ. Tympanic membrane (TM), ossicular chain and inner ear represent a highly complex sound pressure wave receiver, whose dynamics are unmatched by any other sense organ. At the hearing threshold, the pressure and amplitude are a millionfold smaller than the loudest tolerable sound. There, the energy level is so low that a further improvement in sensitivity would be pointless, as then the bouncing of molecules on the TM (by Brownian motion) would be audible. While at the hearing threshold, vibration amplitudes of the TM are in the pico‐ to nanometer range (smaller than the diameter of a hydrogen molecule), the TM is displaced by up to 1 mm during changes in static pressure (blowing your nose, swallowing, etc.). At a sound pressure of 1 Pascal (i.e. 94 dB SPL), the vibratory displacement amplitude of the stapes still measures only 30 nm (in the frequency range 100–1000 Hz) (Hüttenbrink, 1988a, 1995).
This review article will discuss the ‘surgical anatomy’ of the human middle ear and its adjacent areas. In contrast to ‘functional anatomy’, which deals with the question ‘What function does an anatomical structure have and how does its design contribute to this?’, ‘surgical anatomy’ revolves around the following questions: ‘What can go wrong in the human middle ear? Do pathologies and their complications have an anatomical or developmental basis? What are the consequences of the middle ear anatomy for surgery and vice versa?’.
After a technical note on the characteristics of middle ear surgery in general, a short overview of congenital anomalies of the middle ear is given. Thereafter, the surgical aspects of middle ear anatomy are discussed in anatomical order. For the convenience of the reader, each section contains, along with a description of the ‘surgical anatomy’, basic information on the physiology, major pathologies and therapeutic (surgical) strategies.
Middle ear surgery
The tympanic cavity represents the bulk of the middle ear space. The tympanic cavity, however, is not an isolated, air‐filled chamber housed within the temporal bone, but communicates anteriorly with the epipharynx via the auditory (Eustachian) tube, and in the opposite direction connects backward with the mastoid cavity through a recess, the tympanic antrum. These adjacent pneumatic spaces, the mastoid and the Eustachian tube, have a strong impact on the clinical appearance of the tympanic cavity and thus influence middle ear surgery.
For middle ear surgery, familiarity with the interrelationships of the anatomical structures is as essential as knowledge of their individual morphology, as surgery is strongly influenced by the close spatial relations between the different components. During ear surgery, the otosurgeon must be capable of freely rotating a three‐dimensional imaginary picture of the temporal bone. To make things worse, the patient lies flat on his back during surgery (imaginary picture rotated by 90° on the x‐axis) with his head being turned to the non‐operated side (imaginary picture rotated by about 45° on the z‐axis). Moreover, the perspective constantly changes as during ear surgery the surgeon varies his direction of view often and ‘moves around’ the ear with the microscope. In line with a change in the angle of view the projection of anatomic structures changes. For example, working and looking through the ear canal, malleus and incus obscure the view into the oval window niche and the anterior boundary of the vestibular organ. During a transmastoid approach, however, the ossicles obscure the view onto the cochlea and the geniculate ganglion. Especially in malformed ears or in those patients whose middle ears have been operated before, the anatomy and thus the topographic relations of middle ear structures can grossly differ from what one considers a ‘normal’ middle ear.
The complex spatial relations of important structures necessitate an extensive theoretical and practical study of the ear anatomy before an ‘otosurgical career’ is started. It is not sufficient to be proficient in the two‐dimensional anatomy (i.e. study of an anatomical atlas). 3D computer models of temporal bones based on computed tomography scans have been developed recently but they lack anatomical details and haptic elements, and hence they don't play a substantial role in practical training so far. Instead, to conduct ear surgery safely, it is essential that the surgeon trains him‐ or herself by dissecting human temporal bones to become familiar with the complex, three‐dimensional relationships of the structures within the ear. Furthermore, the instruments used for ear surgery are very different to those used in other surgery, and again the surgeon needs to train him‐ or herself on these before operating on a living patient. To make matters worse, all middle ear surgery is performed via the binocular microscope, as required by the tiny dimensions. A surgeon who is used to relate his manipulations to his original (macroscopic) vision is now confronted with magnified movements (and tremor) when he looks through the microscope. Extensive training with temporal bone preparations is mandatory to correlate finger movements to this magnified vision.
Congenital anomalies of the middle ear
Congenital anomalies of the middle ear occur in one in 3000–20 000 newborns. Their clinical and surgical relevance derives from the fact that they are characterized by a deviation not only from normal anatomical development but also from regular function (Kosling et al. 2009). There is such a great variety of malformed parts in the middle ear that only some examples can be given within this chapter. Middle ear anomalies can be unilateral or bilateral and may be broadly classified into minor (only involvement of the middle ear) and major (associated with an involvement of the tympanic membrane and external ear) (Teunissen & Cremers, 1993) or into three degrees (mild, moderate, severe) (Kosling et al. 2009). Especially in malformations associated with syndromes [e.g. Franceschetti (Treacher–Collins), Goldenhar, Klippel–Feil] medical management is a challenge and needs close cooperation between the otosurgeon and other specialities (pediatrics, neurology, genetics, radiology and orthopedics) (Kosling et al. 2009). Minor middle ear anomalies include a change in configuration or size of the tympanic cavity or a reduced distance between anatomical structures of the middle ear and fixated ossicles (stapes ankylosis). In major anomalies, the ossicles are often involved; they are rarely normal and rarely completely missing. The most common isolated ossicle deformity involves the stapes suprastructure in terms of aplasia or hypoplasia, thickening, thinning or fusion of the stapes crura (Bartel‐Friedrich & Wulke, 2007). In each case, the surgeon needs to estimate whether deformed ossicles can perform a normal transmission function or should be replaced. There may be a fusion of joints inside the ossicular chain or a separation of ossicles. Also, the inner ear windows may be involved in middle ear anomalies in terms of a mobile stapes footplate or dysplasia/aplasia of the round or oval window (Kosling et al. 2009). Other anomalies include dehiscence or displacement of the facial nerve, a missing antrum, apneumatized mastoid, and aberrant courses of arteries and veins (Bartel‐Friedrich & Wulke, 2007). Of note, primary cholesteatoma (discussed below) also counts as a congenital middle ear anomaly.
Tympanic membrane
Located at the medial end of the external ear canal, the tympanic membrane (TM, eardrum, membrana tympani) forms the lateral border of the tympanic cavity. It is the only membrane in the human body which is surrounded by air on both sides. Having a thickness of about 0.1 mm and a diameter of about 10 mm, the TM is composed of three layers, with the fibrous stratum (lamina propria) being the dominant component in the middle, giving stability to the membrane. Inside this fibrous stratum, there is a remarkable arrangement of extremely solid collagen fibers, running radially, concentrically and tangentially (Jahnke, 2004). The ability of the collagen fibers to stretch is limited, allowing high compliance with the small displacements occurring at acoustic pressures but resistance to further stretching at higher pressures. Above 400 daPa, the TM behaves like a solid wall and a further increase of atmospheric pressure will not induce any further displacement (bulging) of the TM (Hüttenbrink, 1995).
The fibrous stratum is covered on both sides by two epithelial layers. On the lateral side, this layer is formed by stratified, keratinizing epithelium, a thin continuation of the skin which lines the external ear canal – a circumstance which becomes a critical factor during the development of cholesteatoma (see below), because this disease derives from a TM retraction pocket or TM perforation.
On the medial side, the epithelial layer is formed by cuboidal, mucosal epithelium. The membrane's thickened annulus fibrosus, which consists of fibrocartilage, is anchored in a sulcus of the bony ear canal (Lang, 1992).
Anatomically, the TM can be divided into the tense portion (pars tensa), which constitutes nearly the complete membrane in humans, and the much smaller flaccid portion (pars flaccida, Shrapnell's membrane). The tense portion describes the large area of the membrane below the neck of the malleus, i.e. inferior to the plicae mallearis anterior et posterior – the area encircled by the annulus. In a healthy state the tense portion is transparent, but inflammation, effusion or scarring can lead to a characteristic color change. The flaccid portion covering the incisura Rivini of the lateral epitympanic wall between the processus brevis mallei and the roof of the bony ear canal (tympanic notch) lacks an annulus fibrosus. The TM inserts directly into the bony ear canal here. Unlike the pars tensa, the pars flaccida lacks a central fibrous layer.
The short process of the malleus (processus brevis, also known as the lateral process) is located at the superior end of the manubrium (malleus handle). The manubrium is loosely adherent to the pars tensa, and ends distally in the umbo of the TM. With the umbo of the TM being its most depressed point, the membrane receives a conical shape with a slight outward convex curvature between annulus and manubrium.
Clinically, the TM can be divided into four quadrants, which are separated from each other by an imaginary straight line drawn down the malleus handle and continuing to its lower edge, and a perpendicular line running through the umbo. The correct physiologic position of the TM (balanced pressure situation between outer ear canal and tympanic cavity) is signaled during otoscopy by a triangular reflection of light from the lower anterior quadrant, contacting the umbo. This small area is the only part of the TM which is orientated perpendicular to the incoming light, due to the oblique orientation of the external ear canal and the concave shape of the membrane. In the pre‐binocular‐otoscopic era, the correct position of the light reflex was the only diagnostic clue for the otologist to detect a retraction of the membrane.
From a functional standpoint of hearing, the TM can be considered the vibrational driver of the middle ear apparatus. The hearing process starts with sound pressure waves traveling through the external ear canal, reaching the TM. The vibrations of the air molecules induce a vibration of the TM. The impedance transformation achieved by the middle ear, from vibrations in air to vibrations in the fluid of the cochlea, is the origin of the final vibration of the hair cells within the cochlea. Additionally, the TM provides some amplification of the sound pressure by a few dB due to its curved surfaces (Helmholtz’ catenary principle) (Hüttenbrink, 1988a; Teunissen & Cremers, 1993).
The acoustic properties of the TM are vital for normal middle ear function. The ability of the TM to respond to the smallest sound pressures, and at the same time to withstand very large (atmospheric) pressure fluctuations, is closely linked to its micro‐ and macro‐anatomy. Hence, during surgery of the TM, the special physical characteristics of this organ must be considered.
Cholesteatoma
The TM can be at the root of a common and notorious ear disease: cholesteatoma. Cholesteatoma is defined by an abnormal skin growth into the middle ear space behind the plane of the eardrum. Histologically, cholesteatoma is simply a formation of squamous keratinizing cells, similar to the lining of the external ear canal or the TM. The differences between this and normal skin include the fact that this ‘skin’ has advanced to an area of mucosa, i.e. that lining the tympanic or mastoid cavities, and the fact that its granulating undersurface, the perimatrix, has bone‐destroying capacities (Hüttenbrink, 1994).
