Abstract
Here we present a brief, historical review of research into the mammalian middle ear structures. Most of their essential homologies were established by embryologists, notably including Reichert, during the 19th century. The evolutionary dimension was confirmed by finds of fossil synapsids, mainly from the Karroo of South Africa. In 1913, Ernst Gaupp was the first to present a synthesis of the available embryological and paleontological data, but a number of morphological details remained to be solved, such as the origin of the tympanic membrane. Gaupp favoured an independent origin of the eardrum in anurans, sauropsids, and mammals; we support most of his ideas. The present review emphasizes the problem of how the mammalian middle ear structures that developed at the angle of the lower jaw were transferred to the basicranium; the ontogenesis of extant marsupials provides important information on this question.
Keywords: evolution, Mammalia, middle ear, middle ear ossicles, ontogeny
Introduction
The study of the mammalian middle ear has been one of the central themes of vertebrate morphological research of the last 200 years. The middle ear ossicles have, of course, been known to human anatomists for much longer, as shown by their visual representation in Vesalius (1543). The middle ear ossicles of amphibians, sauropsids (reptiles and birds), and mammals were described in detail by Cuvier (1800) but the observed differences were not considered at that time.
An improved theoretical understanding – beyond mere description – of the delicate middle ear structures only became possible through the comparative embryological approach developed during the first decades of the 19th century, as excellently reviewed by Russell (1916).
Early embryological studies
During the four decades after Cuvier, embryological studies of all classes of vertebrates clarified the homologies of the middle ear structures, which, prior to Owen (1843), were more usually referred to as analogies. Carus (1818) had doubted that the articulation of the lower jaw is identical between mammals and the other vertebrates, and he recognized that the incus (anvil) is homologous to the quadrate of ‘lower’ (i.e. non‐mammalian) vertebrates. Meckel (1820) observed that, in mammals, the embryonic malleus (hammer) develops from the posterior end of a thin rod of cartilage attached to the medial side of the dentary, whereas in non‐mammalian vertebrates this posterior end ossifies as the articular bone; Meckel therefore homologized the malleus with the articular. The embryonic cartilage of the lower jaw is now called ‘Meckel's cartilage’ after its discoverer. These discoveries replaced some earlier homologizations, such as those of Geoffroy‐Saint‐Hilaire (1818).
These finds gained a new importance when Rathke (1825a,b) described Kiemenspalten (gill slits) and Kiemenbögen (gill arches) in embryos of the pig and chick. The discovery of Kiemen‐Anlagen (gill anlagen) stimulated a number of similar studies on many different amniotes, including man. These early studies suggested that the most anterior of the Kiemenbögen represents the mandibular arch and that the second represents the hyal arch (Baer, 1828). The term Kiemenbogen was accordingly replaced by the more general terms Visceralbogen (visceral arch) or Schlundbogen (pharyngeal arch). Huschke (1827, 1828) concluded that the first cleft between the mandibular and the hyal arches (Spritzloch = spiraculum) corresponds to the ear duct (Gehörgang = Eustachian tube, middle ear, and outer ear).
The Reichert–Gaupp theory
Carl Reichert, who had mainly studied the embryogenesis of the second visceral arch (cf. cartilage of Reichert), in 1837 summarized and generalized his own findings and those of colleagues such as Meckel (1820), Carus (1818), Rathke (1825a,b) and Baer (1828). Since then these concepts have (not quite correctly) been collectively referred to as ‘Reichert's theory’. The numerous and often controversial publications were carefully reviewed and summarized by Gaupp (1898), Broman (1899), and Van der Klaauw (1924).
The homology of the incus and malleus with the quadrate and articular, respectively (Fig. 1A) was accepted by most authors, but Huxley (1869) and Parker (1874, 1877) maintained different views: they derived only the malleus from the mandibular arch and aligned the incus with the second visceral or hyal arch. Establishing the homology of the stapes proved to be more complicated, the more so because it was difficult to compare it among different tetrapod groups. Reichert (1837) had homologized the stapes with the columella of amphibians, but he did not precisely define what he meant by ‘columella’ because he did not distinguish an extracolumella (Reichert, 1838). Huxley (1869) and Parker (1874, 1877) considered the stapes as an element isolated from the lateral wall of the labyrinth capsule (see section below on developmental biology). Broman (1899) showed conclusively that the human stapes chondrifies as an ‘annulus stapedius’, which is in contact with the hyal arch (cartilage of Reichert) by means of an interhyal blastema. The ‘annulus stapedius’, which often forms around the stapedial artery, comes into contact with the wall of the labyrinth only later and forms a fenestra ovalis (vestibuli) secondarily. Therefore, embryological evidence clearly supported the case that the stapes is the most proximal portion of the hyal arch (Fig. 1A, see also section on developmental biology). Gaupp (1898) adopted this view contra his earlier viewpoints and these homologies of the three middle ear ossicles were also accepted by Gegenbaur (1898).
Figure 1.
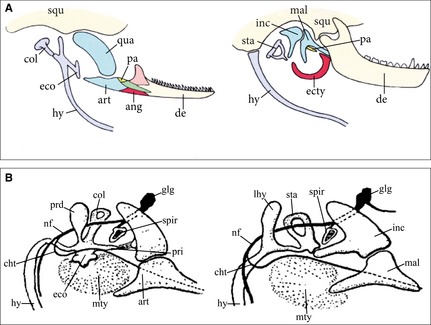
The theory of Reichert. (A) The interpretation by Gaupp (1913: his fig. 144, modified). The primary jaw articulation between quadrate and articular as seen in a schematic juvenile squamate (left side) representing (according to him) the ancestral morphotype; in fact, the quadrate articulates not only with the squamosal but also with the parotic process of the otic capsule lying underneath. The generalized mammalian condition is shown on the right side; homologues are given in the same coloration. Quadrate and articular have evolved into incus and malleus; at least proximal parts of the columella are homologous to the stapes. The primary jaw articulation corresponds to the incudo‐mallear joint, and a secondary jaw joint is formed between the dermal squamosal and dentary; the ectotympanic ring, which provides the frame for the tympanic membrane, is derived from the angular bone. This transition is very well documented in the fossil record. (B) The interpretation of Goodrich (1915); his slightly modified text Fig. 2 is labeled anew to make comparison with the figure of Gaupp easier. Goodrich assumed that the tympanic membrane is homologous in sauropsids and mammals.
The homologizations of the tympanicum and of the prearticular proved to be controversial until way into the 20th century (Fig. 1A). First of all, their specific nature as dermal bones was only understandable after Kölliker (1849) had established two different categories of bone: he distinguished the ‘endochondrial ossifications’ as ‘primary bone’ (Primärknochen) from the ‘Deck‐ oder Belegknochen’; this latter type of bone has been later called ‘dermal bone’ (cf. Romer, 1962; Patterson, 1977; and many others) or ‘exocranial bone’ (Starck, 1979, 1989). Embryologists soon recognized that the tympanicum and prearticular belonged to the mosaic of dermal bones of the lower jaw. Kölliker (1879) suspected that the small exocranial ossification at the anterior process of the malleus is derived from the angular of non‐mammals, and Gaupp (1898) adopted this view. However, Gaupp (1906) recognized that in amniotes an additional dermal bone, which is regularly pierced by the chorda tympani, exists at the ventromedial side of the origin of Meckel's cartilage: he called this the postopercular, and later (Gaupp, 1908, p. 760 ff.) the gonial. Gaupp (1911a) devoted a long study to this element, but only in his 1913 publication did he recognize that Williston (1903), in a study of plesiosaurs, had already named this bony element of the lower jaw the prearticular. Gaupp accepted that prearticular and gonial were the same dermal element, but he retained the name gonial, which he had characterized and defined on a much broader and more profound anatomical level; Goodrich (1930) still used both names.
The tympanicum (in therian mammals called ectotympanicum because there often exists an additional entotympanicum; see review in Maier, 2013) is a dermal bone which in most mammals develops as a horseshoe‐shaped ossification below the labyrinth capsule (Fig. 1A). It provides the frame for the tympanic membrane. Following Gegenbaur, Gaupp (1898) first claimed that it is derived from the quadratojugal of the upper jaw, but Van Kampen (1905) recognized that it is, in fact, an exocranial element of the lower jaw. Van Kampen (1905, fig. 96) favoured the supraangular as its homologue among non‐mammals, but he also considered the angular a possible candidate. Gaupp (1911b, 1913) preferred the angular (Fig. 1A). The homology of the tympanicum with the angular was settled by the craniogenetic study of Palmer (1913), who showed that in the juvenile of the marsupial Perameles the tympanicum looks strikingly similar to the angular of cynodonts.
In mammals, the question of homology of the tympanicum is, of course, closely connected with that of the tympanic membrane. Gaupp (1898, p. 1146, 1911a, 1911b, 1911c, 1913) seems to have been the first author to state explicitly that the eardrums of anurans, reptiles, and mammals are not equivalent and homologous but rather developed three times independently (Gaupp, 1913, p. 304):
‘Die Trommelfelle der Anuren, Sauropsiden, Säuger … stellen Parallelbildungen dar, die sich … von einem gemeinsamen indifferenten Ausgangszustand aus, in dem zwar eine Paukenhöhle bestand, das zwischen ihr und der Haut gelegene Substanzgebiet aber noch nicht zu einer schwingungsfähigen Membran verdünnt war.’1
Gaupp (1913) provided many arguments in favour of this interpretation, mainly relating to the anatomical courses of nerves and muscles. He also integrated the fossil evidence from the then‐known therapsids, mainly taken from Broom (1904, 1911), and he designed a functional scenario in favour of his viewpoint (Fig. 1A). In a simplifying passage he spoke of a ‘supramandibular’ position of the tympanic membrane in sauropsids, whereas it is ‘inframandibular’ in mammals (Gaupp, 1913, p. 299). In recognition of the additions and clarifications given by Ernst Gaupp, who had an outstanding reputation as the founder of modern studies of craniogenesis in amphibians, reptiles, and mammals (Gaupp, 1906), the theory of Reichert has been referred to as the ‘Reichert–Gaupp theory’.
It must not be forgotten that the progress in vertebrate embryology was only made possible by some technical innovations: Reichert and the early authors made their discoveries by means of macroscopic preparations under magnifying lenses. Only serial sections by means of newly developed sledge‐microtomes and microscopic study of the stained section resulted in significant scientific progress and the foundation of the new discipline of microscopic anatomy; three‐dimensional wax‐plate models based on serial sections allowed a much better understanding of complicated embryonic structures (Born, 1876, 1883).
The studies of Edwin Goodrich
Goodrich (1915) studied the early embryological development of the middle ear region in squamates, birds, and mammals (Fig. 1B). This study, which seems to be rarely cited, was based on fairly comprehensive material and it allowed Goodrich to make excellent graphic reconstructions. His special interest was aroused by the peculiar course of the chorda tympani, which is in fact a posttrematic branch of the facial nerve. The chorda tympani runs at the dorsal side of the tympanic cavity, pushed dorsally by a secondarily developing, ventrocaudally situated ‘tympanic diverticulum’, the lateral wall of which eventually forms the tympanic membrane. The position of this diverticulum and its relationships to other structural elements of the middle ear region appear to be very similar in all three amniote groups. The early embryonic stages examined by Goodrich (1915) had also expressed the chondrogenic blastemata of the middle ear ossicles and the hyoid derivatives, and he found great similarities between all three groups; hence, he homologized the mammalian laterohyal with the processus dorsalis and the crus longum incudis with the processus internus (quadratus) of squamates. Further, Goodrich suggested that the extracolumella is secondarily lost in mammals and that the membrana tympani is situated in a very similar position in both groups (Fig. 1B). Therefore, he concluded (Goodrich, 1915, p. 155):
‘…the view of Gaupp that the tympanum of Reptilia is not homologous with that of Mammalia, chiefly because the former is situated above the Meckel's cartilage and the latter below it, seems to me greatly to exaggerate the importance of a comparatively trivial difference.’
However, because he did not follow the ontogenetic process into later developmental stages, he did not explain the different topographic relationships of the anlagen of the tympanic membranes to the bony structures. Goodrich left open the problem of homology of the anuran middle ear elements.
In his Studies on the Structure and Development of Vertebrates, Goodrich (1930) provided an excellent overview of Reichert's theory, including comparative‐anatomical, embryological and paleontological data. Based on his earlier study, he did not make a clear distinction between the tympanic membrane of sauropsids and mammals. He illustrated his concept, which principally accepts the Reichert–Gaupp theory, by a well‐known series of semi‐diagrammatic sketches. Goodrich (1930) described the relevant fossils in his section on the vertebrate skull, with special reference to the secondary jaw articulation in mammals. However, he remained uncertain as to the existence and fate of an extrastapes in early mammals.
The origin of the mammalian tympanic membrane
According to Westoll (1943), the tympanic membranes of anurans and amniotes are homologous, and all the connections of the stapes are derived from the processus opercularis (processus tympanicus) of the hyomandibula of Eusthenopteron. His concept was mainly based on fossil evidence but he also speculated about the expansion of the tympanic cavity: he proposed that the primary tympanic cavity was not associated with the lower jaw, and that the angular cleft housed a ‘submaxillary gland’ and parts of the pterygoideus internus muscle. Westoll's (1943) fairly detailed evolutionary scenario is arguably too hypothetical with regard to many soft tissue structures (such as the tympanic diverticula and muscles), and he did not sufficiently consider the functional problem of whether his small tympanic membranes could have activated the relatively large and massive hyomandibulae.
In a short paper, Westoll (1945) postulated the existence in cynodonts of a small ‘reptilian’ tympanic membrane, situated dorsally behind the quadrate and connected to a small extrastapes. He additionally proposed that the angular cleft was filled with a ‘recessus mandibularis’, i.e. a lateral extension of the pharynx (Fig. 2). This viewpoint was based mainly on the description of the Permian therocephalian Lycaenops by Broom (1936), and on the consideration of some other therapsids. According to Westoll, the lateral wall of this recessus later developed into the definitive membrana tympani (pars tensa), whereas the primary membrane was preserved as the pars flaccida (‘membrana Shrapnelli’).
Figure 2.
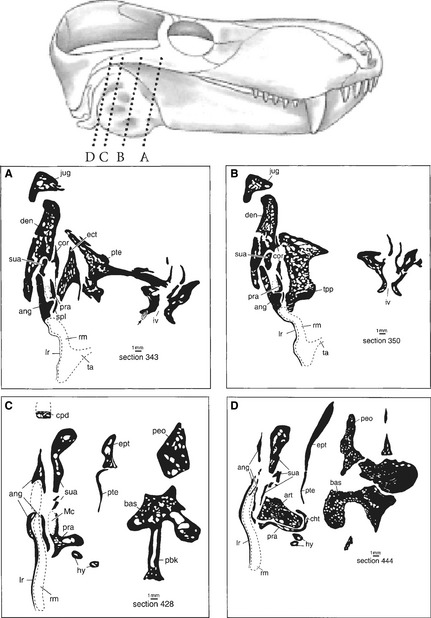
Reconstructed recessus mandibularis (tympanic cavity) in primitive therocephalian therapsids. Above is the skull of Glanosuchus macrops, a scymnosaurine therocephalian (modified from Brink, 1988). Below are four cross‐sections of Glanosuchus sp. drawn from a grinding series housed at the Department of Zoology at the University of Stellenbosch: the section planes are indicated by the stippled lines in the figure of the skull of Glanosuchus as well as by letters A–D. The likely position of Meckel's cartilage is indicated by a dotted line, i.e. it was formed as cartilage; only its posterior end is ossified as articular. In the cross‐sections the hypothetical recessus mandibularis is drawn in by a stippled contour underneath the very thin bony plate of the reflected lamina of the angular. The proximal portions of the hyoids show the mammalian tympanohyal (from Maier & van den Heever, 2002).
Watson (1953) mainly considered data from fossil synapsids. According to him, the pelycosaurs still possessed a large and massive stapes, and there was no tympanic membrane (Watson, 1953, p. 174). The angular cleft was supposed to have contained the anterior pterygoid muscle, after having wrapped around the smooth ventral border of the medial angular body. Following detailed arguments, he concluded that the tympanic membrane was an innovation of therapsids: ‘the characteristic arrangement of the sound‐transmitting apparatus of the mammalian ear arose as an unforseeable result of many changes in the head structure of mammalian ancestors’ (Watson, 1953, pp. 175–6). Parrington (1955) summarized previous studies. He postulated that early therapsids possessed a small tympanic cavity which was reached by an extrastapedial extension of the stapes. The pronounced groove at the back of the squamosal was thought to have housed a fairly long and wide external auditory meatus. He assumed that the gorgonopsid middle ear principally functioned ‘in much the same way as does that of a modern lizard’ (Parrington, 1955, p. 32). The peculiar structuring of the angular was interpreted by him exclusively in connection with the chewing musculature. He rejected the idea of a de novo evolution of a tympanic membrane. Shute (1956) modified some of the postulates of Watson (1953) and Parrington (1955) but he adhered to the assumption that the mammalian eardrum is ‘an extension of the original reptilian drum, and Shrapnell's membrane (is) a new formation consequent upon the retreat of the bodies of the malleus and incus into the middle ear’ (p. 278).