Many cholesteatoma cases start with a TM retraction in the flaccid portion (prospective cholesteatoma), where the lack of the fibrous layer and of the annulus represent a crucial weakness of the TM. Any keratin being produced by the keratinizing cells on the TM and in the outer ear canal is normally transported in an outward direction (epithelial migration). In retraction pockets, however, this self‐cleaning mechanism can become defective. If the keratin is no longer transported over the rim of the retraction, the debris (keratin) accumulates (pre‐cholesteatoma). These masses, which become inflamed, induce a foreign body reaction within the perimatrix, with destruction of all adjacent structures (manifest cholesteatoma). The retraction pockets increase in size and form a ‘cholesteatoma sac’, which advances further into the tympanic cavity. The cholesteatoma sac will destroy structures it comes into contact with due to the constant pressure and the destructive process in the perimatrix tissue.
Despite these pathogenic considerations, there is no unanimously accepted theory for the development of acquired cholesteatoma within the voluminous literature on this issue. Most authors support a theory involving a combination of the precursor retraction pockets and the migration theory (Karmody et al. 2009).
Besides development in the area of Shrapnell's membrane (epitympanic cholesteatoma), retraction may also occur in the tense portion of the TM, with predominant cholesteatoma growth into the tympanic cleft and/or mastoid (Tos, 1988).
Cholesteatomas originating from the TM (following a TM retraction or a defect) are called secondary cholesteatoma and represent 99% of cholesteatoma cases. In contrast, primary cholesteatoma is a congenital dermoid cyst developing behind an intact TM, deriving from the embryonic epidermal crest. Primary cholesteatoma normally becomes symptomatic within the first years of childhood by hearing impairment due to its extension into the middle ear cleft and the destruction of the ossicular chain (Michaels, 1988).
One of the unfortunate characteristics of cholesteatoma is that it never heals by itself, but will gradually expand and destroy adjacent structures. Thus, cholesteatoma is not limited to the TM, but can extend into the middle ear or inner ear or even beyond the temporal bone. Despite its name, cholesteatoma is a ‘benign’ disease (it is not a tumor and does not metastasize) but, as described later, if left untreated it can lead to significant morbidity and even mortality, due to its destructive character.
Tympanic membrane surgery
In treating the majority of pathologies, TM surgery is performed through the ear canal (endaural approach). However, for pathologies located in the anterior parts of the TM, the endaural approach allows only limited access due to the backwards‐sloping anterior wall of the outer ear canal. In these cases, a retroauricular access is advisable. For this, a semicircular skin incision is made slightly dorsal to the auricle skin fold. The auricle is folded anteriorly and the skin canal wall is opened from behind. Often, the otosurgeon is confronted with a narrow bony ear canal or exostoses, protruding into the lumen, which limits the access to the middle ear. To get a sufficient overview and enough space for surgical manipulations, the ear canal must be widened using a drill. For this, skin flaps are created within the ear canal by incision and folded away to expose the bony surface.
During the drilling process, for which diamond burrs are used, the surgeon must be aware of several risks, e.g. opening of the capsule of the temporomandibular joint (anteriorly), damage to an aberrant facial nerve (dorso‐caudally) or touching the TM or the short process of the malleus at the prominentia mallearis (medially). If the TM or any structure of the ossicular chain is touched with the rotating drill, a huge amount of energy (up to 120–140 dB) will be transmitted into the inner ear. This mechanical overload (acoustic trauma) can lead to serious hearing damage.
The most commonly performed ear surgery is a simple incision into the TM (paracentesis) performed with a small sickle knife or trepan.
Indications may be chronic otitis media with effusion or acute otitis media, with the aim to aspirate the fluid from the middle ear. Often, the paracentesis is combined with the insertion of a small tympanostomy tube (grommet) into the TM, which prevents the premature, spontaneous closure of the opening. The grommet remains in situ for a period of several months until it is rejected by epithelial migration. Grommets serve to improve ventilation of the middle ear until the mucoid transformation of the middle ear mucosa has redeveloped into the normal respiratory type.
Although technically simple, paracentesis is not a simple or generally unperilous operation, as important structures can be accidentally injured. For example, in the anterior sections of the TM, puncture of an aberrant, uncovered carotid artery may cause a dramatic complication. The region of the posterior upper quadrant should not be incised, as this carries the risk of damage to the underlying ossicular chain (i.e. the area of the incudostapedial joint). At the lower posterior quadrant, an uncovered high jugular bulb (one of the most common variations of the jugular vein) protruding into the hypotympanum may be accidentally opened, causing significant morbidity and mortality (Atmaca et al. 2014). As the normal jugular bulb is fully encompassed in bone, iatrogenic opening and the heavy bleeding which may result cannot be solved with simple coagulation as in bleeding of smaller and easily accessible veins. Instead, in case of bleeding, the bulb must be covered with connective tissue and autologous cartilage.
Because of these risks of injury to middle ear structures, the best option for paracentesis is to do the incision in the anterior lower quadrant.
Traumatic perforations of the TM are regularly encountered in clinical practice. They are inflicted by direct mechanical trauma (e.g. cotton swab, hair pin, hitting the water surface) or hydraulically via an overpressure in the ear canal (e.g. slap on the ear), although the stability of the healthy membrane with its collagenous fibers is impressive: it can withstand over‐ or under‐pressures up to 1 atm (10 m water column) (Strohm, 1986).
Acute perforations of the TM have the potential to heal without treatment, with up to 80% undergoing spontaneous closure (Farrior, 1983). Spontaneous healing may be supported by a silicone foil, which is placed on the defect to give guidance to the epithelial growth, starting at the rim of the perforation. The otologist, however, has to ensure using the ear microscope that no epithelial remnants or keratin debris has entered the middle ear, and that the epithelial rims are not rolled inside, as this could become the origin of a middle ear cholesteatoma.
If a TM perforation does not heal by itself in a reasonable time, then a chronic inflammation or another pathology of the middle ear may be the underlying cause (chronic otits media perforata simplex: see later). Failure of a TM perforation to heal spontaneously is usually addressed by surgery to close the defect, to improve hearing and to decrease the risk of further middle ear infection through the open TM (Aggarwal et al. 2006). Typical graft materials used for closure of the TM are muscle fascia, perichondrium, thin cartilage slices and fat (for small perforations) (Jahnke, 2004).
Such autologous transplants for reconstructing the TM are typically inserted using an underlay technique (undermining the limbus, lifting the TM, and covering the defect with the graft from the medial side). To be successful (prevention of re‐perforation) it is essential that a TM graft not only covers the perforation but that it is additionally anchored and stabilized on the bony ear canal (Fig. 1). During this procedure, care should be taken not to damage the chorda tympani when lifting the limbus out of its bony sulcus in the posterior region. To avoid this, the middle ear is normally entered more inferiorly and here again, the otosurgeon may be confronted with a high jugular bulb.
Figure 1.
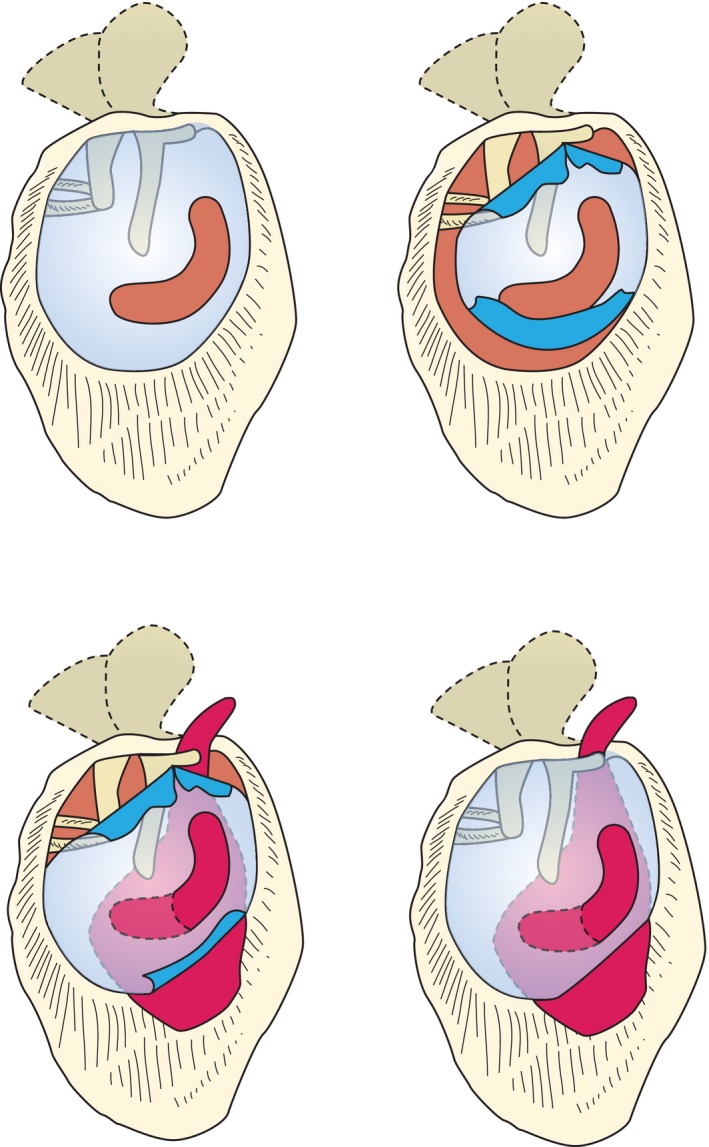
A perforation in the tympanic membrane is closed with a perichondrium transplant in underlay technique, with the transplant being stabilized on the bony ear canal to prevent dislocation.
A few surgeons perform ‘myringoplasty’ using an overlay technique, which requires the positioning of the graft between fibrous stratum and elevated skin of the outer lining. This technique carries a certain risk of covering some of the epithelium with the graft (with consecutive development of a cholesteatoma) or development of scar formation in the anterior angle (called ‘blunting’ due to the resulting blunt anterior tympano‐meatal angle).
With such techniques, successful closure of perforations can be expected in about 95% of ears with normal ventilation. In cases of chronic inflammation of the middle ear mucosa with insufficient gas production, adhesive processes, tympanic fibrosis or defects of the entire TM, the outcome is less good (Zahnert et al. 2000). Hence, if ventilation of the middle ear is reduced, a more stable reconstruction of the TM is needed to avoid re‐perforation. In these cases, cartilage has been proven to be superior over fascia or perichondrium due to its higher mechanical stability (Beutner et al. 2010).
When used with its original thickness, cartilage will result in increased acoustic impedance of the TM because of increased mass and stiffness, thereby having an unfavorable effect on the acoustic transfer characteristics (Zahnert et al. 2000; Jahnke, 2004). Normally, the autologous cartilage is harvested from the tragus or cavum area of the auricle, where its thickness is about 1000 μm. To be suitable for TM reconstruction, cartilage should be cut into slices of 400 μm and less. Varying techniques and modifications for the alignment of the cartilage slices have been described (Beutner et al. 2010).