The paper of Allin (1975) proved to be very influential. Allin provided a fairly complete synopsis (much more detailed than the present one) of paleontological, comparative‐anatomical and functional data and concepts (Fig. 2B). However, he neglected evidence from early embryology, which is difficult to integrate (see section on developmental biology). Allin (1975) agreed with Sushkin (1927) and Watson (1953) that primitive amniotes as well as basal diapsids (captorhinomorphs) and synapsids (pelycosaurs) possessed a relatively massive stapes and show no evidence of a tympanic membrane; in them, the retroarticular process is not conspicuous. According to him, there exists evidence for faint hyostapedial contacts by means of cartilage or ligaments. He saw no conclusive evidence for an extrastapes in fossil synapsids and described the gradual size decrease of the quadrate and its detachment from the squamosal. The angular cleft that opens with the angular gap behind the reflected lamina was, according to Allin (1975), most likely filled by a recessus mandibularis or tympanic cavity, at least in therapsids (Fig. 2): it may have functioned as a resonator chamber from early on. The squamosal sulcus most probably did not house a long and tubular external acoustic meatus, but the depressor mandibulae attaching at the retroarticular process. Most likely, a primary postquadrate tympanic membrane did not exist in ‘cotylosaurs’ (stem amniotes) and primitive synapsids: this character distribution implies that the tympanic membrane of synapsids and diapsids must be of independent origin. Allin (1975) held that the air‐filled angular cleft functioned as a resonating chamber long before a neomorphic tympanic membrane was formed together with the epidermal lining of a fairly shallow external auditory meatus. Posteriorly, the retroarticular process and the hyal may have contributed to the fixation of the tympanic membrane. Allin (1975) estimated that advanced cynodonts had a hearing sensitivity not much inferior to that of mammals. Concomitant with the miniaturization of the postdentary elements, the dentary increased in size and a masseter muscle differentiated, which must have improved biting. However, real chewing probably occurred only in cynodonts that had developed a secondary palate (Maier et al. 1996; Maier, 1999) and complex tooth crowns (Crompton, 1972). These two adaptive trends were possibly coupled and may have enhanced each other, together with decreasing body size, in some lines of late theriodont evolution (Hopson, 1973). Allin (1975) argues that improvement of hearing was the driving factor in the transformation of the lower jaw but he did not completely rule out the possibility of a dual sound‐receiving system with a primary postquadrate membrane, although this was in contradiction to his favoured scenario, as manifested in his fig. 26E.
Parrington (1979) was understandably dissatisfied with the paper of Allin (1975). Next to his own study, he mainly referred to that of Westoll (1943), according to which all contacts of the stapes are derived from the hyomandibula of Eusthenopteron. Allin (1986) and Allin & Hopson (1992) seem to have reacted to Parrington's critique by making surprising compromises: they accepted the ‘dual origin hypothesis’, thereby devaluing some of the careful and consistent arguments in Allin (1975). Allin (1986) seems to contradict himself when he states: ‘Lombard & Bolt (1979) and Carroll (this volume) argue that the common ancestor of synapsids and sauropsids probably did not possess a true tympanic membrane. I agree’ (p. 289). Further down he writes about the squamosal sulcus harbouring a depressor mandibulae muscle: ‘I no longer adhere to this view…’ (Allin, 1986, p. 289). He further writes: ‘Although a tympanic membrane in a position corresponding to that of sauropsids was probably present, there is no direct evidence that a stapedial tympanic process existed’ (Allin, 1986, p. 290). He presented many arguments against the presumed postquadrate drum being functionally meaningful: ‘perhaps it was already vestigial in some advanced cynodonts and in the first mammals’ (Allin, 1986, p. 291). Otherwise, these studies are excellent presentations of the facts and theories of the middle ear evolution of mammals. In contrast, Lombard & Bolt (1979) state that the hypothesis of Gaupp ‘appears to rest on assumptions equally as plausible as those of the standard view’ and they conclude from their extensive analysis of the enormous literature treating this problem that ‘the tympanic membranes and the tympanic processes of the stapes in recent mammals, reptiles + birds, and frogs, are not homologous’ (pp. 19–20). Allin & Hopson (1992) presented a comprehensive review of the fossil evidence connected with the evolution of the mammalian middle ear; these authors again consider the conflicting hypotheses and conclude that two confluent tympanic membranes, as suggested by Westoll (1945) and Allin (1986), would also be evolutionarily possible.
Function of the ear in mammalian ancestors
Maier & van den Heever (2002) published sections of a grinding series of the primitive therocephalian Glanosuchus (Fig. 2), which essentially corroborate the data published by Broom (1936) on the therocephalian Lycaenops and on an unspecified therocephalian by Olson (1944). Figure 2 shows that the reflected lamina covering the angular notch is extremely thin but stabilized by low, radial crests; it seems most likely that the thin bony plate covering the recessus mandibularis already functioned as an inefficient forerunner of the tympanic membrane, although the pressure ratio must have been very low (Rosowski, 1992). These delicate bone structures certainly could not serve as muscle attachments. Maier & van den Heever (2002) concluded that ‘evolutionary optimization is not measured in absolute terms, but by its relation to contemporaneous and sympatric competitors, i.e. it must have been good enough for the Permian world’ (p. 316). Luo & Crompton (1994) carefully analyzed the structural and functional transformation of the quadrate into an incus in advanced cynodonts.
Kermack et al. (1973, 1981) described the well‐preserved material of Morganucodon. They were able to demonstrate that in this basal mammaliaform an almost perfect tympanic cavity must have existed underneath the ear ossicles, but that the whole structural complex of the middle ear was still attached to the angle of the dentary. The secondary jaw articulation between the condylar process of the dentary and the squamosal existed co‐axially, lateral to the primary quadrate‐articular joint (Fig. 3E). The authors (Kermack et al. 1981, p. 138 ff.) calculated sound sensitivity for Morganucodon by dividing the effective area of the tympanum by the area of the footplate and multiplying the quotient by the quadrate lever; they found a transformer ratio of 28.9, which is within the range found in extant mammals. Rosowski & Graybeal (1991) elaborated and refined the functional analysis of the ear structures of Morganucodon and they also concluded that ‘the evidence suggests that the middle and inner ear of Morganucodon functioned much like those of modern small mammals with high‐frequency hearing’ (p. 160). This state of structural transformation in an early mammaliaform represents an almost ideal link between the therapsid and mammalian conditions.
Figure 3.
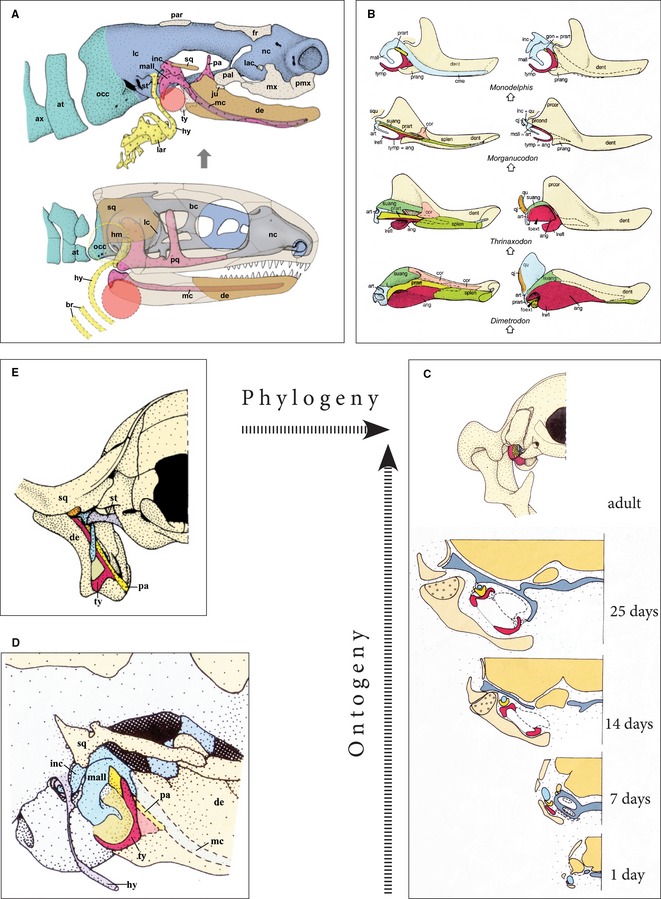
Evolutionary and ontogenetic development of the mammalian middle ear ossicles. (A) Comparison of the skull of a neonatal marsupial (Monodelphis domestica; above) and a basal amniote (below). The exocranium (brown) of the basal amniote is adopted from Captorhinus, and it is combined with a hypothetical ‘reptilian’ endocranium of a fetal stage (blue = neural endocranium, purple = viscerocranium, yellow = hyobranchial skeleton). The hyostylic suspension of the jaws in the basal amniote already shows the future arrangement of the ear ossicles: articular and quadrate (which will become malleus and incus, respectively) forming the primary jaw joint, and hyomandibula (which will become the stapes) inserted into the fenestra ovalis of the otic capsule. Neonatal marsupials retain the primary jaw articulation and the elements of the secondary joint (dentary and squamosal more densely stained) lie still far apart; both are gradually approaching each other in phylogeny and ontogeny, and finally develop an ‘Anlagerungsgelenk’ (‘appositional joint’); the red circle in the basal amniote indicates the future position of the tympanic membrane at the angle of the lower jaw, the yellow circle that of the future tympanic membrane behind the quadrate in squamates (adopted from Maier & Werneburg, 2014). (B) Evolutionary series of fossil synapsids (Dimetrodon, Thrinaxodon, Morganucodon) and postnatal stage of Monodelphis showing the gradual transformation of the angle of the lower jaw into sound‐transmitting middle ear structures (modified from Allin, 1975; and Maier, 1990: medial view on the left, lateral view on the right). (C) Postnatal ontogeny of Monodelphis demonstrating the translocation of the ectotympanic (red colour) from the lower jaw to the skull base (modified from Maier, 1990; adult stage in posterior view, early postnatal stages as cross‐sections). (D) Close‐up of the jaw and ear region in a 7‐day‐old Monodelphis, resembling that of Triassic cynodonts (lateral view; modified from Maier, 1990). (E) Posterior view of the ear region of Morganucodon from the Triassic‐Jurassic boundary, showing that the quadrate and articular elements of the primary jaw joint are functionally replaced by the squamosal and dentary of the secondary articulation (which is more laterally positioned). The primary elements are transformed into incus and malleus, but they are still attached to the lower jaw (modified from Kermack et al. 1981). The hatched arrows indicate that the middle ear structures of the adult marsupial are reached by two pathways: the phylogenetic and the ontogenetic.
The jaw‐joint articular cartilage
The transformation of the quadrate and articular into mammalian middle ear ossicles occurred at the same time as the development of a new secondary jaw articulation between the squamosal and the processus articularis (condyloideus) of the dentary. The gradual approach of these two dermal bones is well‐documented within the fossil record (Broom, 1904; Gaupp, 1911c) and it can also be observed in the craniogenesis of extant mammals (Fig. 3; see below). The primary jaw articulation between quadrate and articular is transformed into the joint between incus and malleus, and a new, secondary joint appears between two dermal bones, the squamosal and the dentary (Fig. 3A,B). The existence of articular cartilage on these dermal bones caused much controversy, which was discussed at length by Gaupp (1913). It has long been known that cartilage, usually called ‘secondary cartilage’, can differentiate in dermal bones (exoskeletal tissue) when functionally necessary in secondary contact zones (hydrostatic pressure according to the ‘principle of causal histogenesis’ by Pauwels, 1960, Kummer, 1985, and Hall, 2005). In postnatal marsupials, the approaching dentary and squamosal (Anlagerungs‐Gelenk or articulation d'apposition, Starck, 1967, p. 413 ff) show no cartilage at first, but after direct contact, cartilage differentiates on the articular process of the dentary first, and much later on the glenoid surface of the squamosal (Maier, 1987, 1990). The heterochronic differentiation of these tissues shows that genetic factors must be involved, in addition to the proposed mechanical responses. Gaupp (1913) and Zeller (1989) noted that monotremes do not possess articular cartilage at the dentary‐squamosal joint at all (cf. Lubosch, 1910), whereas in many placental mammals the condylar process and variable portions of the mandibular ramus can be pre‐formed in cartilage long before any contact with the opposing squamosal. Anthwal et al. (2012) have recently discussed the secondary and articular cartilages in the light of recent research.
The mandibular–basicranial shift
How the middle ear complex was transferred from the lower jaw to the basicranium, where it is typically found in extant mammals, remained to be clarified. Versluys (1903) and Gaupp (1913) attempted to answer that question by postulating that a horizontal anlage of the tympanic would be primitive for extant mammals – but this statement only shifts the problem into the more remote past. Describing the fossil monotreme Teinolophus from the Early Cretaceous, Rich et al. (2005) argued that the medial side of the mandible still showed signs of an attached angular and prearticular; however, Rougier et al. (2005) rejected this interpretation. Maier (1993) pointed out that the anlage of the tympanic is horizontal in both monotremes and placentals (see Van Kampen, 1905), whereas it is vertical and still attached to the mandible in early postnatal ontogeny of marsupials (see Maier, 1987, 1990) (Fig. 3C,D). In very altricial marsupial neonates the anlage of the tympanic bone and its membrane are almost vertical and they are apposed tightly to the medial trough of the dentary. With the postnatal expansion of the brain and the braincase, the elements of the future middle ear are gradually turned downward into an almost horizontal position (Fig. 3C). This repositioning of middle ear elements, which occurs within the first 2–4 postnatal weeks, is only possible because the young are tightly attached to the teats of the mother and because fusion of the lips by a specialized periderm arrests movement of the lower jaw while suckling (Gegenbaur, 1898; Müller, 1968a,b; Maier, 1987). Anthwal et al. (2012) suggested that the disruption of the cartilage of Meckel is also connected with the detachment of the ear ossicles from the cartilage of Meckel. It is only when the fusion of the lips is resolved after some weeks of lactotrophic nutrition that the dentaries swing back into a vertical position – leaving behind the middle ear elements underneath the otic capsule, to which they have become meanwhile attached by ligaments. The inflected angular process of the marsupial dentary appears to reflect these ontogenetic rotations (Fig. 3C). Maier (1987, 1990, 1999) speculated that a similar ontogenetic process may have been responsible for the translocation of the middle ear from the lower jaw to the basicranium, i.e. that this morphogenetic process may have a recapitulatory quality in the sense of Haeckel (1866). Indeed, any model of evolutionary transformation should include a concept of the ontogenetic mechanisms.
Because taxa such as Sinoconodon and Morganucodon from the Triassic‐Jurassic boundary still possessed a ‘mandibular middle ear’ (Fig. 3B,E), its translocation to the basicranium must have occurred later, i.e. during the Jurassic and Cretaceous. Fortunately enough, many excellently preserved fossils, mostly from China, document this important evolutionary step and a series of papers have been devoted to this important question (e.g. Hurum et al. 1996; Rougier et al. 1996; Kielan‐Jaworowska et al. 2004; Luo, 2007, 2011; Ji et al. 2009; Meng et al. 2011; Zhou et al. 2013). It is evident from these studies that basal mammalian taxa such as Yanoconodon (Eutricondonta), Liaconodon (Eutriconodonta), Haldanodon and Castorocauda (Docodonta), Megaconus (Haramyidae), and Maotherium (Symmetrodonta) retained a ‘mandibular tympanic’ (for review see Meng et al. 2003; Luo, 2011 and references therein). It seems probable that the detachment occurred several times independently, in Hadrocodium, Pseudotribosphenida including Monotremata, Multituberculata s.str., and Theria (Rich et al. 2005; Luo, 2011). There is an ongoing discussion about whether the detachment of the tympanic from the dentary in monotremes and therians is homologous or whether it occurred independently, at least twice. The interpretation is influenced by the phylogenetic positioning of the monotremes. As described earlier, the orientation of the tympanic differs between marsupials on the one hand, and monotremes and placentals on the other. We have good reasons to assume that the vertical position in early ontogeny (found in marsupials) is the plesiomorphic state of the groundplan of therians, because it closely resembles the evolutionary stage of adult cynodonts and of Morganucodon. This character distribution would imply that at least some phases of the ontogenetic and phylogenetic process occurred independently. Zeller (1989, 1993) also favoured a convergent detachment of the tympanicum in monotremes.
On the existence of ossified Meckel's cartilage
It is puzzling that in a number of early mammalian fossil taxa the anterior process of the malleus is very elongated. Subsequent to its description in the Cretaceous eutriconodonts Repenomamus and Gobiconodon by Wang et al. (2001), most authors considered this bony structure to represent an ossified Meckel's cartilage: Morganucodon (Luo, 2007, 2011; Ji et al. 2009), Yanoconodon, Maotherium (Ji et al. 2009), Sinoconodon (Luo, 2011), Megaconus (Zhou et al. 2013); Agilodocodon (Meng et al. 2015); Meng et al. (2003), who reviewed the phenomenon first, also described it in Zhangheotherium. In Fig. 2 is shown that the cartilage of Meckel of Glanosuchus is only indicated as empty contour.
According to Stadtmüller (1936a), Meckel's cartilage persists in some lissamphibians. It seems to be generally retained as a thin cartilaginous rod in lepidosaurs (Versluys, 1936), but it disappears in all birds and mammals (Marinelli, 1936; Stadtmüller, 1936b). In extant mammals, Meckel's cartilage has mechanical and formative functions only in very early embryonic stages, but these roles are soon taken over by the adjacent dermal bones and this very thin rod of cartilage becomes resorbed in early postnatal ontogeny.