Tympanic cavity
The tympanic cavity is the chamber between the external ear canal and the inner ear, which forms the essential major part of the middle ear cavity. It is lined by a cuboidal to columnar mucosal epithelium with scattered goblet cells. The distance between nearly all of the important anatomical structures is <10 mm (Wysocki & Skarzynski, 1998), which necessitates the use of a microscope for manipulations. Like a cube, the tympanic cavity is ‘bounded by six not too clearly demarcated walls’ (Anson, 1992) in each direction. The otosurgeon faces delicate structures and corners.
The superior paries tegmentalis (tegmen tympani) separates the tympanic cavity from the middle cranial fossa, forming the roof of one and the floor of the other compartment. The tegmen tympani is often composed of only a very thin bony layer, thus posing the risk of dura injury when manipulating in this area, or spread of a middle ear infection to the intracranial space. Besides, the tegmen tympani can be disrupted in many instances: acquired, by cholesteatoma and chronic otitis media, following a trauma, by iatrogenic damage or spontaneously. A disrupted tegmen with a possible defect of the dura can result in cerebrospinal fluid leakage and herniation of the meninges (meningocele) or brain (meningoencephalocele) into the middle ear cavity. Hence, during middle ear surgery, any soft formation next to the middle cranial fossa must be cautiously examined with palpations of the underlying intact bone and should not just be excised and removed.
The inferior paries jugularis represents the floor of the tympanic cavity (hypotympanum). Normally, this floor consists of bone but it might also be irregularly excavated by some air‐filled cells. Right underneath the floor, the jugular bulb (fossa of the internal jugular vein) lingers and can be inadvertently opened by the otosurgeon, possibly leading to the heavy and troublesome venous bleeding referred to earlier.
The medial paries labyrinthica is mostly represented by the labyrinth capsule, i.e. the lateral cochlear wall with its basal turn forming the promontory here. The oval window (vestibular window; fenestra vestibuli) lies dorso‐superior to the promontory with the round window (fenestra cochleae) caudal to it (Lang, 1992). While the oval window is closed by the stapes footplate, the round window is sealed only by a membrane. Opening the labyrinth carries the risk of perilymph leakage, leading to hearing loss or even deafness.
The posterior border (paries mastoidea) is indistinctly circumscribed by the sinus tympani. Its superior part opens into the mastoid via the antrum area.
The anterior border is called the paries caroticus, a name signaling that this region allows only cautious manipulations, as opening of an exposed carotid artery might have life‐threatening consequences. However, the carotid artery is usually not seen during middle ear surgery, as it is normally completely covered with bone and significant pathologies are rare in this region. Superior and lateral to the carotid artery, the Eustachian tube, which is lined by pseudostratified, ciliated columnar epithelium with scattered goblet cells, opens into the tympanic cavity. Medial and parallel to the tube, the tensor tympani muscle runs in its bony semicanal.
The lateral paries membranaceus is essentially formed by the TM and its bony frame, described earlier.
Clinically, the tympanic cavity can be divided into three levels: hypo‐, meso‐ and epitympanum. The hypotympanum (level below the TM) houses no functional elements. Its clinical and surgical relevance derives from the risk of opening the jugular bulb here and from middle ear diseases originating at the level of the mesotympanum which can advance into this area and may be difficult to treat. The mesotympanum (level of the TM) houses the long processes of malleus and incus, the stapes, oval and round window, facial nerve and parts of the middle ear muscles or their tendons. The epitympanum (level above the TM) houses the body of the malleus and the incus, and thus the bulk of the ossicular chain.
Chronic otitis media
The most common disease affecting the integrity of the ossicular chain is the chronic inflammation of the middle ear, referred to as chronic otitis media (COM). It is defined by a defect of the TM which does not heal spontaneously and persists over time. Within an older terminology, COM was divided into two subtypes: (i) otitis media mesotympanalis (tubo‐tympanic disease) with the tympanic annulus not being involved and (ii) otitis media epitympanalis (attico‐antral disease), which equates to cholesteatoma. In fact, acute otitis media with or without effusion is not connected with the development of cholesteatoma (Tos, 1988).
COM without cholesteatoma is defined by a chronic defect of the TM and may have various underlying causes, including bacterial colonization of the mucosa, mucosal dysfunction with insufficient aeration of the middle ear, tubal dysfunction and lack of local immunocompetence; there may be an anatomical or genetic basis to these problems. The characteristic appearance of COM without cholesteatoma is the central perforation of the TM (not involving the annulus fibrosus), possibly associated with chronic, non‐fetid otorrhea and conductive hearing loss. Independent of the existence of cholesteatoma, COM has the potential not only to induce a TM perforation but also to destroy the ossicular chain. Surgical treatment may therefore vary between simple closure of the TM perforation and extensive surgery with mastoidectomy and ossiculoplasty with insertion of a middle ear prosthesis.
The middle ear transfer function may also be impeded by tympanosclerosis, a chronically scarring process which can lead to conductive hearing loss due to sclerotic fixation of the ossicles. It is usually caused by recurrent chronic inflammation of the middle ear. There may also be isolated sclerosis of the TM (myringosclerosis), as a sign of previous inflammations; this can, however be acoustically asymptomatic. Surgical treatment of tympanosclerosis for hearing improvement is mostly ineffective. Removal of the sclerotic masses has, if anything, only a temporary effect, as refixation inevitably reoccurs. Furthermore, microfractures of the stapes footplate, induced by the ‘cleaning’ manipulations, carry the risk of an inner ear complication (hearing loss due to labyrinthitis) (Austin, 1988). Therefore, most otosurgeons no longer attempt to perform surgery for a tympanosclerotic conductive hearing loss, but recommend conventional or implantable hearing aids for hearing restoration.
Cholesteatoma in the tympanic cavity
As mentioned before, cholesteatoma has the potential to extend beyond the TM into the middle ear, where it can lead to serious impairment not only of the ossicular chain but of any other nearby anatomical structure. Within the middle ear, cholesteatoma appears as a white mass, consisting of squamous epithelium which grows like a ball or sometimes like wallpaper and has an osseo‐destructive character. In secondary cholesteatoma, there is always a connection to the TM, in the majority of cases taking the form of a retraction of Shrapnell's membrane. From here, the ‘cholesteatoma sac’ can reach deep into the middle ear and mastoid. The speed at which the cholesteatoma destroys the middle ear structures sometimes correlates with its grade of inflammation. As no conservative treatment option exists, cholesteatoma always has to be surgically removed. Only if surgery has to be postponed, may conservative treatment with meticulous cleaning of the debris (removal of the ‘foreign body’) and drying the sac, together with microscopic control of the progression, be an option. If left untreated, the cholesteatoma will continue to grow, destroying any structure it comes into contact with. Hence, although being a slow‐advancing disease, cholesteatoma can lead to a multitude of awkward complications. To begin with, depending on its point of origin, it impairs or destroys the ossicular chain, leading to conductive hearing loss. Later on, destruction of the bony facial canal wall can result in a slowly progressive facial palsy. With constant growth, cholesteatoma can thin out the otherwise very hard bone of the labyrinth, resulting in a labyrinthine fistula, which is located most often in the lateral semicircular canal due to its anatomical proximity to the middle ear cleft. This defect in the bony wall of the labyrinth is associated with vertigo and the characteristic fistula symptome (a pressure rise in the external ear canal is transmitted via the cholesteatoma sac into the opened labyrinth and will induce nystagmus and vertigo). Further progression of the inflammation may lead to sensory hearing loss to the point of deafness. With further advancement, by the labyrinthitis through the inner ear canal, or directly across the tegmen or the posterior fossa, the cholesteatoma can reach the brain and if so, it can be the trigger (by means of a super‐inflammation) for serious, life‐threatening conditions such as meningitis, encephalitis or brain abscesses (Fisch, 1982).
Surgically, cholesteatoma must be removed by first fully exposing the diseased region (drilling away the surrounding bone) and then removing the cholesteatoma, preferably as an intact ‘cholesteatoma sac’. If the cholesteatoma has developed out of the epitympanic area, the ear canal is first circumferentially enlarged (wide exposure) and then an atticotomy is performed by thinning out or drilling away the scutum (lateral attic wall) using a diamond burr. Thereafter, the cholesteatoma can be exposed in the whole epitympanic area – from the aditus ad antrum, beyond the fossa incudis towards the anterior epitympanic recess above the auditory tube (supratubal recess) (Fig. 2). If cholesteatoma has advanced further posteriorly, then surgery must extend to the mastoid.
Figure 2.
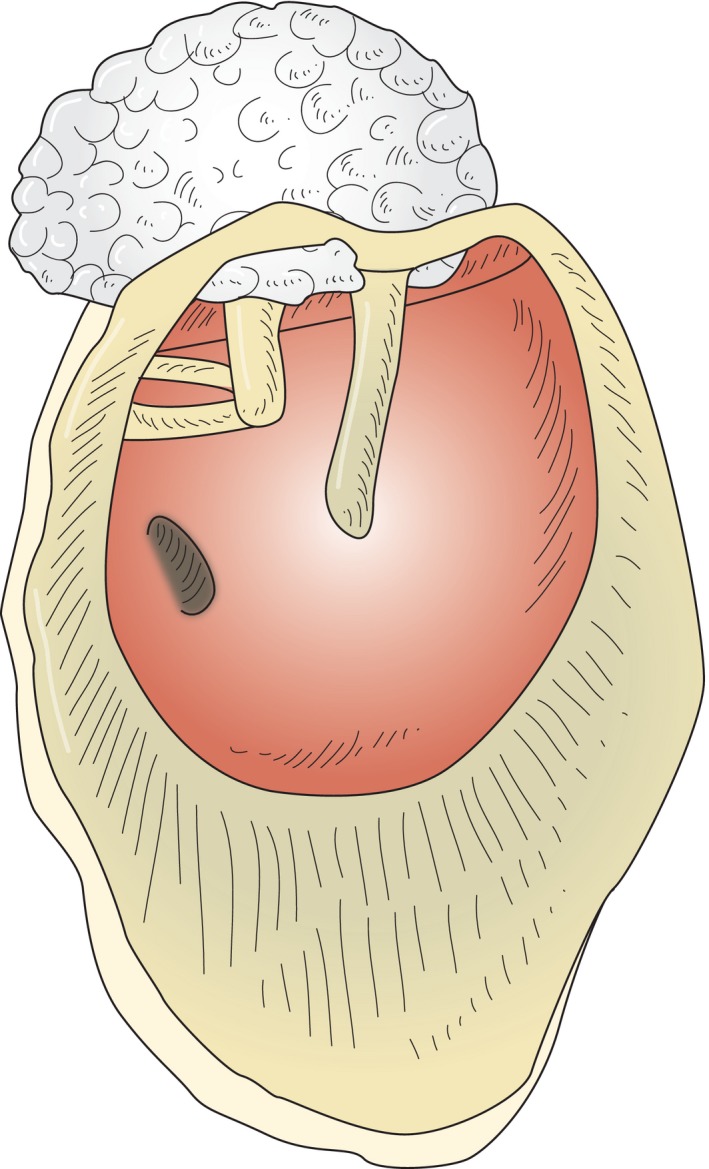
Development of a cholesteatoma: keratinizing squamous epithelium advancing from the superior TM into the epitympanic recess of the middle ear (schematic drawing without TM).