The identification of the elongated anterior process of the malleus in certain fossil taxa as an ossified Meckel's cartilage seemed to be questionable, on the basis of its dimensions. To investigate this a little further, we measured the diameter of Meckel's cartilage in a few amniotes of which histological serial sections of perinatal stages were to hand (Table 1). In all studied mammals, birds, and squamates the diameter of Meckel's cartilage does not exceed 0.5 mm. In all available postnatal stages of the opossum Monodelphis domestica younger than 4 weeks its diameter is < 0.17 mm (see Maier, 1987). In a neonate rabbit (Oryctolagus cuniculus), its diameter is about 0.2 mm. Furthermore, it can be gleaned from the figures of Zeller (1989) that in a nestling of the platypus Ornithorhynchus (70 mm CRL), Meckel's cartilage is around 0.5 mm thick and in a Crocodylus (105 mm TL) 0.3 mm. In Homo we observed a significant increase during early fetal life, but in most other cases the absolute thickness of Meckel's cartilage remains more‐or‐less constant in absolute terms, i.e. it decreases relative to head size (Table 1).
Table 1.
Diameter of Meckel's cartilage of selected amniote species representing Squamata, Aves, and Mammalia (Marsupialia and Placentalia). Measurements were taken on histological serial sections of early ontogenetic stages near the posterior end of the dentary. All specimens are housed in the Institut für Evolution und Ökologie, Universität Tübingen, Germany. Fetuses were stained in our histology lab with standard Azan. For abbreviations see text
| Class/order | Species | Size/age | Meckel's cartilage diameter |
|---|---|---|---|
| Squamata | Heloderma suspectum | HL 19 mm | 0.19 mm |
| Squamata | Lacerta lepida | TL 78 mm | 0.27 mm |
| Squamata | Pogona vitticeps | HL 12.5 mm | 0.19 mm |
| Squamata | Pogona vitticeps | HL 14 mm | 0.19 mm |
| Squamata | Varanus acanthurus | HL 17 mm | 0.25 mm |
| Squamata | Varanus niloticus | HL 33 mm | 0.38 mm |
| Aves | Calidris sp. | HL 14 mm | 0.20 mm |
| Aves | Columba livia | HL 25 mm | 0.20 mm |
| Marsupialia | Monodelphis domestica | CRL 10 mm | 0.17 mm |
| Marsupialia | Monodelphis domestica | CRL 18 mm | 0.17 mm |
| Marsupialia | Monodelphis domestica | CRL 34 mm | 0.16 mm |
| Marsupialia | Monodelphis domestica | CRL 63 mm | < 0.1 mm |
| Placentalia | Crocidura russula | Neonate | 0.13 mm |
| Placentalia | Crocidura russula | 7 days | 0.13 mm |
| Placentalia | Homo sapiens | CRL 37 mm | 0.30 mm |
| Placentalia | Homo sapiens | CRL 42 mm | 0.34 mm |
| Placentalia | Homo sapiens | CRL 100 mm | 0.50 mm |
| Placentalia | Homo sapiens | HL 63 mm | 0.46 mm |
| Placentalia | Oryctolagus cuniculus | CRL 108 mm | 0.20 mm |
| Placentalia | Sus scrofa | CRL 56 mm | 0.44 mm |
| Placentalia | Sus scrofa | CRL 83 mm | 0.44 mm |
| Placentalia | Tupaia glis | CRL 59 mm | 0.25 mm |
We assume that an ossified Meckel's cartilage would not be thicker than observed in our investigated taxa, because we have no evidence of secondary growth and we never observed any ossification (apart from the symphyseal region and the corpus of the malleus). Indeed, we observed gradual resorption at later postnatal stages. This means that the skeletal element of Meckel, be it cartilage or bone, has the size and shape of a thin thread. We are convinced that the structures regarded as ‘ossified Meckel's cartilage’ in the paleontological literature (see especially Wang et al. 2001) are in fact way too thick – although no measurements are given in the studies referred to. We therefore suspect that the skeletal element in question is an elongated prearticular (gonial). However, Anthwal et al. (2012) have recently reviewed a number of developmental studies that show that the cartilage of Meckel can ossify under certain abnormal conditions.
Thus, it makes no sense to call a structure ‘paedomorphic’ that never existed as an ossification in embryos or fetuses (Luo, 2007; Anthwal et al. 2012), i.e. retaining an embryonic state (Sewertzoff, 1931; DeBeer, 1937; Gould, 1977) – it is simply a plesiomorphic retention of an old fetal developmental pattern. If anything, an ossified cartilage of Meckel would have to be called ‘peramorphic’ or ‘hypermorphotic’ (McKinney & McNamara, 1991).
Developmental biology, developmental genetics, and phylogeny
Developmental biology has become a very important field of morphological research in recent years and it continues to produce an enormous number of publications, which are difficult for the outsider to follow (cf. Wolpert et al. 1998; Gilbert, 2014). Only a few points concerning the embryogenesis of the middle ear can be mentioned in this review. Recently, Takechi & Kuratani (2010) and Anthwal et al. (2012) have provided excellent reviews covering both traditional and modern approaches to an understanding of the mammalian middle ear. Thompson et al. (2012) have studied the relationships of the stapes and the otic capsule in special mice strains (Wnt1cre/Dicer) and they found a dual origin of the footplate of the stapes in the mouse. O'Gorman (2005) noted that his ‘processus brevis’ (probably identical with the official anatomical name processus lateralis) is formed by second arch tissues, supporting its homology with the processus retroarticularis of non‐mammalian amniotes (Mason, 2013; critically discussed the finds of O'Gorman). This is not too surprising because this process is the insertion point of the musculus depressor mandibulae (cf. Allin, 1975: plate 6E), which is innervated by the facial nerve and whose tendons contain second arch material (Köntges & Lumsden, 1996). Sienknecht (2013) and Kitazawa et al. (2015) compared experimentally the developmental genetics of birds and mammals (chicken and mouse); the latter could clearly support the independent origin of the tympanic membrane in diapsids and mammals. However, this experimental approach was not fine‐grained enough to tell anything about a possible double tympanic membrane, as suggested by Westoll (1945) and Allin (1986).
These studies are most valuable additions to the traditional anatomical (mostly embryological) and palaeontological approach because they include new data. So far, most of the results appear to fit well into the Reichert–Gaupp theory. Principally, this experimental research has to obey the same methodological rules as the traditional studies. That means that it has to interpret its data within the theoretical framework of comparative ontogeny and phylogenetic systematics, i.e. to distinguish between homologous and convergent structures and processes (Patterson, 1982, 1988; DePinna, 1991; Hall, 1994).
Due to the enormous technical expense, so far only a few species have been included in studies of developmental genetics (mainly chicken and mouse) and taxonomic differences have been hardly considered. But we know that early developmental processes can vary considerably. One only has to think of the different types of egg cells and modes of cell division (blastulation). Thompson et al. (2012) have recently discussed this with respect to the composition of the stapes. In recent years, the group of Ralph Sommer (cf. Rudel & Sommer, 2003; Zheng et al. 2005; Schlager et al. 2006) has demonstrated that the cellular pathways can vary considerably in closely related nematodes and in other invertebrates.
Conclusion
The intricate interrelationship between ontogeny and phylogeny, which was already noticed and discussed in pre‐Darwinian times (Baer, 1828), was pressed by Haeckel (1866) into the dogma of the ‘biogenetic law’. Critical minds soon pointed out that the aspect of palingenetic recapitulation does not sufficiently accentuate the active role of ontogeny. Garstang (1922) corrected some viewpoints of Haeckel by saying that ‘phylogeny is the procession of ontogenies along a given phyletic line of modification’ and ‘ontogeny does not recapitulate phylogeny: it creates it’ (p. 98). Sewertzoff (1931) developed his concept of ‘phyllembryogenesis’ in order to underline the importance of early ontogenesis for evolutionary change. DeBeer (1958) also clearly stated that ‘phylogeny is … due to modified ontogeny’ and ‘new characters may appear at all stages of ontogeny, and by heterochrony they may be retarded or accelerated so as to appear later or earlier in subsequent ontogenies’ (p. 170).
Irrespective of the relationships between phylogeny and ontogeny, it can be stated that the concepts of modern morphology, including its terminology, are essentially based on the results of comparative ontogenetic studies that begun in the first decades of the 19th century. This approach made use of the new methods and techniques of histology and microscopic anatomy – as well as of Darwinian and Haeckelian phylogeny. New details regarding the differentiation of tissues (histogenesis) and organs (organogenesis) could be incorporated within comparative and phylogenetic studies; adult structures became much better understood. Already at the turn of the century the outlines of modern comparative anatomy of vertebrates had emerged (Hertwig, 1906). The anatomy of the head region gained its modern shape mainly by the pioneering articles of Gaupp (1906, 1913). Then, the increasing number of fossils had to be integrated into this new model of evolutionary morphology. Fossils mainly added to the knowledge of skeletal structures of adult stages, but they provided the concepts of comparative ontogeny with the dimension of geological time (‘deep time’). Goodrich (1930) and Broom (1936) were important in this period because they were able to integrate both embryology and paleontology. At the end of this first period of modern anatomy and paleontology, the handbook of Bolk et al. (1931–1939) represents an impressive synthesis. It is evident that phylogenetic systematics and cladistics on the one hand (Hennig, 1966; Wägele, 2000) and developmental biology and genetics on the other (Gilbert, 2014) provide most valuable additions to the traditional corpus of knowledge.
Anatomical abbreviations
Because most figures are adopted from previous publications, the labeling is not always consistent.
- ang,
angular
- art,
articular
- at,
atlas
- ax,
axis
- bas,
basisphenoid
- bc,
brain capsule
- br,
branchial bars
- cht,
chorda tympani
- cme,
cartilago Meckeli (Meckel's cartilage)
- col,
columella
- cor,
coronoid
- pd,
coronoid process of dentary
- CRL,
crown–rump length
- de/den/dent,
dentary
- eco,
extracolumella
- ect,
ectopterygoid
- ecty,
ectotympanic
- ept,
epipterygoid (alisphenoid)
- foext,
fossa externa
- fr,
frontal
- glg,
ganglion geniculi (facialis)
- gon,
gonial
- HL,
head length
- hm,
hyomandibula
- hy,
hyoid
- inc,
incus
- ju/jug,
jugal
- iv,
interpterygoid vacuity
- lac,
lacrimal
- lar,
larynx
- lc,
labyrinth capsule
- lhy,
laterohyal
- lr/lrefl,
lamina reflexa (reflected lamina)
- mal/mall,
malleus
- mc/Mc,
Meckel's cartilage
- mty,
membrana tympani
- mx,
maxillary
- nc,
nasal capsule
- nf,
nervus facialis
- occ,
occipital
- pa,
processus ascendens palatoquadrati
- pal,
palatine
- par,
parietal
- pbk,
parabasisphenoid keel
- peo,
perioticum
- pmx,
premaxillary
- pq,
palatoquadrate
- pra/prart,
prearticular
- prang,
processus angularis
- prcond,
processus condylaris
- prcor,
processus coronoideus
- prd,
processus dorsalis
- pri,
processus internus
- pte,
pterygoid
- qj,
quadratojugal
- qu/qua,
quadrate
- rm,
recessus mandibularis
- spir,
spiraculum
- splen/spl,
splenial
- sq/squ,
squamosal
- st/sta,
stapes
- sua/suang,
surangular
- ta,
tuba auditiva
- TL
total length
- Tpp,
transversal process of pterygoid
- ty/tymp,
tympanic (ectotympanic)
Note
[The tympanic membranes of anurans, sauropsids, mammals cannot be derived from each other, but were developed in parallel … from an undifferentiated common ancestor, in which existed a tympanic cavity, but in which the tissue between the cavity and the skin was not yet thinned out to a vibrating membrane] (translated by WM).
References
- Allin EF (1975) Evolution of the mammalian middle ear. J Morphol 147, 403–438. [DOI] [PubMed] [Google Scholar]
- Allin EF (1986) The auditory apparatus of advanced mammal‐like reptiles and early mammals In: The Ecology and Biology of Mammal‐Like Reptiles. (eds Hotton N, MacLean PD, Roth JJ, Roth EC.), pp. 283–294, Washington, DC: Smithsonian Institution Press. [Google Scholar]
- Allin EF, Hopson JA (1992) Evolution of the auditory system in Synapsida (‘mammal‐like reptile’ and primitive mammals) as seen in the fossil record In: The Evolutionary Biology of Hearing. (eds Webster DB, Fay RR, Popper AN.), pp. 587–614, New York: Springer‐Verlag. [Google Scholar]
- Anthwal N, Joshi L, Tucker AS (2012) Evolution of the mammalian middle ear and jaw: adaptations and novel structures. J Anat 222, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer CE (1828) Ueber Entwickelungsgeschichte der Thiere, Beobachtung und Reflexion. Koenigsberg: Borntraeger. [Google Scholar]
- Bolk L, Göppert E, Kallius E, et al. (eds) (1931. –1939) Handbuch der Vergleichenden Anatomie der Wirbeltiere, Vols 1–6. Berlin: Urban & Schwarzenberg. [Google Scholar]
- Born G (1876) Ueber die Nasenhoehlen und den Thränennasengang der Amphibien. Morph Jb 2, 577–646. [Google Scholar]
- Born G (1883) Die Plattenmodellirmethode. Arch Mikrosc Anat 22, 584–599. [Google Scholar]
- Brink AS (1988) Illustrated Bibliographical Catalogue of the Synapsida. Handbook 10, Part II. Pretoria: Government Printer. [Google Scholar]
- Broman I (1899) Die Entwickelungsgeschichte der Gehörknöchelchen beim Menschen. Anat Hefte I. Abt 34–37, 507–670. [Google Scholar]
- Broom R (1904) On the structure of the theriodont mandible, and on its mode of articulation with the skull. Proc Zool Soc Lond 1904, 490–498. [Google Scholar]
- Broom R (1911) On the structure of the skull in cynodont reptiles. Proc Zool Soc Lond 1911, 893–925. [Google Scholar]
- Broom R (1936) On the structure of the skull in the mammal‐like reptiles of the suborder Therocephalia. Philos Trans B 226, 1–42. [Google Scholar]
- Carus CG (1818) Lehrbuch der Zootomie. Leipzig: Gerhard Fleischer. [Google Scholar]
- Crompton AW (1972) Postcanine occlusion in cynodonts and tritylodontids. Bull Br Mus Nat Hist Geol 21, 29–71. [Google Scholar]
- Cuvier G (1800) LeÇons d'anatomie comparée. Tome II. Paris: Baudouin. [Google Scholar]
- DeBeer G (1937) The Development of the Vertebrate Skull. Oxford: Clarendon Press. [Google Scholar]
- DeBeer G (1958) Embryos and Ancestors. Oxford: Clarendon Press. [Google Scholar]
- DePinna MCC (1991) Concepts and tests of homology in the cladistic paradigm. Cladistics 7, 367–394. [Google Scholar]
- Garstang W (1922) The theory of recapitulation. A critical re‐statement of the biogenetic law. J Linn Soc Lond Zool 35, 81–101. [Google Scholar]
- Gaupp E (1898) Ontogenese und Phylogenese des schall‐leitenden Apparates bei den Wirbeltieren. Erg Anat Entwicklungsgesch 8, 900–1149. [Google Scholar]
- Gaupp E (1906) Die Entwicklung des Kopfskelettes In: Handbuch der vergleichenden und experimentellen Entwickelungslehre der Wirbeltiere, Vol. III. (ed. Hertwig O.), pp. 573–874, Jena: Fischer. [Google Scholar]
- Gaupp E (1908) Zur Entwickelungsgeschichte und vergleichenden Morphologie des Schädels von Echidna aculeata var. typica. Richard Semon Zool Forschungsreisen 3, 539–788. [Google Scholar]
- Gaupp E (1911a) Beiträge zur Kenntnis des Unterkiefers der Wirbeltiere. I. Der Processus anterior (Folii) des Hammers der Säuger und das Goniale der Nichtsäuger. Anat Anz 39, 97–135. [Google Scholar]
- Gaupp E (1911b) Beiträge zur Kenntnis des Unterkiefers der Wirbeltiere. II. Die Zusammensetzung des Unterkiefers der Quadrupeden. Anat Anz 39, 433–473. [Google Scholar]
- Gaupp E (1911c) Beiträge zur Kennnis des Unterkiefers der Wirbeltiere. III. Das Problem der Entstehung eines ‘sekundären’ Kiefergelenks bei den Säugern. Anat Anz 39, 609–666. [Google Scholar]
- Gaupp E (1913) Die Reichertsche Theorie. Arch Anat Entwicklungsgesch (Suppl.) 1912, 1–416. [Google Scholar]
- Gegenbaur C (1898) Vergleichende Anatomie der Wirbelthiere, Vol. I. Leipzig: Engelmann. [Google Scholar]
- Geoffroy‐Saint‐Hilaire E (1818) Philosophie anatomique. Vol. I. Pièces osseuses des organes respiratoires. Paris: Méquignon‐Marvis; (reprint). [Google Scholar]
- Gilbert SF (2014) Developmental Biology, 10th edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- Goodrich ES (1915) The chorda tympani and middle ear in reptiles, birds, and mammals. Q J Microsc Sci 61, 137–160. [Google Scholar]
- Goodrich ES (1930) Studies on the Structure and Development of Vertebrates. London: MacMillan. [Google Scholar]
- Gould SJ (1977) Ontogeny and Phylogeny. Cambridge, MA: Harvard University Press. [Google Scholar]
- Haeckel E (1866) Generelle Morphologie der Organismen. Berlin: Georg Reimer. [Google Scholar]
- Hall BK. (ed.) (1994) Homology. The Hierarchical Basis of Comparative Biology. London: Academic Press. [Google Scholar]
- Hall BK (2005) Bones and Cartilage. Developmental and Evolutionary Skeletal Biology. Amsterdam: Elsevier Academic Press. [Google Scholar]