During cholesteatoma surgery, the surgeon has to adopt an operative principle similar to surgery of carcinoma: if only one cholesteatoma cell is missed and is not removed, the cholesteatoma may re‐occur (residual cholesteatoma). Due to the very variable clinical presentation and local extension of the cholesteatoma, the surgical approach, excision and reconstruction need to be tailored to the anatomy and extent of the disease in each individual patient (Sade, 1993). The primary goal of surgery is eradication of disease and prevention of recurrence. Preservation or reconstruction of hearing is an important but secondary consideration (Kim et al. 2006).
Ossicular chain and middle ear muscles
Together with the TM, the ossicular chain with hammer (malleus), anvil (incus), and stirrup (stapes) forms the sound‐conducting apparatus. The three ossicles, which are a characteristic feature of the mammalian middle ear, are connected by delicate synovial articulations: the malleo‐incudal and incudo‐stapedial joints (Mason, 2013).
The malleus is divided into head (caput mallei), neck (collum mallei), short process (processus brevis) and handle (manubrium mallei). The connection of the malleus handle with the TM is mostly loose, with a small cartilaginous insert on the end of the short process. At the umbo, however, where the collagen fibers of the TM converge, the attachment is extremely stable, and can only be cut with sharp instruments. The head of the malleus articulates with the body of the incus in a characteristic saddle‐shaped joint.
The incus consists of body (corpus incudis), short process (crus breve) and long process (crus longum), which is capped with the lenticular apophysis. The lenticular apophysis is connected to the long process via a very delicate bony pedicle. The stapes consists of head (caput stapedis), two legs (crus anterior and crus posterior) and footplate (basis stapedis). Whereas the head of the stapes articulates with the lenticular apophysis of the incus in a shallow, ball‐and‐socket cartilaginous joint, the footplate has a fibrous articulation with the rim of the oval window by means of the annular ligament. All ossicles and the entire tympanic cavity are lined with single‐layered epithelium (Anson, 1992).
When TM and ossicles are set into vibration, they conduct the sound via the oval window into the fluid‐filled inner ear, where a traveling wave in the cochlea is elicited, stimulating the hair cells. The functional principle for the sound‐conducting apparatus is to overcome the difference in acoustic impedance between the air in the ear canal and the liquid‐filled inner ear. This goal is attained mainly by the pressure amplification between the large TM and the smaller footplate. Besides this hydraulic amplification, a minor pressure gain is provided by the curvature of the TM; the catenary principle, first described by Helmholtz (Tonndorf & Khanna, 1970; Killion & Dallos, 1979). The lever effect, as the third pressure amplification (due to the different lengths of manubrium mallei and long incudal process), was for a long time considered another important means of amplifying pressure, but this has not been substantiated in the human middle ear. It was based on the concept of the rotation of malleus and incus around a fixed axis, which is anatomically located between the anterior mallear ligament and the tip of the short incudal process. This form of rotation around an anatomically defined axis is, however, only seen in response to changes in atmospheric pressure (Hüttenbrink, 1988b). For sound pressure vibrations, the functional rotation axis varies with frequency (at different frequencies, axes align through the umbo or outside the ossicular chain, or are even orientated along the handle of the malleus) (Hüttenbrink, 1995).
Besides this acoustic function of the middle ear with its vibrations in molecular‐sized amplitudes, many anatomical details exhibit a construction principle for the non‐acoustical function of the middle ear in ambient atmospheric pressure variations. Evolutionary pressure had to follow the physical law that a sensitive hearing detector (with its function as the predominant warning organ) had to be constructed in a manner so as to also withstand the million‐times larger pressure changes of ambient air pressure without a disastrous loss of function. This minor purpose of some designs of middle ear components is for example visible in the fact that for sound transmission, only a columella‐like strut, connecting TM and stapes footplate, transmits sound as efficiently as the articulated, three‐ossicular chain, which has been proven in numerous temporal bone experiments (Hüttenbrink, 1995), and which is the basis of our prosthetic reconstructions of the sound‐conducting apparatus. Nature also shows this principle in the impressive acoustic sensitivity of the owl's ear, with its simple columella transmission. Therefore, both components of our air‐pressure environment – sound and atmospheric pressures – have to be related to understand the anatomy, design and function of the middle ear.
When air pressure changes exceed approximately 100 mN, the malleus–incus joint is decoupled due to the minute gliding resistance of the hyaline cartilage.
Following the intricate construction of the ossicles, their suspensory ligaments and joint design, the inward–outward displacement of the malleus handle, as induced by changes of ambient static air pressure at the TM (with displacements million of times larger than at sound pressure) is transformed at the incus to a predominantly upward–downward and anterior–posterior movement in the plane of the incudo‐stapedial joint. This gliding movement results in decoupling of the stapes from the malleus and incus, and separates the pressure‐sensitive inner ear from the outer world air pressure changes. Hence, the joints of the middle ear ossicles can be considered a protective mechanism for the inner ear (Hüttenbrink, 1988a).
Although tiny, the middle ear joints are based on the same construction principles as the big articulations of the body. The joint surfaces between the ossicles are covered with hyaline cartilage, and the joint capsules are characterized by a surprising quantity of elastic fibers. Similar to all other hyaline articulations in the human body, the cartilage is deprived of blood supply. Its nutrition is provided by the synovial fluid, which has to circulate inside the joint cleft. Hyaline cartilage needs movement, friction and intermittent pressure. Immobilization of a joint results in arthritic degeneration with the loss of gliding capacities and, in the middle ear, loss of the protective function. In all cartilaginous joints of the body, this movement and circulation of the synovial fluid is provided by the antagonistic pull of the attached muscles. Following this basic construction principle of nature, it can be hypothesized that the two middle ear muscles, the M. tensor tympani and the M. stapedius, have the same purpose (Hüttenbrink, 1995).
Theories on the acoustic function of the muscles, however, are still controversial. A tensor tympani contraction does not alter the sound transmission across the middle ear (Fisch & Schultheiss, 1963; Mills et al. 2006). And a pull of the stapedius muscle has only a minor acoustic effect, with a slight decrease in the low frequencies and a few dB improvement in the high frequencies (Hüttenbrink, 1995).
The muscles run only partially through the tympanic cavity with their tendons. The tensor tympani muscle has its origin on the cartilaginous and bony wall of the Eustachian tube. At the processus cochleariformis, the muscle tendon turns 90° to reach the upper part of the manubrium mallei. The sheath of the tendon is very thick; its collagenous fibers form the malleo‐cochleariform ligament, which stabilizes the malleus (and the central part of the TM) against a pressure‐induced outward displacement (Hüttenbrink, 1989).
The tensor tympani muscle is innervated by a branch of the mandibular nerve. Its contraction is elicited only by non‐acoustic stimuli such as an air puff to the orbit or other trigeminal stimulation (Fisch & Schultheiss, 1963; Mills et al. 2006). The inward movement of the malleus handle caused by tensor contraction results, like a change in air pressure, in a gliding at the malleo‐incudal and incudo‐stapedial joints (Hüttenbrink, 1995).
The stapedius muscle arises from the posterior wall of the tympanic cavity below the mastoidal portion of the facial nerve. Its tendon runs through the opening of the eminentia pyramidalis and reaches the stapes head. The muscle is innervated by a branch of the facial nerve. At contraction, the stapes tilts backwards in the oval window and the annular ligament becomes stretched. This rocking movement of the stapes suprastructure results in an outward movement of the anterior pole and an inward movement of the posterior pole due to the rotational axis running through the center of the footplate. Consequently, there is no alteration of the pressure in the cochlear fluids (no net volume displacement). Instead, the movement induces a maximum gliding in the incudo‐stapedial joint. The small acoustic effect is due to the stiffening of the annular ligament. In fact, the function of the middle ear is much more complicated than these simple anatomical considerations suggest (see Mason, this issue) but these concepts provide a good starting point towards understanding the functional effects of middle ear pathologies and surgery.
Reconstruction of the ossicular chain
Often, the osteo‐destructive character of cholesteatoma involves the fragile bony structures of the ossicular chain. It begins with a bony erosion and ends with anything up to complete destruction of any parts of the ossicular chain in contact with the cholesteatoma. Unlike other bones, the ossicles have no capacity of self‐regeneration, and thus any discontinuity of the ossicular chain can only be repaired with reconstructive surgery, i.e. ossiculoplasty (Sade, 1993; Hüttenbrink, 1994).
A variety of materials have been inserted into the defective ossicular chain. They are often tolerated by the middle ear, which is considered an immunologically privileged space. For example, dentin (teeth) or keratin (toenails) can work well as reconstructive material in the middle ear, but they have been abandoned in favor of modern materials.
Some surgeons sculpture prostheses from the bone of the patient's squama occipitalis during the ear surgery. These prostheses are cheap but the preparation is time‐consuming and some aspects are less favorable (low stability in inflammation, risk of bony fixation to the walls of the middle ear cleft). The use of cartilage as material for ossicular chain reconstruction has been abandoned, as its structure softens over time. This characteristic favors its use for tympanic membrane repair but for the sound transmission, a rigid material is mandatory (Beutner & Hüttenbrink, 2009).
Today, if possible, the use of autogenous (the patient's own) ossicles is considered the gold standard. The remnants (mostly incus body, rarely malleus head) are resculptured and inserted as a prosthesis on top of the stapes. If the ossicles are destroyed or compromised due to cholesteatoma invasion, modern alloplastic materials are used instead. Titanium prostheses are favored by many surgeons, as they are well tolerated and they have superior mechanical characteristics (light weight and stiffness). Over the years, other materials have been popular, including other metals (gold, platinum, nickel‐titanium), ceramics, cements (hydroxyapatite, aluminum oxide, bioglass) or polymers (teflon, plexiglas, polyamide). Some are still in use by surgeons familiar with them, whereas others did not stand the test of time and were abandoned, e.g. some plastic materials which induced a foreign body reaction and were destroyed or rejected over time (Jahnke, 2004).
Considering differing materials and forms, the number of different middle ear prostheses easily exceeds 100. The design of the prostheses can be divided into three main types depending on the defect to be reconstructed: (i) defect of the lenticular apophysis (Fig. 3), (ii) defect of the incus (Fig. 4), (iii) additional defect of the stapes suprastructure with only the stapes footplate in the oval window remaining intact (Fig. 5). Isolated defects of the malleus (body or handle) occur very rarely and don't need a special type of alloplastic prosthesis for reconstruction.
Figure 3.