- Hennig W (1966) Phylogenetic Systematics. Urbana: University of Illinois Press. [Google Scholar]
- Hertwig O. (ed.) (1906) Handbuch der vergleichenden und experimentellen Entwickelungslehre der Wirbeltiere. 3 Volumes. Jena: Gustav Fischer. [Google Scholar]
- Hopson JA (1973) Endothermy, small size, and the origin of mammalian reproduction. Am Nat 107, 446–452. [Google Scholar]
- Hurum JH, Presley R, Kielan‐Jaworowska Z (1996) The middle ear in multituberculate mammals. Acta Palaeontol Pol 41, 253–275. [Google Scholar]
- Huschke E (1827) Über die Kiemenbogen und Kiemengefässe beym bebrüteten Hühnchen. Isis 20, 401–403. [Google Scholar]
- Huschke E (1828) Über die Kiemenbögen am Vogelembryo. Isis 21, 160–164. [Google Scholar]
- Huxley TH (1869) On the representatives of the malleus and the incus of the Mammalia and the other Vertebrata. Proc Zool Soc Lond 37, 391–408. [Google Scholar]
- Ji Q, Luo Z‐X, Zhang X, et al. (2009) Evolutionary development of the middle ear in Mesozoic therian mammals. Science 326, 278–281. [DOI] [PubMed] [Google Scholar]
- Kermack KA, Musset F, Rigney HW (1973) The lower jaw of Morganucodon . Zool J Linn Soc 53, 87–175. [Google Scholar]
- Kermack KA, Musset F, Rigney HW (1981) The skull of Morganucodon . Zool J Linn Soc 71, 1–158. [Google Scholar]
- Kielan‐Jaworowska Z, Cifelli RL, Luo Z‐X (2004) Mammals from the Age of Dinosaurs. New York: Columbia University Press. [Google Scholar]
- Kitazawa T, Takechi M,HirasawaT, et al. (2015) Developmental genetic bases behind the independent origin of the tympanic membrane in mammals and diapsids. Nat Commun 6, 6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker A (1849) Allgemeine Betrachtungen über die Entstehung des knöchernen Schädels der WirbeltiereLeipzig:Berichte der königlichen zootomischen Anstalt zu Würzburg, 1847/1848.
- Kölliker A (1879) Grundriss der Entwickelungsgeschichte des Menschen und der höheren Tiere, 2nd edn Leipzig: Engelmann. [Google Scholar]
- Köntges G, Lumsden A (1996) Rhomencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229–3242. [DOI] [PubMed] [Google Scholar]
- Kummer B (1985) Kausale Histogenese der Gewebe des Bewegungsapparates und funktionelle Anpassung In: Benninghoff Anatomie, 14th edn, Vol. I. (ed. Staubessand J.), pp. 199–213, Berlin: Urban & Schwarzenberg. [Google Scholar]
- Lombard RE, Bolt JR (1979) Evolution of the tetrapod ear: an analysis and re‐interpretation. Biol J Linn Soc 11, 19–76. [Google Scholar]
- Lubosch W (1910) Bau und Entstehung der Wirbeltiergelenke. Jena: Fischer. [Google Scholar]
- Luo Z‐X (2007) Transformation and diversification in early mammal evolution. Nature 450, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Luo Z‐X (2011) Developmental patterns in Mesozoic evolution of mammal ears. Annu Rev Ecol Evol Syst 42, 355–380. [Google Scholar]
- Luo Z‐X, Crompton AW (1994) Transformation of the quadrate (incus) through the transition from non‐mammalian cynodonts to mammals. J Vertebr Paleontol 14, 341–374. [Google Scholar]
- Maier W (1987) Der Processus angularis bei Monodelphis domestica (Didelphidae, Marsupialia) und seine Beziehungen zum Mittelohr: Eine ontogenetische und evolutionsmorphologische Untersuchung. Gegenb Morphol Jahrb 133, 123–161. [PubMed] [Google Scholar]
- Maier W (1990) Phylogeny and ontogeny of mammalian middle ear structures. Neth J Zool 40, 55–74. [Google Scholar]
- Maier W (1993) Cranial morphology of the common therian ancestor, as suggested by the adaptations of neonate marsupials In: Mammal Phylogeny, Vol. 1. (eds Szalay FS, Novacek MJ, McKenna MC.), pp. 165–181, New York: Springer. [Google Scholar]
- Maier W (1999) On the evolutionary biology of early mammals – with methodological remarks on the interaction between ontogenetic adaptation and phylogenetic transformation. Zool Anz 238, 55–74. [Google Scholar]
- Maier W (2013) The entotympanic in late fetal Artiodactyla (Mammalia). J Morphol 274, 926–939. [DOI] [PubMed] [Google Scholar]
- Maier W, van den Heever J (2002) Middle ear structures in the Permian Glanosuchus sp. (Therocephalia, Therapsida), based on thin sections. Mitt Mus Nat kd Berlin (Geowiss Reihe) 5, 309–318 (Festschrift für H.‐P. Schultze). [Google Scholar]
- Maier W, Werneburg I (2014) Schlüsselereignisse der organismischen Makroevolution. Zürich: Scidinge Hall. [Google Scholar]
- Maier W, van den Heever J, Durand F (1996) New therapsid specimens and the origin of the secondary hard and soft palate. J Zool Syst Evol Res 34, 9–19. [Google Scholar]
- Marinelli W (1936) Kranium und Visceralskelett der Sauropsiden. II. Vögel In: Handbuch der Vergleichenden Anatomie der Wirbeltiere IV. (eds Bolk L, Göppert E, Kallius E, Lubosch W.), pp. 809–838, Berlin: Urban & Schwarzenberg. [Google Scholar]
- Mason MJ (2013) Of mice, moles and guinea pigs: functional morphology of the middle ear in living mammals. Hear Res 301, 4–18. [DOI] [PubMed] [Google Scholar]
- McKinney ML, McNamara KJ (1991) Heterochrony. The Evolution of Ontogeny. New York: Plenum Press. [Google Scholar]
- Meckel JF (1820) Handbuch der menschlichen Anatomie, Vol. 4. Halle: Buchhandlung des Halleschen Waisenhauses. [Google Scholar]
- Meng J, Hu Y‐M, Wang Y‐Q, et al. (2003) The ossified Meckel's cartilage and internal groove in Mesozoic mammaliaforms: implications to origin of the definitive mammalian middle ear. Zool J Linn Soc 138, 431–448. [Google Scholar]
- Meng J, Wang Y, Li C (2011) Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature 472, 181–185. [DOI] [PubMed] [Google Scholar]
- Meng Q‐J, Ji Q, Zhang Y‐G, et al. (2015) An arboreal docodont from the Jurassic and mammaliaform ecological diversification. Science 347, 764–768. [DOI] [PubMed] [Google Scholar]
- Müller F (1968a) Zur Phylogenese des sekundären Kiefergelenks. Rev Suisse Zool 75, 373–414. [PubMed] [Google Scholar]
- Müller F (1968b) Die transitorischen Verschlüsse in der postnatalen Entwicklung der Marsupialia. Acta Anat 71, 581–624. [PubMed] [Google Scholar]
- O'Gorman S (2005) Second branchial arch lineages of the middle ear wild‐type and Hoxa2 mutant mice. Dev Dyn 234, 124–131. [DOI] [PubMed] [Google Scholar]
- Olson EC (1944) Origin of mammals based upon cranial morphology of the therapsid suborders. Geol Soc Am Spec Pap 55, 1–130. [Google Scholar]
- Owen R (1843) Lectures on comparative anatomy, London: Lonman, Brown, Green and Longmans. [Google Scholar]
- Palmer WR (1913) Note on the lower jaw and ear ossicles of a foetal Perameles . Anat Anz 43, 510–515. [Google Scholar]
- Parker WK (1874) On the structure and developmennt of the skull in the pig (Sus scrofa). Philos Trans Biol 164, 289–336. [Google Scholar]
- Parker WK (1877) On the structure and development of the skull of the urodelous Amphibia. Philos Trans R Soc Lond B Biol Sci 167, 529–597. [Google Scholar]
- Parrington FR (1955) On the cranial anatomy of some gorgonopsids and the synapsid middle ear. Proc Zool Soc Lond 125, 1–40. [Google Scholar]
- Parrington FR (1979) The evolution of the mammalian middle and outer ears: a personal review. Biol Rev 54, 369–387. [DOI] [PubMed] [Google Scholar]
- Patterson C (1977) Cartilage bones, dermal bones and membrane bones, or the exoskeleton versus the endoskeleton In: Problems in Vertebrate Evolution. (eds Andrews SM, Miles RS, Walker AD.), pp. 77–121, London: Academic Press. [Google Scholar]
- Patterson C (1982) Morphological characters and homology In: Problems of Phylogenetic Reconstruction. (eds Joysey KA, Friday AE.), pp. 21–74, London: Academic Press. [Google Scholar]
- Patterson C (1988) Homology in classical and molecular biology. Mol Biol Evol 5, 603–625. [DOI] [PubMed] [Google Scholar]
- Pauwels F (1960) Eine neue Theorie über den Einfluß mechanischer Reize auf die Differenzierung der Stützgewebe. Z Anat Entwicklungsgesch 121, 478–515. [PubMed] [Google Scholar]
- Rathke H (1825a) Kiemen bei Säugethieren. Isis 1825, 747. [Google Scholar]
- Rathke H (1825b) Kiemen bei Vögeln. Isis 1825, 1100. [Google Scholar]
- Reichert C (1837) Ueber die Visceralbogen der Wirbelthiere im allgemeinen und deren Metamorphosen bei den Vögeln und Säugethieren. Müllers Arch Physiol 1837, 120–222. [Google Scholar]
- Reichert C (1838) Vergleichende Entwickelungsgeschichte des Kopfes der nackten Amphibien nebst den Bildungsgesetzen des Wirbelthier‐Kopfes. Koenigsberg: Gebrüder Borntraeger. [Google Scholar]
- Rich TH, Hopson JA, Musser AM, et al. (2005) Independent origins of middle ear bones in monotremes and therians. Science 307, 910–914. [DOI] [PubMed] [Google Scholar]
- Romer AS (1962) The Vertebrate Body, 3rd edn Philadelphia: Saunders. [Google Scholar]
- Rosowski JJ (1992) Hearing in transitional mammals: predictions from the middle ear anatomy and hearing capabilities of extant mammals In: The Evolutionary Biology of Hearing. (eds Webster DB, Fay RR, Popper AN.), pp. 615–632, New York: Springer. [Google Scholar]
- Rosowski JJ, Graybeal A (1991) What did Morganucodon hear? Zool J Linn Soc 101, 131–168. [Google Scholar]
- Rougier GW, Wible JR, Novacek MJ (1996) Middle‐ear ossicles of Kryptobataar dashzevegi (Mammalia, Multituberculata): implications for mammaliamorph relationships and evolution of the auditory apparatus. Am Mus Novit 3187, 1–43. [Google Scholar]
- Rougier GW, Forasiepi AM, Martinelli AG (2005) Comment on ‘independent origins of middle ear bones in monotremes and therians’ (II). Science 309, 1492. [DOI] [PubMed] [Google Scholar]
- Rudel D, Sommer RJ (2003) The evolution of developmental mechanisms. Dev Biol 264, 15–37. [DOI] [PubMed] [Google Scholar]
- Russell ES (1916) Form and Function. London: Murray; (reprint by University of Chicago Press, 1982). [Google Scholar]
- Schlager B, Röseler W, Zheng M, et al. (2006) HAIRY‐like transcription factors and the evolution of the nematode vulva equivalence group. Curr Biol 16, 1386–1394. [DOI] [PubMed] [Google Scholar]
- Sewertzoff AN (1931) Studien über die Reduktion der Organe der Wirbeltiere. Zool Jahrb Anat Ontogen 53, 611–699. [Google Scholar]
- Shute CCD (1956) The evolution of the mammalian eardrum and tympanic cavity. J Anat 90, 261–281. [PMC free article] [PubMed] [Google Scholar]
- Sienknecht UJ (2013) Developmental origin and fate of middle ear structures. Hear Res 301, 19–26. [DOI] [PubMed] [Google Scholar]
- Stadtmüller F (1936a) Kranium und Visceralskelett der Stegocephalen und Amphibien In: Handbuch der Vergleichenden Anatomie der Wirbeltiere IV. (eds Bolk L, Göppert E, Kallius E, Lubosch W.), pp. 501–698, Berlin: Urban & Schwarzenberg. [Google Scholar]
- Stadtmüller F (1936b) Kranium und Visceralskelett der Säugetiere In: Handbuch der Vergleichenden Anatomie der Wirbeltiere IV. (eds Bolk L, Göppert E, Kallius E, Lubosch W.), pp. 839–1016, Berlin: Urban & Schwarzenberg. [Google Scholar]
- Starck D (1967) Le crâne des mammifères In: Traité de Zoologie, XVI (1). (ed. Grassé P‐P.), pp. 405–549, Paris: Masson. [Google Scholar]
- Starck D (1979) Vergleichende Anatomie der Wirbeltiere, Vol. 2. Das Skeletsystem. Berlin: Springer. [Google Scholar]
- Starck D (1989) Considerations on the nature of skeletal elements in the vertebrate skull, especially in mammals In: Trends in Vertebrate Morphology. (eds Splechtna H, Hilgers H.), pp. 375–385, Stuttgart: Gustav Fischer Verlag. [Google Scholar]
- Sushkin PP (1927) On the modifications of the mandibular and hyoid arches and their relations to the brain‐case in the early Tetrapoda. Palaeont Z 8, 263–321. [Google Scholar]
- Takechi M, Kuratani S (2010) History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. J Exp Zool (Mol Dev Evol) 314B, 417–433. [DOI] [PubMed] [Google Scholar]
- Thompson H, Ohazama A, Sharpe PT, et al. (2012) The origin of the stapes and relationship to the otic capsule and oval window. Dev Dyn 241, 1396–1404. [DOI] [PubMed] [Google Scholar]
- Van der Klaauw CJ (1924) Bau und Entwickelung der Gehörknöchelchen. Erg Anat Entwicklungsgesch 25, 565–622. [Google Scholar]
- Van Kampen PN (1905) Die Tympanalgegend des Säugetierschädels. Morph Jahrb 34, 321–722. [Google Scholar]
- Versluys J (1903) Die mittlere und äussere Ohrsphäre der Lacertilia und Rhynchocephalia. Zool Jahrb Morphol 12, 161–406. [Google Scholar]
- Versluys J (1936) Kranium und Visceralskelett der Sauropsiden. I. Reptilien In: Handbuch der Vergleichenden Anatomie der Wirbeltiere IV. (eds Bolk L, Göppert E, Kallius E, Lubosch W.), pp. 699–808, Berlin: Urban & Schwarzenberg. [Google Scholar]
- Vesalius A (1543) De humani corporis fabrica (reprint Wiesbaden: Marix, 2004, pp. 167).
- Wägele J‐W (2000) Grundlagen der Phylogenetischen Systematik. Munich: Verlag Friedrich Pfeil. [Google Scholar]
- Wang Y, Hu Y, Meng J, et al. (2001) An ossified Meckel's cartilage in two Cretaceous mammals and origin of the mammalian middle ear. Science 294, 357–361. [DOI] [PubMed] [Google Scholar]
- Watson DMS (1953) Evolution of the mammalian ear. Evolution 7, 159–177. [Google Scholar]
- Westoll TS (1943) The hyomandibular of Eusthenopteron and the tetrapod middle ear. Proc R Soc Lond B Biol Sci 131, 393–414. [Google Scholar]
- Westoll TS (1945) The mammalian middle ear. Nature 155, 114–115. [Google Scholar]
- Williston SW (1903) North American plesiosaurs Pub Field Columb Mus, Geol Series, Vol. II, pp. 1–77. [Google Scholar]
- Wolpert L, Beddington R, Brockes J, et al. (1998) Principles of Development. Oxford: Oxford University Press. [Google Scholar]
- Zeller U (1989) Die Entwicklung und Morphologie des Schädels von Ornithorhynchus anatinus (Mammalia: Prototheria: Monotremata). Abh Senckenb Naturforsch Ges 545, 1–188. [Google Scholar]
- Zeller U (1993) Ontogenetic evidence for cranial homologies in monotremes and therians, with special reference to Ornithorhynchus In: Mammal Phylogeny, Vol. I. (eds Szalay FS, Novacek MJ, McKenna MC.), pp. 95–107, New York: Springer. [Google Scholar]
- Zheng M, Messerschmidt D, Jungblut B, et al. (2005) Conservation and diversification of Wnt signaling function during the evolution of nematode vulva development. Nat Genet 37, 300–304. [DOI] [PubMed] [Google Scholar]
- Zhou C‐F, Wu S, Martin T, et al. (2013) A Jurassic mammaliaform and the earliest mammalian evolutionary adaptations. Nature 500, 163–167. [DOI] [PubMed] [Google Scholar]