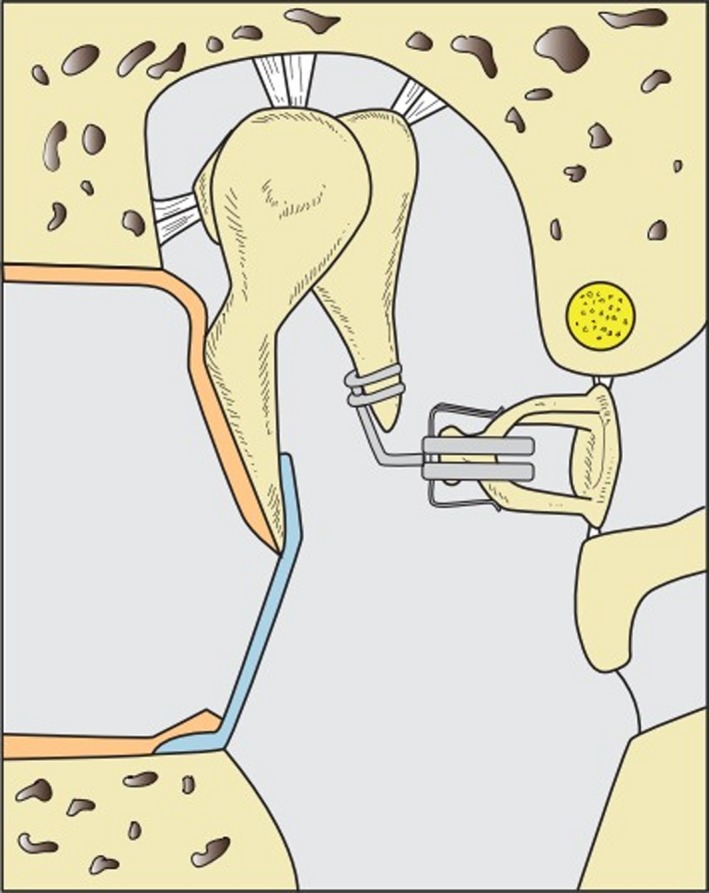
A defect of the of the long incus process is bridged with an angular titanium prosthesis. Some surgeons also use a drop of bone cement, but long‐term stability, especially at its contact zone at the incus necrotic bone, is obscure.
Figure 4.
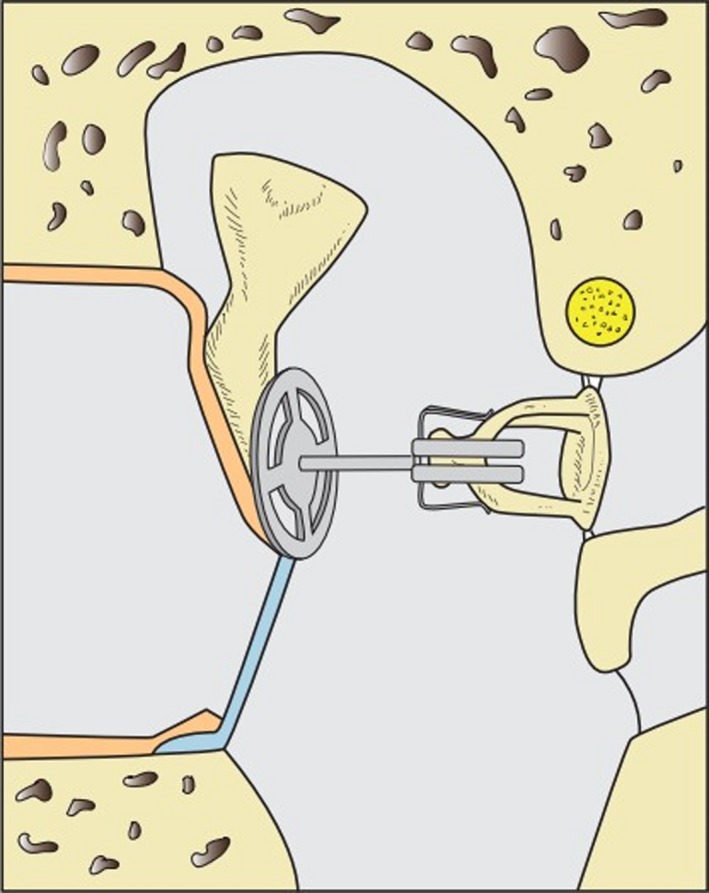
If the incus is defect, a partial ossicular replacement prosthesis (PORP) is inserted between TM (with or without contact to the malleus) and stapes.
Figure 5.
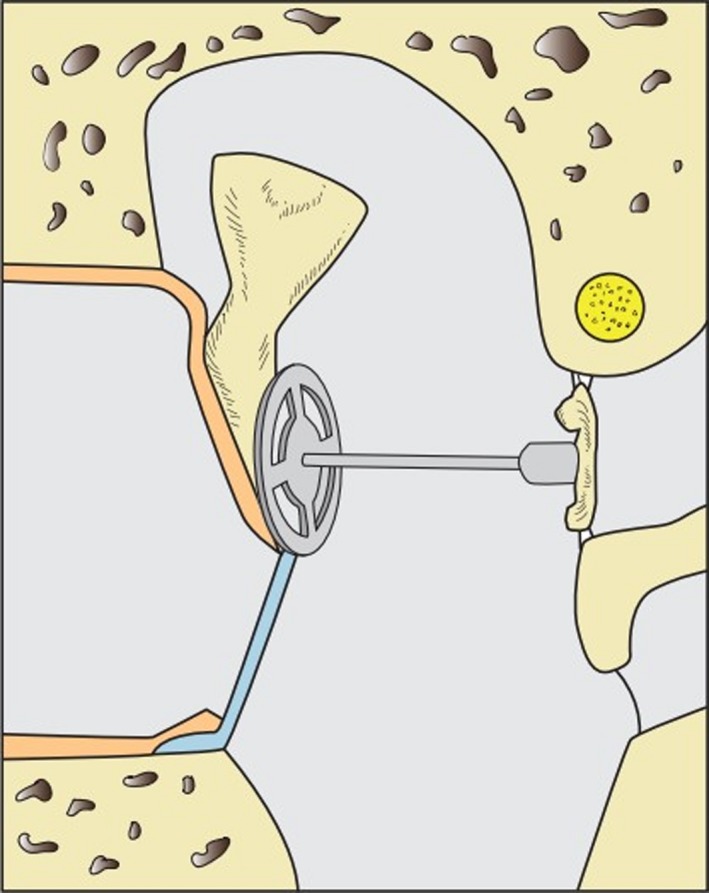
If the stapes suprastructure is defect, a total ossicular replacement prosthesis (TORP) connects the malleus handle/TM with the stapes footplate.
All these middle ear prostheses can only overcome a conductive hearing loss due to a defect of the middle ear sound‐conducting apparatus. If the inner ear itself is the origin of the hearing loss (pure sensorineural hearing loss), then middle ear prostheses are obsolete, as the function of the middle ear is not impeded. They can only restore the transmission of sound and are not capable of increasing the sound energy In cases of mild to moderate hearing losses, conventional hearing aids (which do increase the sound energy) are suitable. They emit the amplified sound into the ear canal via an earmold and are worn externally behind the ear (BTE). Implantable hearing aids are a novel alternative but they are only indicated for a small group of patients, mostly if there is combined hearing loss (middle and inner ear deficits) or if a BTE cannot be worn due to chronic ear canal inflammation (Beutner & Hüttenbrink, 2009). In high‐grade sensorineural hearing loss, cochlear implants may be an option. Here, an electrode is inserted via the round window into the cochlea's scala tympani to stimulate the auditory nerve directly and electrically, rather than generating mechanical movements (TM, ossicles, perilymph wave), the perception of which requires intact hair cells in the cochlea. Currently, such electrodes have a functional length of 20–30 mm and a diameter of around 1 mm (Fig. 6).
Figure 6.
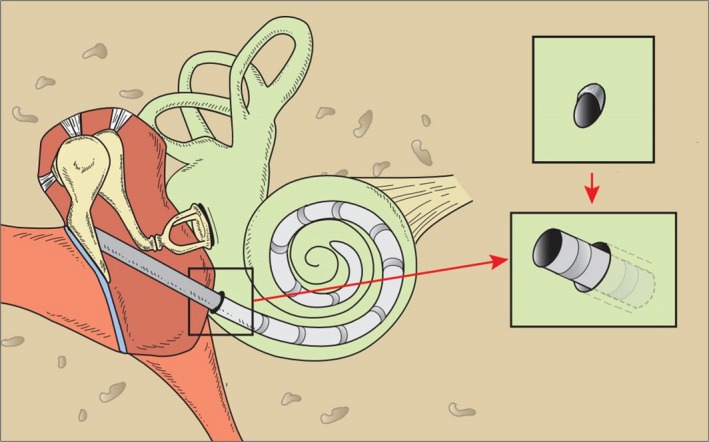
In patients suffering from high grade sensorineural hearing loss or complete deafness, a cochlear implant electrode is inserted into the cochlea to directly stimulate the acoustic nerve. Prior to insertion of the electrode via the round window into the scala tympani, the bony lip of the round window has to be drilled away to fully expose the round window membrane.
Otosclerosis and stapes surgery
Sclerotic formations may seriously constrain the function of the ossicular chain, as vibration is significantly impeded even though the structure of the ossicles remains anatomically intact. Here, tympanosclerosis has to be distinguished from otosclerosis. Otosclerosis is a local disorder, starting at the bony labyrinth and the oval window, and resulting in fixation of the stapes footplate. Its origin is unknown. Histologically, it is characterized by abnormal remodeling with resorption of the bony otic capsule and replacement with a hypercellular woven bone that becomes sclerotic over time. Despite being a disease of the inner ear, otosclerosis patients mainly suffer from conductive hearing loss, as the disease leads to progressive fixation of the stapes in the oval window. Aside from conventional hearing aids, stapes surgery (stapesplasty) is a therapeutic option to restore hearing for the patients. During stapesplasty, the stapes suprastructure is removed and a small hole (about 0.5 mm) is made within the stapes footplate. Thereafter, a piston prosthesis is attached to the long process of the incus (which should still show an unimpaired movement) with its lower end reaching through the footplate perforation into the inner ear perilymph (Fig. 7). Movements of the TM and the incus will then lead to a vibration of the stapes piston and induce a traveling wave in the perilymph.
Figure 7.
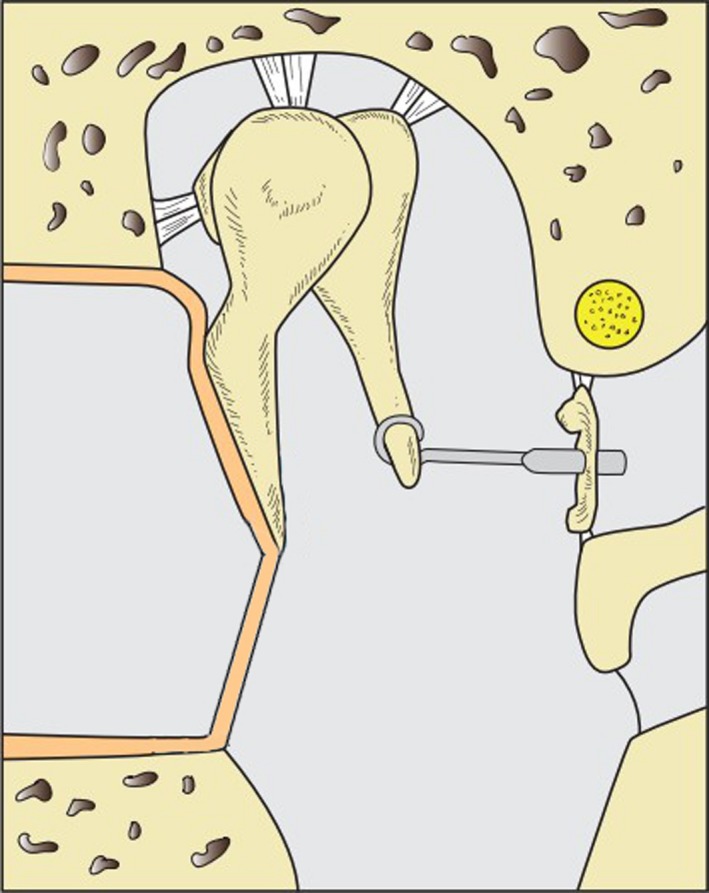
In otosclerosis, the ankylosed stapes is replaced by a piston prosthesis which is attached to the incus long process and its piston inserted into the inner ear perilymph through a perforation in the stapes footplate.
Stapes surgery is remarkably different from regular middle ear surgery, as the inner ear space will be opened and the risk of postoperative deterioration of hearing and deafness is much higher. When perforating the stapes footplate (which can be performed with a trepan, a burr or a laser), the surgeon has to know the exact anatomy and relative distance to the inner ear otolith organs. Minimal distances from the stapes footplate to both the utriculus and the sacculus are slightly < 0.5 mm, less at the superior border, larger below the posterior pole. As after insertion a stapes piston can move in‐ and outwards up to 0.5 mm due to atmospheric pressure variations, care should be taken to insert it not deeper than approxmately 0.5 mm into the vestibule to obtain sufficient distance towards the sacculus. To prevent the piston from being pulled out of the perforation during an outward movement of the ossicular chain/TM, for example when sneezing, performing the Valsalva maneuver, flying or diving, a piston length of 4.5 mm will provide a sufficiently deep insertion in most cases.
An inward movement of the ossicular chain, together with the prosthesis, can become dangerous if the piston is too long or positioned anterior–superiorly. If the piston comes into contact or impales the macular organs, the patient will suffer from abrupt, massive vertigo during the surgical manipulations. In the long postoperative term, if the vestibular organ is not destroyed by this trauma, a permanent or recurrent dizziness can become an intolerable condition. Surgical revision with extraction of the piston is the only solution, but the complete loss of sound transmission due to the missing stapes/piston will ensue.
Whereas malleus and incus are relatively similar in shape across ears in general, the stapes may show considerable anatomic variations in the form of very thin and delicate crura, deflection towards the promontory or towards the facial nerve, or a slight curving along its long axis. The anchorage of the stapes in the oval niche is crucial for the integrity of the inner ear and thus the acoustic organ. Surgery at the stapes is always accompanied by the risk of stapes tilting and dislocation, e.g. when cutting through the incudo‐stapedial joint, clipping a prosthesis onto the stapes head or when cholesteatoma matrix has to be separated from the stapes crura (Hüttenbrink, 1993). Occasionally, the facial nerve is not covered with bone in its canal, most often in its tympanal course right above the oval window niche. In these cases, the nerve may prolapse over the niche, tilting the stapes towards the promontory and partially covering the stapes footplate. Any surgical manipulation, especially in an inflamed, granulomatous middle ear near the assumed position of the facial nerve, must take into account this vulnerability of an uncovered nerve: any soft structure in this region has to be addressed potentially as being the nerve, until the opposite is proven!
In stapes surgery, a nerve prolapse can pose a significant risk for manipulations in the oval niche and can even make it impossible to place a piston when the oval niche is completely filled by the soft nerve masses. Fortunately, this is a very rare condition.
Another rare anatomic variation, which is mostly discovered fortuitously during middle ear surgery, is a persistent stapedial artery. In humans, it has normally disappeared by the time of birth (see Mason, this issue). Its occurrence can perturb any middle ear surgery but it represents a particularly troublesome situation during stapes surgery. In most cases, a persistent stapedial artery will be asymptomatic, but some case reports describe an association with tinnitus of pulsating type. Concern about coagulation of the stapedial artery used to be commonplace because of the risk of facial palsy, hearing loss, hemiplegia and even tabetic syndrome. Some of the concerns might be speculative, originating from anatomical considerations and not based on clinical case reports. In fact, several successful coagulations with no complications have been reported (Hitier et al. 2013).
Round window surgery
When cautiously touching the stapes with a needle, a bulging of the round window membrane can be visualized, corresponding to the perilymph motion of the cochlea. This ‘round window reflex’ informs the surgeon that the stapes is not fixed in the oval window (as it may be in patients with otosclerosis) and that vibrations of the stapes should therefore result in an effective perilymph wave. Often, scar tissue or a pseudomembrane, i.e. a mucosal fold (the membrana fenestra secundaria), obliterates the round window niche and obscures the view onto the true round window membrane (independent of the surgical approach) and must cautiously be removed first.
Whereas the oval window is normally clearly distinguished, the round window partially hides behind an overhanging ridge from the promontory, the so‐called subiculum promontorii. In certain cases, especially with congenital anomalies, atresia of the round window occurs (Toth et al. 2006).
The round window is the primary access to the inner ear for implantation of a cochlear implant (CI) or for positioning of an implantable hearing aid. Therefore, it is necessary to expose the round window membrane fully, and hence to remove the subiculum promontorii. Insertion of the CI electrode cable is performed via the round window membrane to minimize the mechanical trauma to the sensitive mechanoreceptors in the inner ear. This is a prerequisite for preserving hair‐cell integrity and residual hearing for modern, combined electro‐acoustic stimulation (EAS). The former practice was to insert the electrode via a cochleostomy, through a hole drilled into the thick bony wall of the lower turn of the cochlea, and resulted in much greater mechanical trauma. Drilling away the bony lip and hence baring the round window membrane is also a prerequisite when an implantable hearing aid is inserted into the middle ear, and an efficient contact of the transducer/vibrator with the inner ear fluid via the round window membrane must be established. This drilling, however, implies the risk of touching the membrane with the burr, which can result in serious inner ear hair cell trauma due to the mechanical overload. Therefore, utmost caution, best visibility and anatomical knowledge is needed for this surgical step, considering that the drilling manipulations have to be performed via the limited space (keyhole surgery) of a ‘posterior tympanotomy’. Here, the surgeon drills a small triangular passage between the mastoid portion of the facial nerve, the posterior wall of the outer ear canal containing the chorda tympani and the so‐called bony ridge of the attachment of the tip of the incudal short process (Fig. 8).
Figure 8.
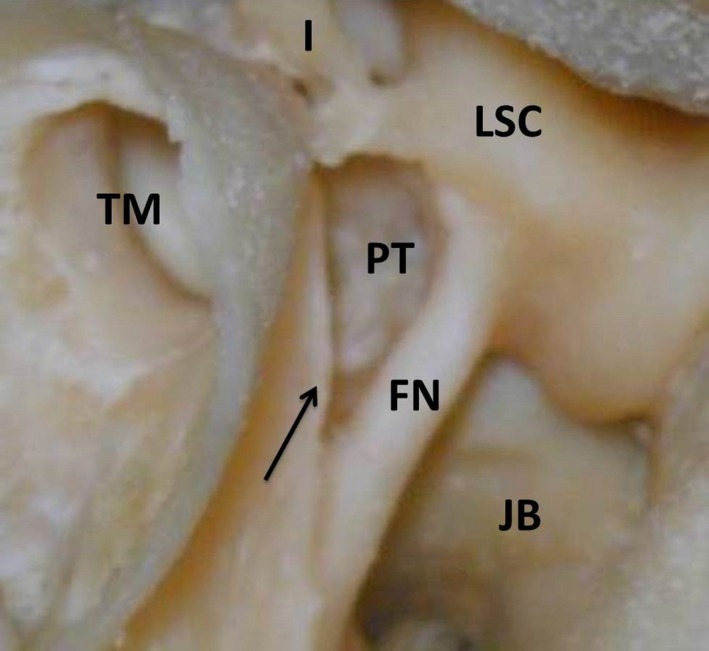
Picture of a temporal bone. A posterior tympanotomy (PT) is drilled as a triangular passage from the mastoid to the tympanic cavity, bordered by the facial nerve (FN) and the chorda tympani (arrow). I, Incus; LSC, lateral semicircular canal; JB, jugular bulb, TM, tympanic membrane.
Dorso‐caudal to the round window niche, below the ponticulus (a bony bar projecting from the processus pyramidalis towards the promontory), the tympanic sinus (sinus tympani) opens underneath the curve of the facial nerve. The depth of the tympanic sinus varies and in exceptional cases may extend far below the facial nerve, so that it can be opened even below the horizontal semicircular canal, dorsal to the facial nerve. Hence, cholesteatomas advancing into the tympanic sinus and the hypotympanum are a surgical challenge because these areas are difficult to reach. Removal of the cholesteatoma matrix has to be performed working around a facial nerve with only a thin bony coverage left, sometimes with the additional use of an endoscope.
Facial nerve and chorda tympani
There are a number of nerves and smaller blood vessels for the vascular supply and innervation of the middle ear. The neural structures with the most surgical relevance, however, mainly cross the middle ear with no or only minor functional contribution to the ear, i.e. the facial nerve and the chorda tympani.
The facial nerve runs through the whole of the temporal bone and is always close to any surgical approach to the middle ear. Right after the nerve has formed its second (outer) ‘knee‐bend’ (geniculum nervi facialis) near to the cochleariform process in the anterior region of the middle ear, it runs along the back wall of the tympanic cavity cranial to the oval window within its bony canal (Fallopian canal). It then continues dorsally, inferior to the lateral semicircular canal (prominentia canalis semicircularis lateralis), heading towards the stylomastoid foramen, where it exits the skull. Hence, throughout middle ear surgery, whether in the tympanic or in the mastoid cavity, the surgeon has to be aware of the nerve and know its exact location. Fortunately, iatrogenic injury to the facial nerve, which would result in facial palsy, is very rare during middle ear surgery because of the extensive training on temporal bones (and hence experience) an otosurgeon undertakes before operating on a patient. Special attention must be drawn to the facial nerve when working in the area of the stapes footplate and when drilling in the mastoid below the semicircular canal, i.e. when performing a posterior tympanotomy. Normally, the nerve is covered with bone while running in its bony canal. However, dehiscence of the bony canal with an uncovered nerve is the most common anatomical abnormality of the nerve. The anatomical course of the facial nerve through the temporal bone is relatively consistent.
During any middle ear surgery, the surgeon is confronted with the course of the chorda tympani. In chronic otitis media and, especially, in cases of cholesteatoma, the chorda tympani sometimes has to be ‘sacrificed’ (cut through) to gain higher safety for full eradication of the disease (i.e. if the chorda tympani is embedded in the cholesteatoma). Sometimes, especially in the endaural approach towards the stapes in otosclerosis surgery, the chorda tympani has to be stretched and moved aside to gain better access to the middle ear and especially to the ossicular chain. Although the chorda is flexible and hence moving it temporarily aside is possible, this stretching may result in taste disturbances. Fortunately, these tend to disappear after a short time, as the sense of taste is transmitted by a total of six nerves on both sides of the mouth (nervi VII, IX, X), which after a while recoup the full sense. Still, the chorda may be accidentally injured, for example when removing parts of the bony ear canal in the dorsal aspect. Likewise, drying up of the nerve fibers resulting from the steady hot light of the surgical microscope or simply by contacting the bare nerve with a thick suction tube may harm the nerve function.
Mastoid cavity
The mastoid cavity is a diverticulum of the tympanic cavity projecting into the mastoid region of the skull. It is composed mainly of aerated, pneumatized cells and houses or borders important elements such as the facial nerve, the semicircular canal system, the jugular vein, and the medial and posterior cranial fossa. Surgery of the tympanic cavity always requires consideration of the mastoid and vice versa. Hence, during most mastoidectomies the surgeon always checks and opens the tympanic cavity, either via the antrum cells above the horizontal semicircular canal or via drilling of a posterior tympanotomy, through which the surgeon gains access from the mastoid cavity to the posterior middle ear cleft.
Like the tympanic cavity, the mastoid cavity can be the center of an acute ear infection, i.e. acute mastoiditis. Unlike acute otitis media, which is most often treated conservatively (with antibiotics), acute mastoiditis necessitates prompt surgery in the form of a mastoidectomy. Especially in children and immuno‐compromised patients, the infection can spread into the bone and the ensuing osteomyelitis can be the source of serious complications (facial nerve paralysis, development of an abscess, sinus thrombosis, meningitis, encephalitis). During a mastoidectomy, all inflamed pneumatized cells and the ostitic bone in the mastoid have to be completely removed. Besides this radical eradication of all infected material, a free drainage from the mastoid and air circulation from the middle ear towards the mastoid have to be established.
Mastoid surgery
Sometimes, the bulk of a cholesteatoma has extended into the mastoid cavity. Depending on the origin of the retraction pocket, it may stretch from the antrum to the supralabyrinthine region and further laterally up to the level of the cortical bone and posteriorly to the sinus dura angle. Huge cholesteatomas can fill and destroy the complete mastoid down to its tip. Removal of the cholesteatoma extension backwards may therefore require a mastoidectomy. Normally, both transmeatal and transmastoidal approaches are performed together for this purpose, with the posterior wall of the external meatus separating these two approaches. This ‘canal wall up’ or ‘intact canal wall’ technique is particularly appropriate when the mastoid cavity is relatively large in size and shows a good pneumatization with healthy mucosa. If, however, the mastoid is very narrow, with sparse pneumatization or practically no cells at all, and if no sufficient ventilation of the mastoid cavity can be expected (due to chronically inflamed or even destroyed mucosa), then often preference is given to a ‘canal wall down’ technique. Here, the posterior wall of the external meatus is completely removed, leading to a ‘radical cavity’, comprising the external auditory canal and the mastoid. One of the major advantages of the removal of the posterior wall is the optimal overview on pathologies (inflammatory processes or cholesteatoma), which can in most cases now be thoroughly and reliably eradicated, even in hidden spaces such as the sinus tympani. Residual cholesteatomata developing from remaining epithelial cells occur more frequently in the canal wall up technique and are most often found in the shadow of the posterior wall. Of note, cholesteatoma treatment must always be individualized and there is no one‐plan‐fits‐all strategy in middle ear surgery. Hence, both canal wall up and canal wall down methods have their place in cholesteatoma surgery (Tos & Lau, 1989).
As with middle ear surgery, thorough knowledge of the anatomy is an essential prerequisite of mastoid surgery. Notably, mastoid pneumatization is reduced in many patients suffering from chronic otitis media (Rajati et al. 2013). In cases of extensive pneumatization of the mastoid, neighboring structures like the dura towards the middle and posterior cranial fossae, the sigmoid sinus or the facial nerve may lack a bony hull and are then covered only by connective tissue. The identification and exposure of the dura is often a meaningful step during middle ear surgery as it represents an important anatomical landmark. Sometimes, uncovering the dura is even mandatory, e.g. to exclude an epidural abscess in cases of mastoiditis. The integrity of the dura may be harmed by inflammation or by surgery, and in the event of liquorrhoea, a duraplasty with multilayered covering, using cartilage, absorbable fibrin sealant patches and fibrin glue is necessary.
Exposing the antrum is a routine step performed during mastoidectomy to visualize the middle ear from the dorso‐cranial level and to enhance mastoid ventilation. The surgeon has to act with extreme caution when enlarging the antrum and removing the bone right above the main body of the incus because of the risk of damage to the inner ear when an intact ossicular chain is touched with the drill. In some patients a bony petrosquamosal lamina, starting from the articular fossa, extending above the middle ear and running inferiorly lateral to the facial canal, proceeding to the mastoid apex, can impede the approach of the surgeon. Anatomically, this so‐called Körner's septum represents the persistence of the petrosquamosal suture line, dividing the mastoid process into a superficial squamous portion and a deep petrous portion. If persistent, Körner's septum may create problems or complications during mastoidectomy, such as a ‘false antrum’ being entered or the facial nerve being injured.
For performing a posterior tympanotomy (opening between facial nerve and posterior wall of external ear canal), the relationships of the facial nerve (dorsally), the incudal short process (superiorly) and the chorda tympani (anteriorly) have to be precisely known by the surgeon who aims to drill away all bone between the aforementioned structures. Gaining access to the middle ear by this approach enables the surgeon to operate in the hypotympanum, the sinus tympani and in the region of the round window without lifting up the TM, as described earlier.
If the purpose of a mastoidectomy is eradication of a disease (i.e. otitis media or cholesteatoma), then the drilling out and removal of all cells in the mastoid is a prerequisite to prevent disease recurrence. Here, supralabyrinthine cells, cells at the sinus‐dura‐angle (Citelli cells) and retrosinusoid cells are of practical significance. Cells may be located directly on the mastoidal portion of the facial nerve (Pogany cells) and in cases of mastoiditis, their inflammation can progress into the facial canal with the danger of facial palsy. The obligatory surgical decompression of the bony canal to relieve pressure and inflammation from the nerve can pose problems when the granulation tissue in these cells has to be separated from the inflamed epineurium of the facial nerve.
Reconstruction techniques
After eradication of the disease, the surgeon must deal with the reconstruction of the middle ear space. In the earlier days of middle ear surgery, creating radical cavities was the standard technique in greater cholesteatoma surgery. A disadvantage of this technique has always been the higher rate of chronic discharge from the large cavity as compared with the intact canal wall technique, where the external meatus remains intact. If the radical cavity later on epithelializes again, then the cavity skin will almost inevitably become inflamed due to the unfavorable ventilation of the cavity, resulting from the discrepancy between a narrow opening of the external ear canal and a wide bottom (mastoid + ear canal) (Beutner et al. 2007).
As a consequence, radical cavities are nowadays partially obliterated after eradication of the cholesteatoma, thus reducing the volume of the cavity and restoring a favorable ventilation ratio through the equally enlarged ear canal entrance. For obliterating the mastoid, bone paste, obtained during the drilling process of the healthy cortical bone and temporarily harvested in antibiotic solution, is used as a first reconstruction layer. Thereafter, the bone dust is covered with thick slices of cartilage while the TM is reconstructed using perichondrium and thin cartilage plates. Long‐term results of this technique are very convincing, with a significant reduction of the postoperative discharge and, at the same time, a significant drop of the rates of residual and recurrent disease (Beutner et al. 2007).
Eustachian tube
Typically, textbooks on otolaryngology report three important functions of the tube for the middle ear: ventilation, clearance and protection (against bacterial spread from the nasopharynx and against autophonia). However, whether the auditory tube really has a function in middle ear ventilation is actually controversial. A large number of tubal tests (i.e. Valsalva, Politzer maneuver, etc.) as well as abundant literature about the importance of tubal function in the development of middle ear diseases exists, but the ‘tubal dysfunction’ in respect to aeration and pressure control of the middle ear has never been proven nor convincingly defined (Tos, 1988; Hüttenbrink, 1994). Most studies trying to analyze tubal function are at high risk of bias and subject to multiple limitations (Norman et al. 2014). Although a Valsalva maneuver practically can lead to pressure equalization in the middle ear, this is most probably not the primal conception of the tube. As a matter of fact, no animal can perform a Valsalva maneuver! Of note, the gas components in the middle ear correspond in composition to ‘mixed venous blood’, but not to the contents of atmospheric air (Felding et al. 1987; Hergils & Magnuson, 1990; Sade & Luntz, 1990). In fact, the gas exchange between the tympanic cavity and the blood vessels of the middle ear mucosa is bi‐directional, possibly also explaining the over‐pressure in the middle ear in the morning after a long‐term non‐opening of the tube (Sade, 2001; Kania et al. 2006). Hence, middle ear pressure can be balanced without a tube. Other studies failed to prove that there is a gas shift at all from the nasopharynx to the tympanic cavity during phases of tube opening, or even showed that swallowing led to de‐aeration of the middle ear rather than aeration (Cohen et al. 2009). Hence, all studies investigating the tube function for the middle ear show complex results which are difficult to interpret (Pau, 2011; Norman et al. 2014).
In contrast, the relevance of the tube for the clearance of the middle ear is much more obvious, as no mucosa‐filled, aerated cavity in the human body comes without a tube for clearance purposes of the mucus, which is also produced by the mucoid epithelium in the middle ear. Despite the controversial results, a wide variety of therapeutic approaches, more or less invasive, have been or still are electively performed to improve a ‘tubal dysfunction’. However, from the studies available, it is not possible to draw conclusions regarding the effectiveness of any of the interventions; moreover, there is insufficient evidence to recommend a trial of any particular intervention (Norman et al. 2014).
As indicated above, there is a lack of evidence that any treatment of a dysfunctional auditory tube is beneficial – not to mention the difficulty of measuring the tube function accurately and coming up with a reliable diagnosis. If during middle ear surgery the otosurgeon discovers scarring in the area of the tube opening in the tympanic cavity, he/she will understandably remove it, though this will probably not ensure a long‐lasting and effective drainage or even ventilation of the tympani cavity. Currently, ventilation or aeration is regarded as crucial for the well‐being and function of the middle ear and especially the sound‐conducting apparatus. In earlier days, middle ear surgery was sometimes even postponed when there were additional pathologies in the area of the nasopharynx, nose or nasal sinuses. However, the surgical principle ‘nose before ear’, which aimed to optimize the tubal function and hence the conditions of the middle ear, did not withstand the test of time, as it did not significantly change the course of middle ear diseases. In children, however, middle ear surgery is still regularly combined with adenoidectomy (surgical removal of the adenoids/pharyngeal tonsil), because of the more horizontal course of the auditory tube in children and the effect of chronically inflamed lymphatic tissue near the tubal orifice.
Cholesteatoma growth in the anterior part of the tympanic cavity can be hazardous if the matrix advances into the Eustachian tube, from where it has to be meticulously removed. The practice of removing the superficial layer of the bone if it is covered with cholesteatoma (to maximize the chances of full eradication), is difficult in the anterior parts of the tympanic cavity, as on the one hand the mucosa must be preserved here and as on the other hand the internal carotid artery lurks closely behind the bony wall.
Conclusion
Taking into account all of the above, surgical anatomy of the middle ear has a number of distinctive features in comparison with other surgical practices in view of the complex anatomy, the tiny structures and the associated risk profile. Prior to surgery on a patient, any middle ear surgeon needs practical training on temporal bones, as two‐dimensional drawings in an anatomy atlas can only work as a rough guide. The aim of middle ear surgery is to eradicate a disease or improve hearing, or often a combination of both. This way, middle ear surgery can have a substantial positive impact on a patient's quality of life. On the other side, middle ear surgery can be associated with a number of severe complications such as hearing impairment and deafness, development of tinnitus or vertigo and even facial palsy.
Conflict of interest
None.
References
- Aggarwal R, Saeed SR, Green KJM (2006) Myringoplasty. J Laryngol Otol 120, 429–432. [DOI] [PubMed] [Google Scholar]
- Anson BDJ (1992) Surgical Anatomy of the Temporal Bone. New York: Raven Press. [Google Scholar]
- Atmaca S, Elmali M, Kucuk H (2014) High and dehiscent jugular bulb: clear and present danger during middle ear surgery. Surg Radiol Anat 36, 369–374. [DOI] [PubMed] [Google Scholar]
- Austin DF (1988) Reconstructive techniques for tympanosclerosis. Ann Otol Rhinol Laryngol 97, 670–674. [DOI] [PubMed] [Google Scholar]
- Bartel‐Friedrich S, Wulke C (2007) Classification and diagnosis of ear malformations. GMS Curr Top Otorhinolaryngol Head Neck Surg 6, Doc05. [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Hüttenbrink KB (2009) Passive and active middle ear implants. GMS Curr Top Otorhinolaryngol Head Neck Surg 8, Doc09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Stumpf R, Zahnert T, et al. (2007) Long‐term results following mastoid obliteration in canal wall down tympanomastoidectomy. Laryngorhinootologie 86, 861–866. [DOI] [PubMed] [Google Scholar]
- Beutner D, Hüttenbrink K, Stumpf R, et al. (2010) Cartilage plate tympanoplasty. Otol Neurotol 31, 105–110. [DOI] [PubMed] [Google Scholar]
- Cohen D, Raveh D, Peleg U, et al. (2009) Ventilation and clearance of the middle ear. J Laryngol Otol 123, 1314–1320. [DOI] [PubMed] [Google Scholar]
- Farrior JB (1983) Incisions in tympanoplasty: anatomic considerations and indications. Laryngoscope 93, 75–86. [DOI] [PubMed] [Google Scholar]
- Felding JU, Rasmussen JB, Lildholdt T (1987) Gas composition of the normal and the ventilated middle ear cavity. Scand J Clin Lab Invest Suppl 186, 31–41. [PubMed] [Google Scholar]
- Fisch U (1982) Intracranial complications of cholesteatoma In: Cholesteatoma & Mastoid Surgery. (eds Sade J.), pp. 369–379, Amsterdam: Kugler Publication. [Google Scholar]
- Fisch U, Schultheiss G (1963) Electromyographic studies on the human stapedial muscle. Acta Otolaryngol 56, 287–297. [DOI] [PubMed] [Google Scholar]
- Hergils L, Magnuson B (1990) Human middle ear gas composition studied by mass spectrometry. Acta Otolaryngol 110, 92–99. [DOI] [PubMed] [Google Scholar]
- Hitier M, Zhang M, Labrousse M, et al. (2013) Persistent stapedial arteries in human: from phylogeny to surgical consequences. Surg Radiol Anat 35, 883–891. [DOI] [PubMed] [Google Scholar]
- Hüttenbrink KB (1988a) The mechanics of the middle‐ear at static air pressures: the role of the ossicular joints, the function of the middle‐ear muscles and the behaviour of stapedial prostheses. Acta Otolaryngol Suppl 451, 1–35. [DOI] [PubMed] [Google Scholar]
- Hüttenbrink KB (1988b) The fixation theory of middle ear muscle function. Laryngol Rhinol Otol (Stuttg) 67, 404–411. [PubMed] [Google Scholar]
- Hüttenbrink KB (1989) The functional significance of the suspending ligaments of the ear ossicle chain. Laryngorhinootologie 68, 146–151. [PubMed] [Google Scholar]
- Hüttenbrink KB (1993) Manipulating the mobile stapes during tympanoplasty: the risk of stapedial luxation. Laryngoscope 103, 668–672. [DOI] [PubMed] [Google Scholar]
- Hüttenbrink KB (1994) Chronic Otitis media In: Oto‐Rhino‐Laryngologie in Klinik und Praxis. (eds Naumann H, Helms J, Herberhold C, Kastenbauer E.), pp. 601–632, Stuttgart: Georg Thieme Verlag. [Google Scholar]
- Hüttenbrink K (1995) The function of the ossicular chain and of the muscles of the middle ear. Eur Arch Otol Rhinol Laryngol (Suppl 1), 1–52. [Google Scholar]
- Jahnke K (2004) Middle Ear Surgery: Recent Advances and Future Directions. Stuttgart: Georg Thieme Verlag. [Google Scholar]
- Kania RE, Herman P, Tran Ba HP, et al. (2006) Role of nitrogen in transmucosal gas exchange rate in the rat middle ear. J Appl Physiol 101, 1281–1287. [DOI] [PubMed] [Google Scholar]
- Karmody CS, Northrop CC, Levine SR (2009) The incudostapedial articulation: new concepts. Otol Neurotol 30, 990–997. [DOI] [PubMed] [Google Scholar]
- Killion M, Dallos P (1979) Impedance matching by the combined effects of the outer and middle ear. J Acoust Soc Am 66, 599–602. [Google Scholar]
- Kim HH, Battista RA, Kumar A, et al. (2006) Should ossicular reconstruction be staged following tympanomastoidectomy. Laryngoscope 116, 47–51. [DOI] [PubMed] [Google Scholar]
- Kosling S, Omenzetter M, Bartel‐Friedrich S (2009) Congenital malformations of the external and middle ear. Eur J Radiol 69, 269–279. [DOI] [PubMed] [Google Scholar]
- Lang J (1992) Klinische Anatomie des Ohres. Vienna: Springer. [Google Scholar]
- Mason MJ (2013) Of mice, moles and guinea pigs: functional morphology of the middle ear in living mammals. Hear Res 301, 4–18. [DOI] [PubMed] [Google Scholar]
- Michaels L (1988) Origin of congenital cholesteatoma from a normally occurring epidermoid rest in the developing middle ear. Int J Pediatr Otorhinolaryngol 15, 51–65. [DOI] [PubMed] [Google Scholar]
- Mills JH, Khariwala SS, Weber PC (2006) Anatomy and Physiology of Hearing In: Bailey BJ, Johnson JT. and Newlands SD, Eds., Head & Neck Surgery: Otolaryngology. 4th edn, vol. 2 Philadelphia: Lippincott Williams & Wilkins, pp. 1883–1903. [Google Scholar]
- Norman G, Llewellyn A, Harden M, et al. (2014) Systematic review of the limited evidence base for treatments of Eustachian tube dysfunction: a health technology assessment. Clin Otolaryngol 39, 6–21. [DOI] [PubMed] [Google Scholar]
- Pau HW (2011) Eustachian tube and middle ear mechanics (in German). HNO 59, 953–963. [DOI] [PubMed] [Google Scholar]
- Rajati M, Shahabi A, Haghir H, et al. (2013) The distance of the sigmoid sinus and the middle fossa dura from the external auditory canal in chronic otitis media. Surg Radiol Anat 35, 477–480. [DOI] [PubMed] [Google Scholar]
- Sade J (1993) Treatment of cholesteatoma and retraction pockets. Eur Arch Otorhinolaryngol 250, 193–199. [DOI] [PubMed] [Google Scholar]
- Sade J (2001) Hyperectasis: the hyperinflated tympanic membrane: the middle ear as an actively controlled system. Otol Neurotol 22, 133–139. [DOI] [PubMed] [Google Scholar]
- Sade J, Luntz M (1990) Middle ear as a gas pocket. Ann Otol Rhinol Laryngol 99, 529–534. [DOI] [PubMed] [Google Scholar]
- Strohm M (1986) Trauma of the middle ear. Clinical findings, postmortem observations and results of experimental studies. Adv Otorhinolaryngol 35, 1–254. [PubMed] [Google Scholar]
- Teunissen EB, Cremers WR (1993) Classification of congenital middle ear anomalies. Report on 144 ears. Ann Otol Rhinol Laryngol 102, 606–612. [DOI] [PubMed] [Google Scholar]
- Tonndorf J, Khanna SM (1970) The role of the tympanic membrane in middle ear transmission. Ann Otol Rhinol Laryngol 79, 743–753. [DOI] [PubMed] [Google Scholar]
- Tos M (1988) Incidence, etiology and pathogenesis of cholesteatoma in children. Adv Otorhinolaryngol 40, 110–117. [DOI] [PubMed] [Google Scholar]
- Tos M, Lau T (1989) Hearing after surgery for cholesteatoma using various techniques. Auris Nasus Larynx 16, 61–73. [DOI] [PubMed] [Google Scholar]
- Toth M, Alpar A, Patonay L, et al. (2006) Development and surgical anatomy of the round window niche. Ann Anat 188, 93–101. [DOI] [PubMed] [Google Scholar]
- Wysocki J, Skarzynski H (1998) Distances between the cochlea and adjacent structures related to cochlear implant surgery. Surg Radiol Anat 20, 267–271. [DOI] [PubMed] [Google Scholar]
- Zahnert T, Hüttenbrink KB, Murbe D, et al. (2000) Experimental investigations of the use of cartilage in tympanic membrane reconstruction. Am J Otol 21, 322–328. [DOI] [PubMed] [Google Scholar]


